Development and Validation of a Prediction Model for Positive Findings of Preoperative Flexible Bronchoscopy in Patients with Peripheral Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
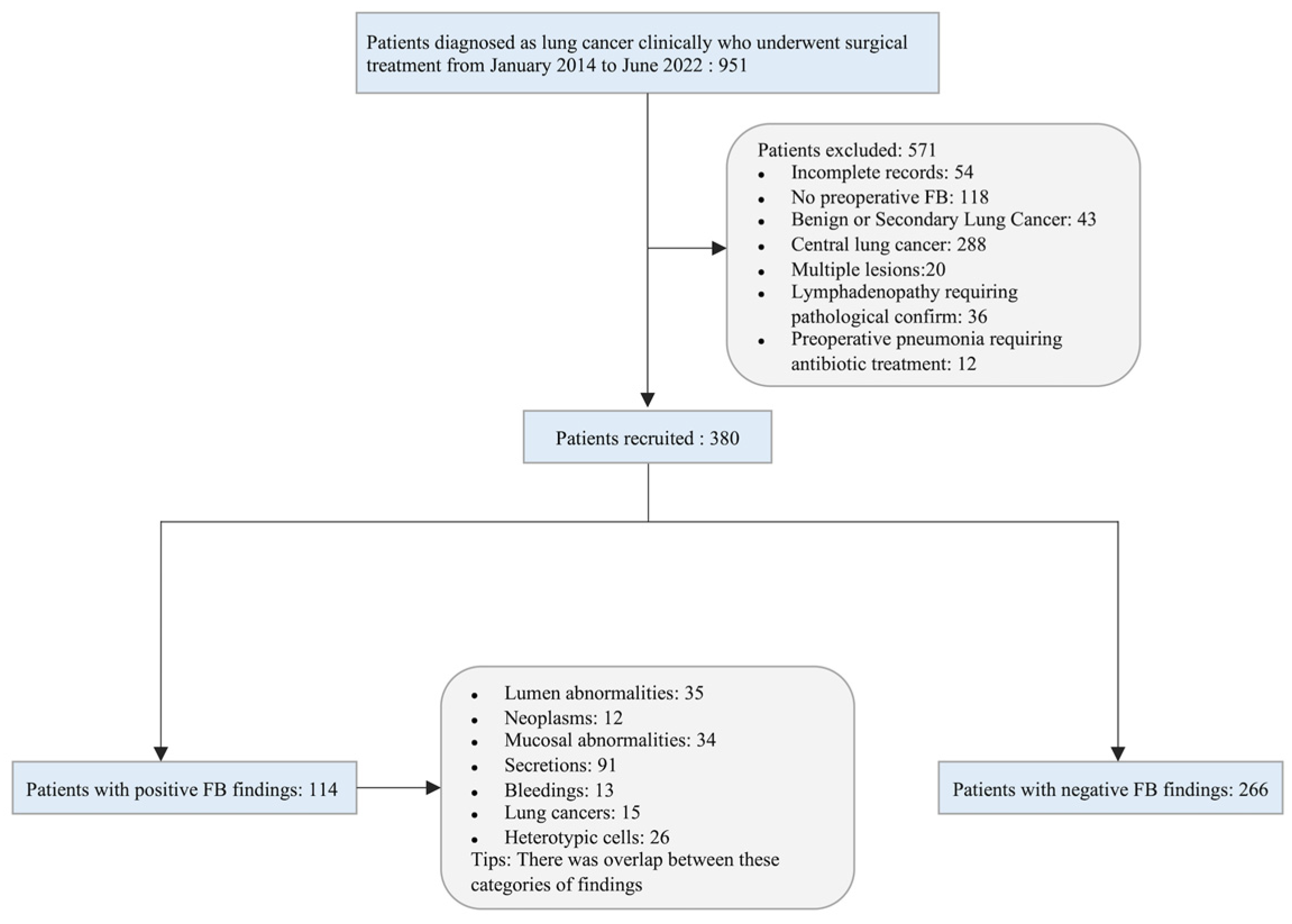
2.1. Study Population
2.2. Procedure of Preoperative Flexible Bronchoscopy
2.3. Positive FB Findings
2.4. Development and Validation of the Model
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Population
3.2. Outcomes
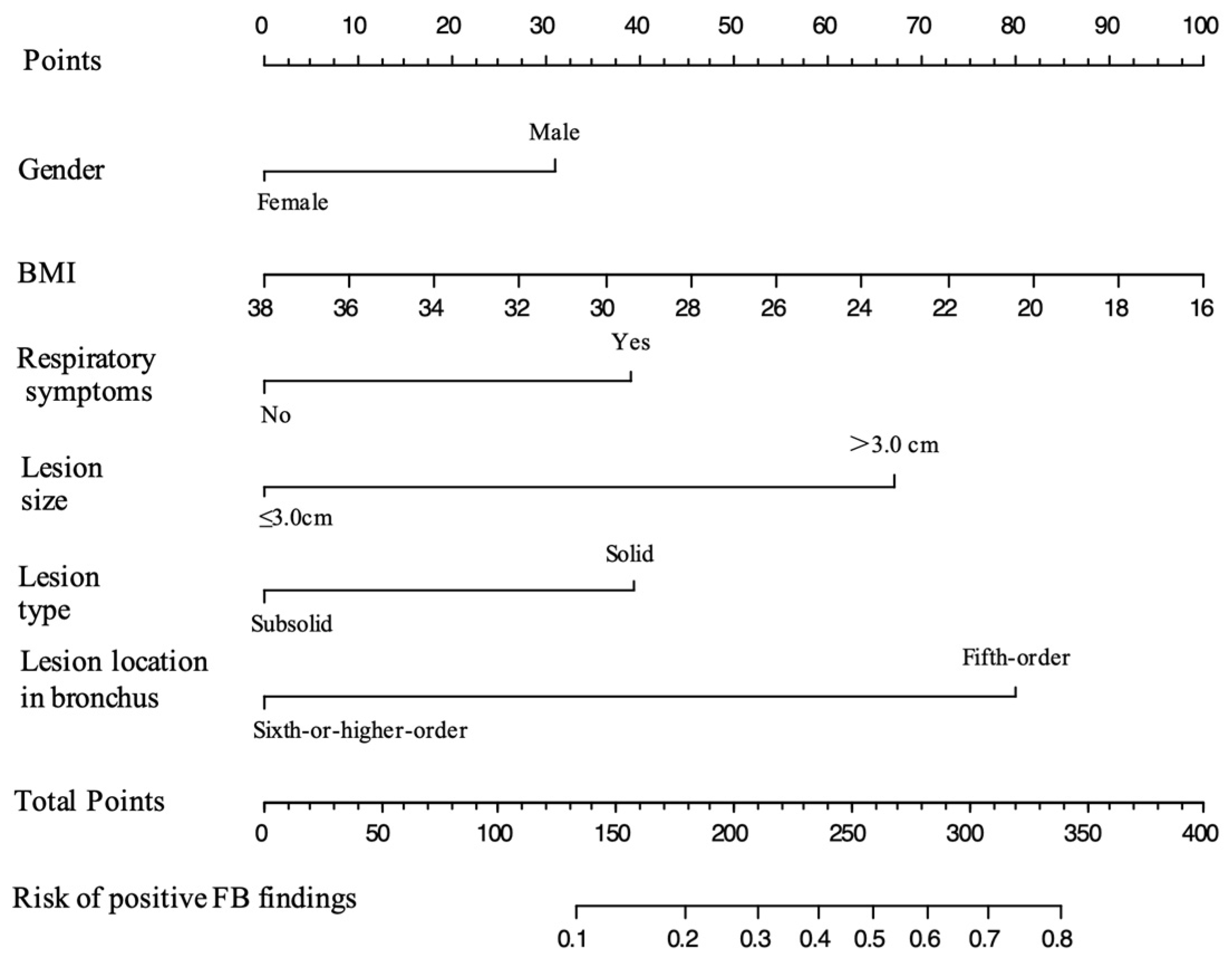
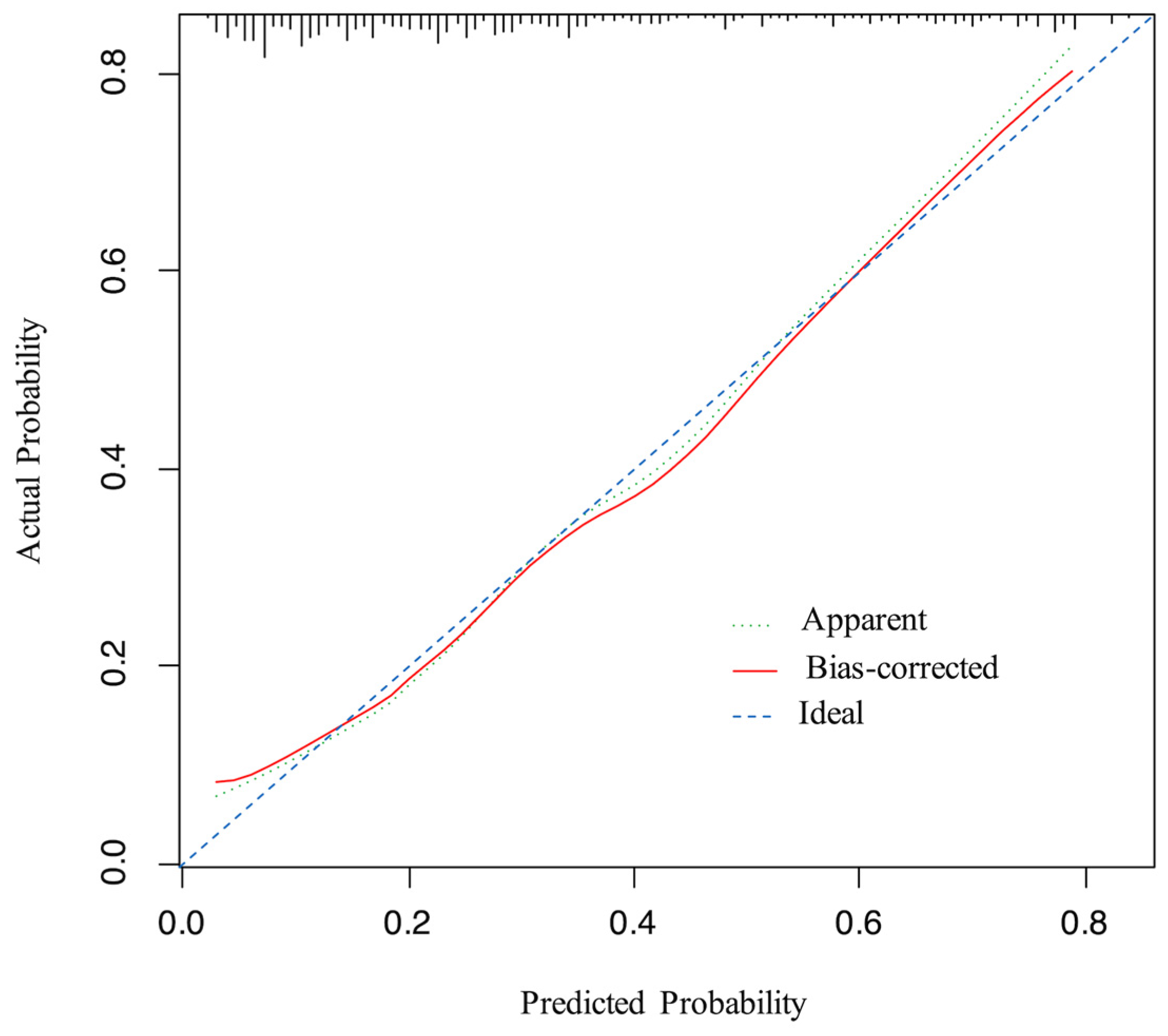
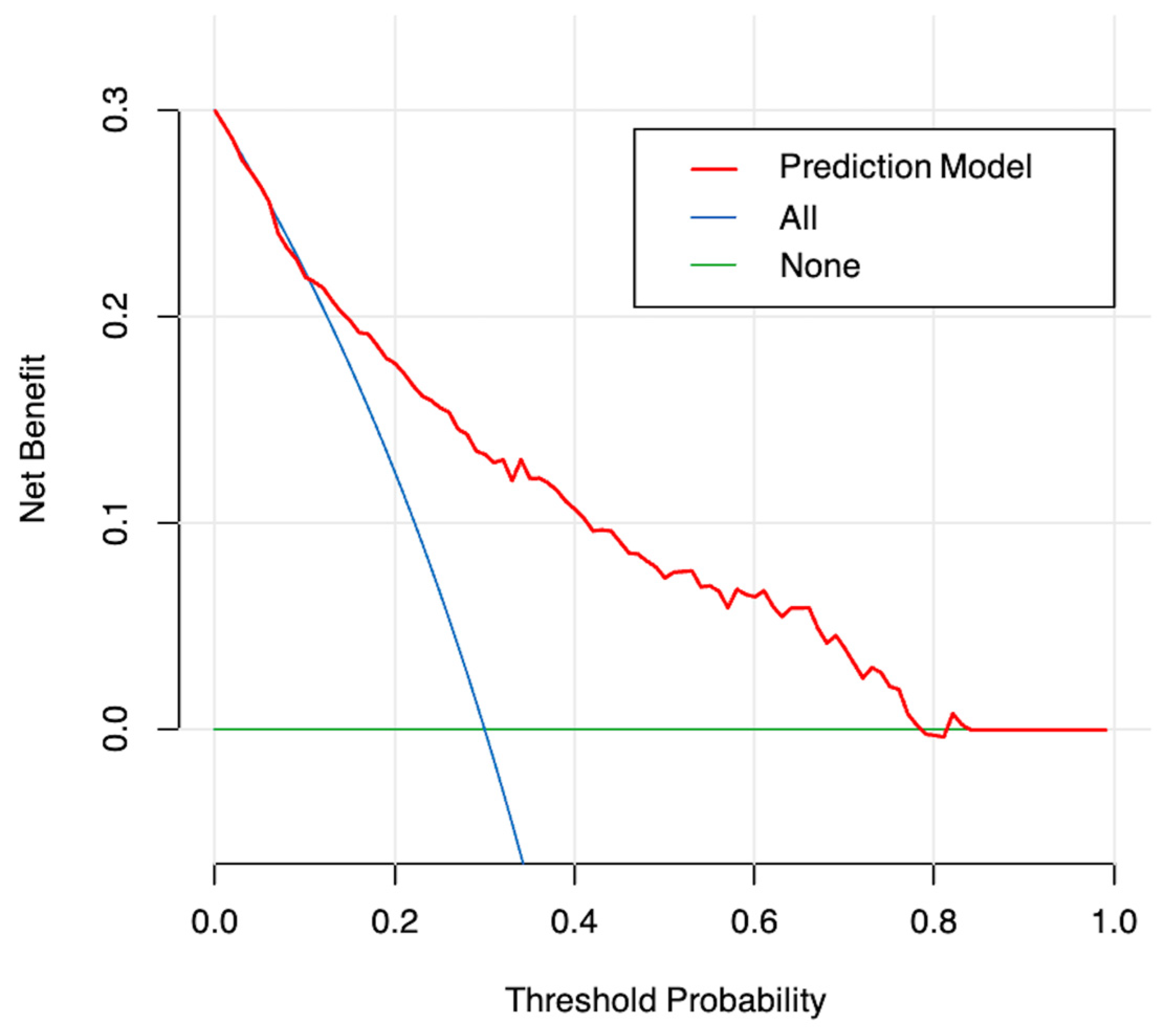
3.3. Development and Validation of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Jain, P.; Arroliga, A.C.; Matthay, R.A. Bronchoscopy and Needle Biopsy Techniques for Diagnosis and Staging of Lung Cancer. Clin. Chest Med. 2002, 23, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Goeckenjan, G.; Sitter, H.; Thomas, M.; Branscheid, D.; Flentje, M.; Griesinger, F.; Niederle, N.; Stuschke, M.; Blum, T.; Deppermann, K.-M.; et al. Prevention, Diagnosis, Therapy, and Follow-up of Lung Cancer. Pneumologie 2011, 65, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L.; Anglade, M.W.; Baker, E.L.; White, C.M.; Kluger, J.; Coleman, C.I. Use of N-Acetylcysteine to Reduce Post-Cardiothoracic Surgery Complications: A Meta-Analysis. Eur. J. Cardiothorac. Surg. 2009, 35, 521–527. [Google Scholar] [CrossRef]
- Odor, P.M.; Bampoe, S.; Gilhooly, D.; Creagh-Brown, B.; Moonesinghe, S.R. Perioperative Interventions for Prevention of Postoperative Pulmonary Complications: Systematic Review and Meta-Analysis. BMJ 2020, 368, m540. [Google Scholar] [CrossRef]
- D’Journo, X.B.; Rolain, J.M.; Doddoli, C.; Raoult, D.; Thomas, P.A. Airways Colonizations in Patients Undergoing Lung Cancer Surgery. Eur. J. Cardio-Thorac. Surg. 2011, 40, 309–319. [Google Scholar] [CrossRef]
- NCCN Guidelines: Non-Small Cell Lung Cancer, Version 2. 2022. Available online: http://www.nccn.org/ (accessed on 15 March 2022).
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of Individuals with Pulmonary Nodules: When Is It Lung Cancer? Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef]
- Jhun, B.W.; Um, S.-W.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Lee, K.S.; Han, J.; Kim, J. Preoperative Flexible Bronchoscopy in Patients with Persistent Ground-Glass Nodule. PLoS ONE 2015, 10, e0121250. [Google Scholar] [CrossRef]
- Ye, T.; Chen, Z.; Ma, D.; Chen, S.; Xia, G.; Zhang, Y.; Li, H.; Zhang, Y.; Luo, X.; Miao, L.; et al. Is Flexible Bronchoscopy Necessary in the Preoperative Workup of Patients with Peripheral CT1N0 Subsolid Lung Cancer?—A Prospective Multi-Center Cohort Study. Transl. Lung Cancer Res. 2021, 10, 1635–1641. [Google Scholar] [CrossRef]
- Silvestri, G.A. Bronchoscopy for the Solitary Pulmonary Nodule. Chest 2012, 142, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.K.; Walkenstein, M.D.; Steinbach, A.; Aranson, R. The Role of Staging Bronchoscopy in the Preoperative Assessment of a Solitary Pulmonary Nodule. Chest 1993, 104, 94–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jo, K.-W.; Kim, H.; Kim, D.; Kim, Y.-H.; Park, S.-I.; Choi, S.; Choi, C.-M. Value of Flexible Bronchoscopy for the Preoperative Assessment of NSCLC Diagnosed Using Percutaneous Core Needle Biopsy. Thorac. Cardiovasc. Surg. 2014, 62, 593–598. [Google Scholar] [CrossRef]
- Torrington, K.G.; Kern, J.D. The Utility of Fiberoptic Bronchoscopy in the Evaluation of the Solitary Pulmonary Nodule. Chest 1993, 104, 1021–1024. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Zhang, Y.; Chen, S.; Li, Y.; Yu, Y.; Sun, Y.; Chen, H. Is Bronchoscopy Necessary in the Preoperative Workup of a Solitary Pulmonary Nodule? J. Thorac. Cardiovasc. Surg. 2015, 150, 36–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the Diagnosis of Lung Cancer. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef]
- Travis, W.D.; Asamura, H.; Bankier, A.A.; Beasley, M.B.; Detterbeck, F.; Flieder, D.B.; Goo, J.M.; MacMahon, H.; Naidich, D.; Nicholson, A.G.; et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1204–1223. [Google Scholar] [CrossRef]
- Moon, Y.; Lee, K.Y.; Sung, S.W.; Park, J.K. Differing Histopathology and Prognosis in Pulmonary Adenocarcinoma at Central and Peripheral Locations. J. Thorac. Dis. 2016, 8, 169. [Google Scholar]
- Sakurai, H.; Asamura, H.; Watanabe, S.; Suzuki, K.; Tsuchiya, R. Clinicopathologic Features of Peripheral Squamous Cell Carcinoma of the Lung. Ann. Thorac. Surg. 2004, 78, 222–227. [Google Scholar] [CrossRef]
- Schwarz, C.; Schonfeld, N.; Bittner, R.C.; Mairinger, T.; Russmann, H.; Bauer, T.T.; Kaiser, D.; Loddenkemper, R. Value of Flexible Bronchoscopy in the Pre-Operative Work-up of Solitary Pulmonary Nodules. Eur. Respir. J. 2013, 41, 177–182. [Google Scholar] [CrossRef]
- Waxman, A.B. Flexible Bronchoscopy: Indications, Contraindications, and Consent. In Introduction to Bronchoscopy; Ernst, A., Herth, F.J.F., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 95–101. ISBN 978-1-316-08418-2. [Google Scholar]
- Han, N.J.; Song, K.-S.; Lee, K.H.; Seo, J.B.; Lee, J.S.; Lim, T.-H.; Kang, G.H. Superficial Endobronchial Lung Cancer: Radiologic-Pathologic Correlation. Korean J. Radiol. 2002, 3, 229. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Brokx, H.A.P.; Postmus, P.E.; Sutedja, T.G. Dual Digital Video-Autofluorescence Imaging for Detection of Pre-Neoplastic Lesions. Lung Cancer 2007, 58, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Asmitanand, T.; Ren, H.; Wu, F.; Zhang, Y.; Li, X.; Di, L.; Song, Z.; Yang, T.; Chen, T.; et al. Fiber-Optic Bronchoscope and Detection of Lung Cancer: A Five Year Study. Neoplasma 2012, 59, 201–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sereno, M.; Esteban, I.R.; Zambrana, F.; Merino, M.; Gómez-Raposo, C.; López-Gómez, M.; Sáenz, E.C. Squamous-Cell Carcinoma of the Lungs: Is It Really so Different? Crit. Rev. Oncol. Hematol. 2012, 84, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Ioanas, M.; Angrill, J.; Baldo, X.; Arancibia, F.; Gonzalez, J.; Bauer, T.; Canalis, E.; Torres, A. Bronchial Bacterial Colonization in Patients with Resectable Lung Carcinoma. Eur. Respir. J. 2002, 19, 326–332. [Google Scholar] [CrossRef]
- Tsuboi, E.; Ikeda, S.; Tajima, M.; Shimosato, Y.; Ishikawa, S. Transbronchial Biopsy Smear for Diagnosis of Peripheral Pulmonary Carcinomas. Cancer 1967, 20, 687–698. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 380) | Positive Findings (n = 114) | Negative Findings (n = 266) | p Value |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Male | 232 (61.1) | 86 (75.4) | 146 (54.9) | |
| Female | 148 (38.9) | 28 (24.6) | 120 (45.1) | |
| Age in years | 0.046 | |||
| Mean (SD) | 61.8 (8.6) | 63.2 (8.1) | 61.3 (8.7) | |
| BMI | 0.002 | |||
| Mean (SD) | 24.29 (3.47) | 23.44 (3.41) | 24.65 (3.43) | |
| Smoking | 0.011 | |||
| Yes | 164 (43.2) | 61 (53.5) | 103 (38.7) | |
| No | 216 (56.8) | 53 (46.5) | 163 (61.3) | |
| History of chronic lung diseases | 0.246 | |||
| Yes | 84 (22.1) | 30 (26.3) | 54 (20.3) | |
| No | 296 (77.9) | 84 (73.7) | 212 (79.7) | |
| Respiratory symptoms | <0.001 | |||
| Yes | 105 (27.6) | 52 (45.6) | 53 (19.9) | |
| No | 275 (72.4) | 62 (54.4) | 213 (80.1) | |
| Lesion size | <0.001 | |||
| ≤3.0 cm | 250 (65.8) | 46 (40.4) | 204 (76.7) | |
| >3.0 cm | 130 (34.2) | 68 (59.6) | 62 (23.3) | |
| Lesion type | <0.001 | |||
| Solid | 303 (79.7) | 104 (91.2) | 199 (74.8) | |
| Subsolid | 77 (20.3) | 10 (8.8) | 67 (25.2) | |
| Lesion location in bronchi | <0.001 | |||
| Fifth order | 234 (61.6) | 94 (82.5) | 140 (52.6) | |
| Sixth-order or higher | 146 (38.4) | 20 (17.5) | 126 (47.4) | |
| Lesion location in lobe | 0.867 | |||
| RUL | 118 (31.1) | 37 (32.5) | 81 (30.5) | |
| RML | 18 (4.7) | 7 (6.1) | 11 (4.1) | |
| RLL | 90 (23.7) | 26 (22.8) | 64 (24.1) | |
| LUL | 103 (27.1) | 28 (24.6) | 75 (28.2) | |
| LLL | 51 (13.4) | 16 (14.0) | 35 (13.2) |
| Variable | Univariable Analysis | |
|---|---|---|
| Odds ratio (95% Confidence Interval) | p-Value | |
| Gender | ||
| Male | Ref. | |
| Female | 0.396 (0.240–0.640) | <0.001 |
| Age | 1.027 (1.001–1.055) | 0.047 |
| BMI | 0.897 (0.835–0.960) | 0.002 |
| Smoking | ||
| No | Ref. | |
| Yes | 1.821 (1.171–2.844) | 0.008 |
| History of chronic lung diseases | ||
| No | Ref. | |
| Yes | 1.402 (0.833–2.331) | 0.196 |
| Respiratory symptoms | ||
| No | Ref. | |
| Yes | 3.371 (2.098–5.442) | <0.001 |
| Lesion size | ||
| ≤3.0 cm | Ref. | |
| >3.0 cm | 4.864 (3.055–7.830) | <0.001 |
| Lesion type | ||
| Solid | Ref. | |
| Subsolid | 0.286 (0.133–0.555) | <0.001 |
| Lesion location in bronchi | ||
| Fifth order | Ref. | |
| Sixth-order or higher | 0.236 (0.135–0.398) | <0.001 |
| Lesion location in lobe | ||
| RUL | Ref. | |
| RML | 1.393 (0.479–3.830) | 0.526 |
| RLL | 0.889 (0.485–1.615) | 0.701 |
| LUL | 0.817 (0.454–1.461) | 0.497 |
| LLL | 1.000 (0.485–2.013) | 0.998 |
| Variable | Multivariable Analysis | Factors Selected for Model | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Gender | ||||
| Male | Ref. | Ref. | ||
| Female | 0.454 (0.218–0.944) | 0.034 | 0.582 (0.332–1.006) | 0.055 |
| Age | 1.019 (0.989–1.051) | 0.226 | ||
| BMI | 0.921 (0.852–0.994) | 0.037 | 0.924 (0.854–0.996) | 0.043 |
| Smoking | ||||
| No | Ref. | |||
| Yes | 0.680 (0.340–1.353) | 0.271 | ||
| Respiratory symptoms | ||||
| No | Ref. | Ref. | ||
| Yes | 2.00 (1.140–3.479) | 0.015 | 1.975 (1.129–3.436) | 0.016 |
| Lesion size | ||||
| ≤3.0 cm | Ref. | Ref. | ||
| >3.0 cm | 3.126 (1.813–5.427) | <0.001 | 3.212 (1.869–5.560) | <0.001 |
| Lesion type | ||||
| Solid | Ref. | Ref. | ||
| Subsolid | 0.486 (0.211–1.040) | 0.074 | 0.505 (0.221–1.070) | 0.087 |
| Lesion location in bronchi | ||||
| Fifth order | Ref. | Ref. | ||
| Sixth-order or higher | 0.234 (0.126–0.418) | <0.001 | 0.248 (0.135–0.438) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, Z.; Li, S.; Zhang, H.; Yao, S.; Li, Y.; Chen, J. Development and Validation of a Prediction Model for Positive Findings of Preoperative Flexible Bronchoscopy in Patients with Peripheral Lung Cancer. Curr. Oncol. 2023, 30, 315-325. https://doi.org/10.3390/curroncol30010025
Li D, Li Z, Li S, Zhang H, Yao S, Li Y, Chen J. Development and Validation of a Prediction Model for Positive Findings of Preoperative Flexible Bronchoscopy in Patients with Peripheral Lung Cancer. Current Oncology. 2023; 30(1):315-325. https://doi.org/10.3390/curroncol30010025
Chicago/Turabian StyleLi, Dongyu, Zaishan Li, Shaolei Li, Hongbing Zhang, Siqing Yao, Yi Li, and Jun Chen. 2023. "Development and Validation of a Prediction Model for Positive Findings of Preoperative Flexible Bronchoscopy in Patients with Peripheral Lung Cancer" Current Oncology 30, no. 1: 315-325. https://doi.org/10.3390/curroncol30010025
APA StyleLi, D., Li, Z., Li, S., Zhang, H., Yao, S., Li, Y., & Chen, J. (2023). Development and Validation of a Prediction Model for Positive Findings of Preoperative Flexible Bronchoscopy in Patients with Peripheral Lung Cancer. Current Oncology, 30(1), 315-325. https://doi.org/10.3390/curroncol30010025





