Identification of a Novel N7-Methylguanosine-Related LncRNA Signature Predicts the Prognosis of Hepatocellular Carcinoma and Experiment Verification
Abstract
1. Introduction
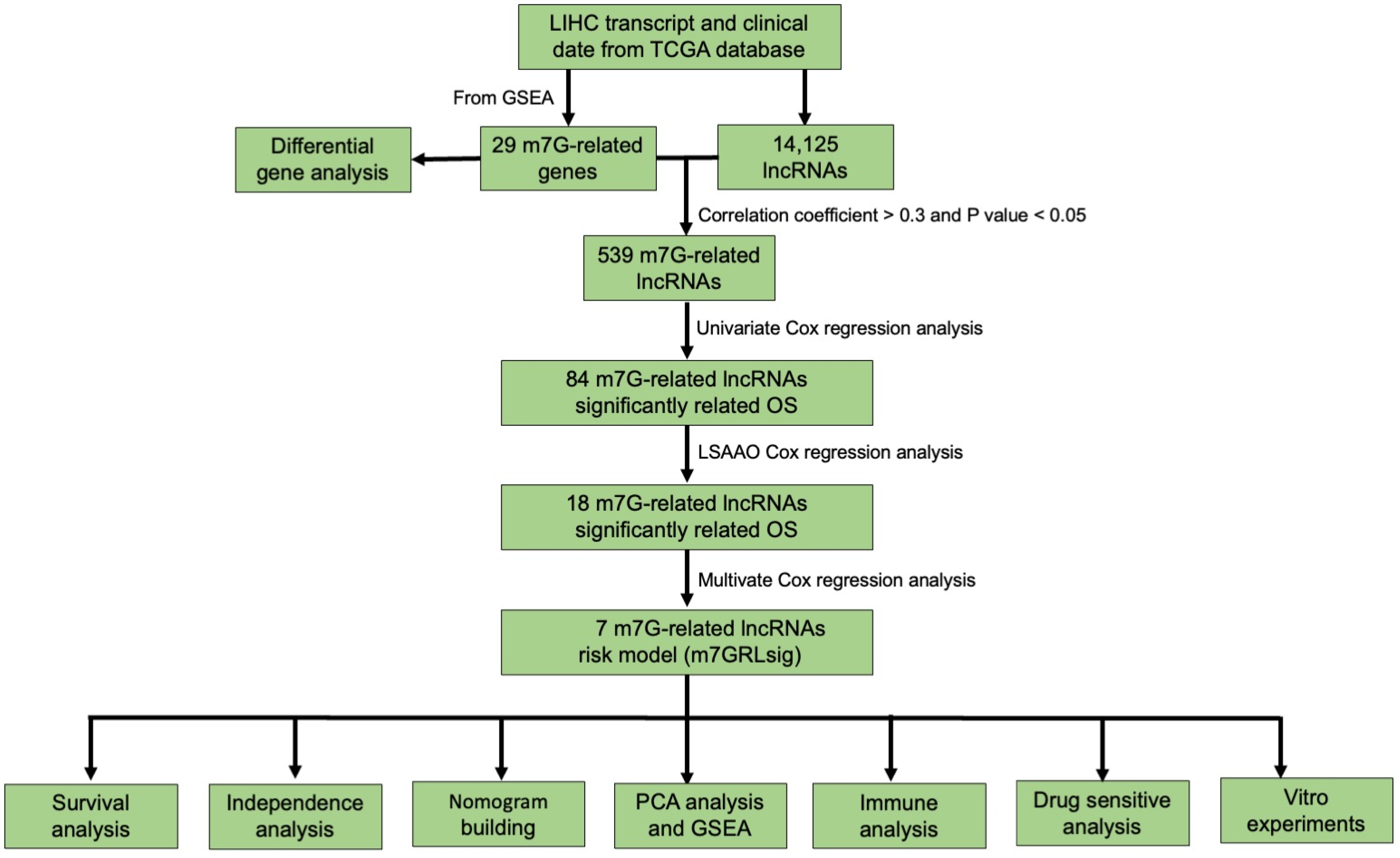
2. Materials and Methods
2.1. Patients and Datasets
2.2. M7G-Related Genes Selection
2.3. Establishment of N7-Methylguanosine-Related lncRNA Signature (m7GRLsig)
2.4. Nomogram Construction
2.5. Immune Landscape Investigation
2.6. Drug Sensitivity Analyses
2.7. Cell Culture
2.8. PCR for Quantitative Reverse Transcription (qRT-PCR)
2.9. Cell Transfection
2.10. Proliferation Assay
2.11. Transwell Analysis
2.12. Statistical Analysis
3. Results
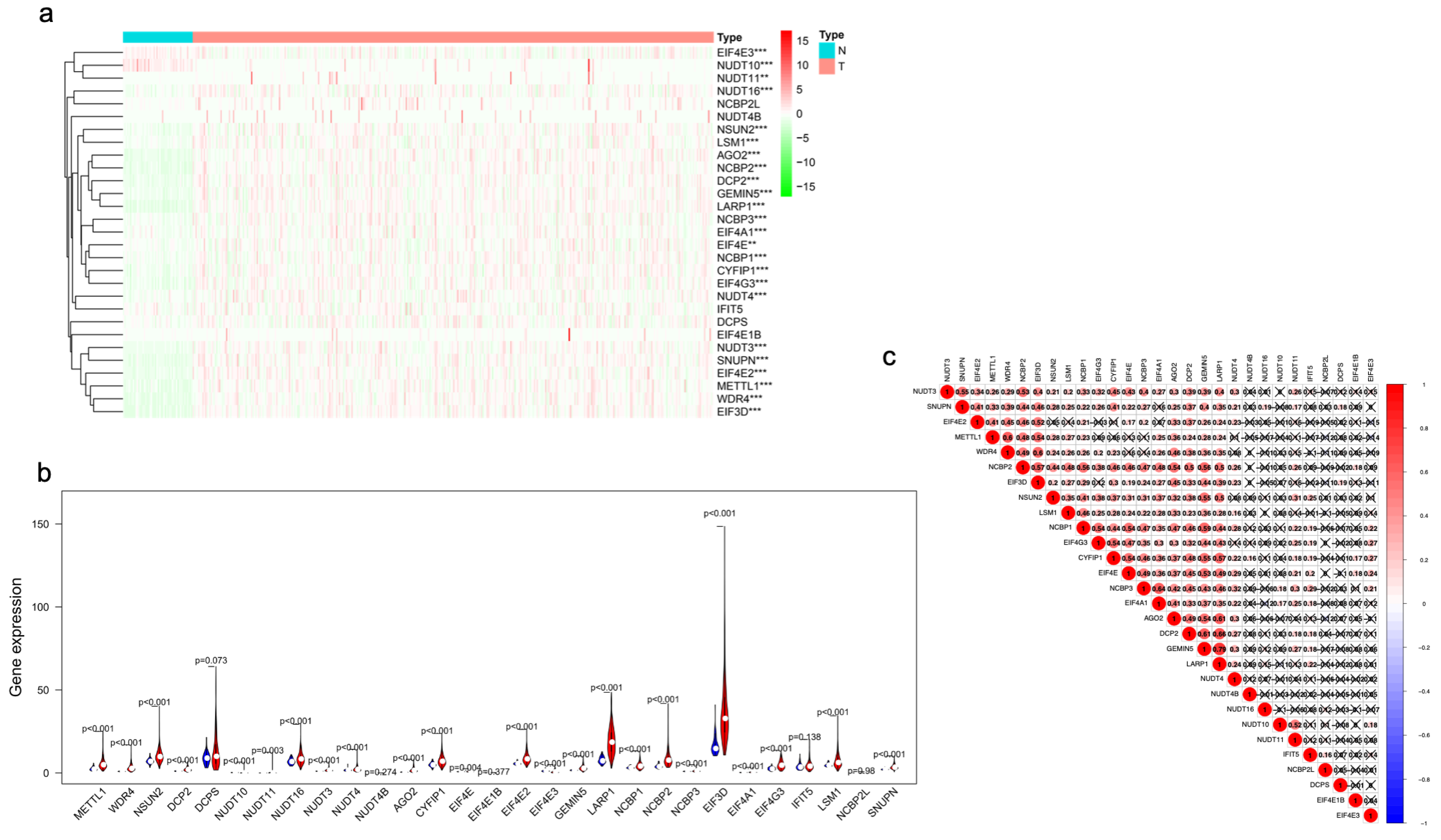
3.1. Differentially Expressed m7G-Related Genes in HCC
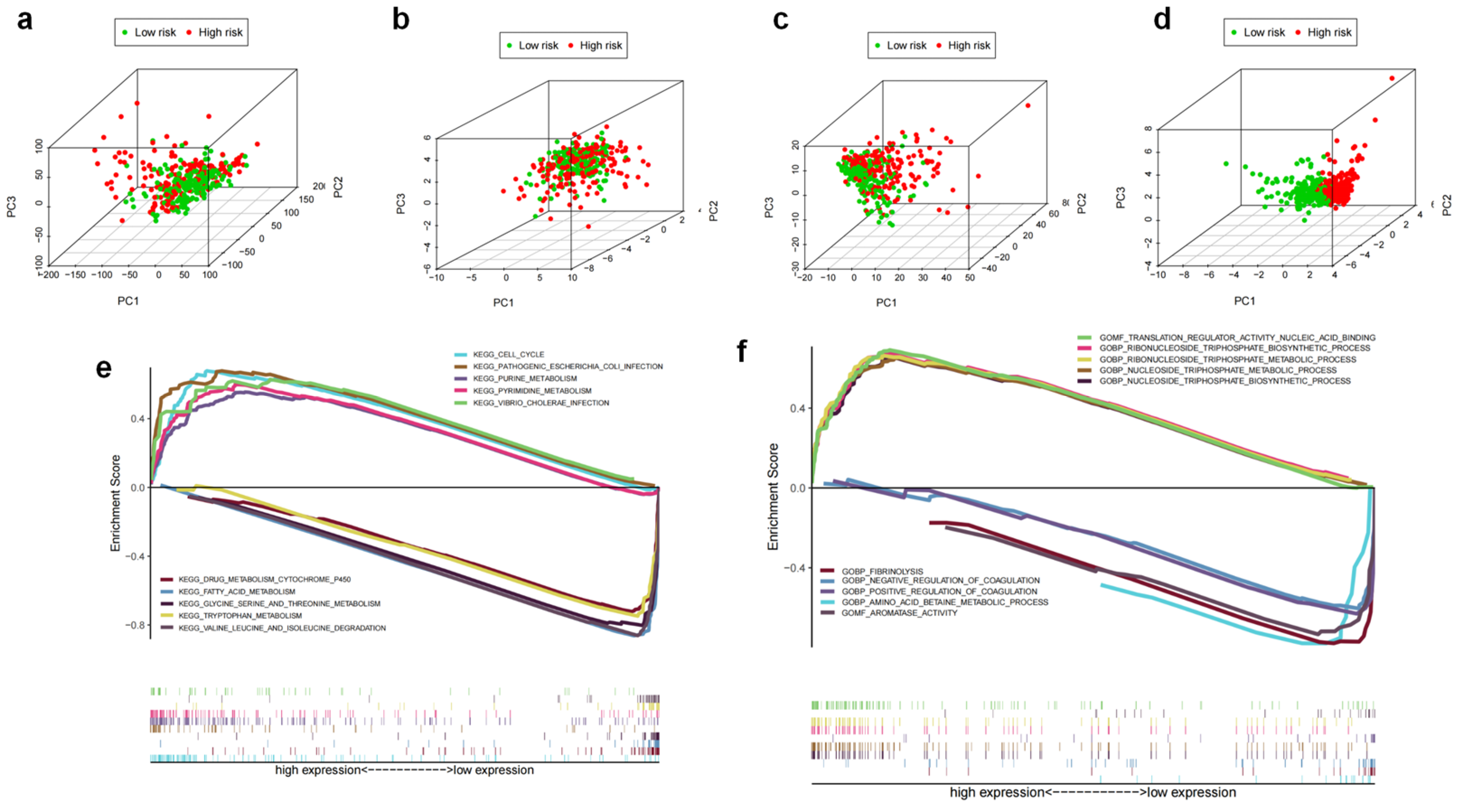
3.2. Identification of the m7G-Related LncRNA Predictive Signature
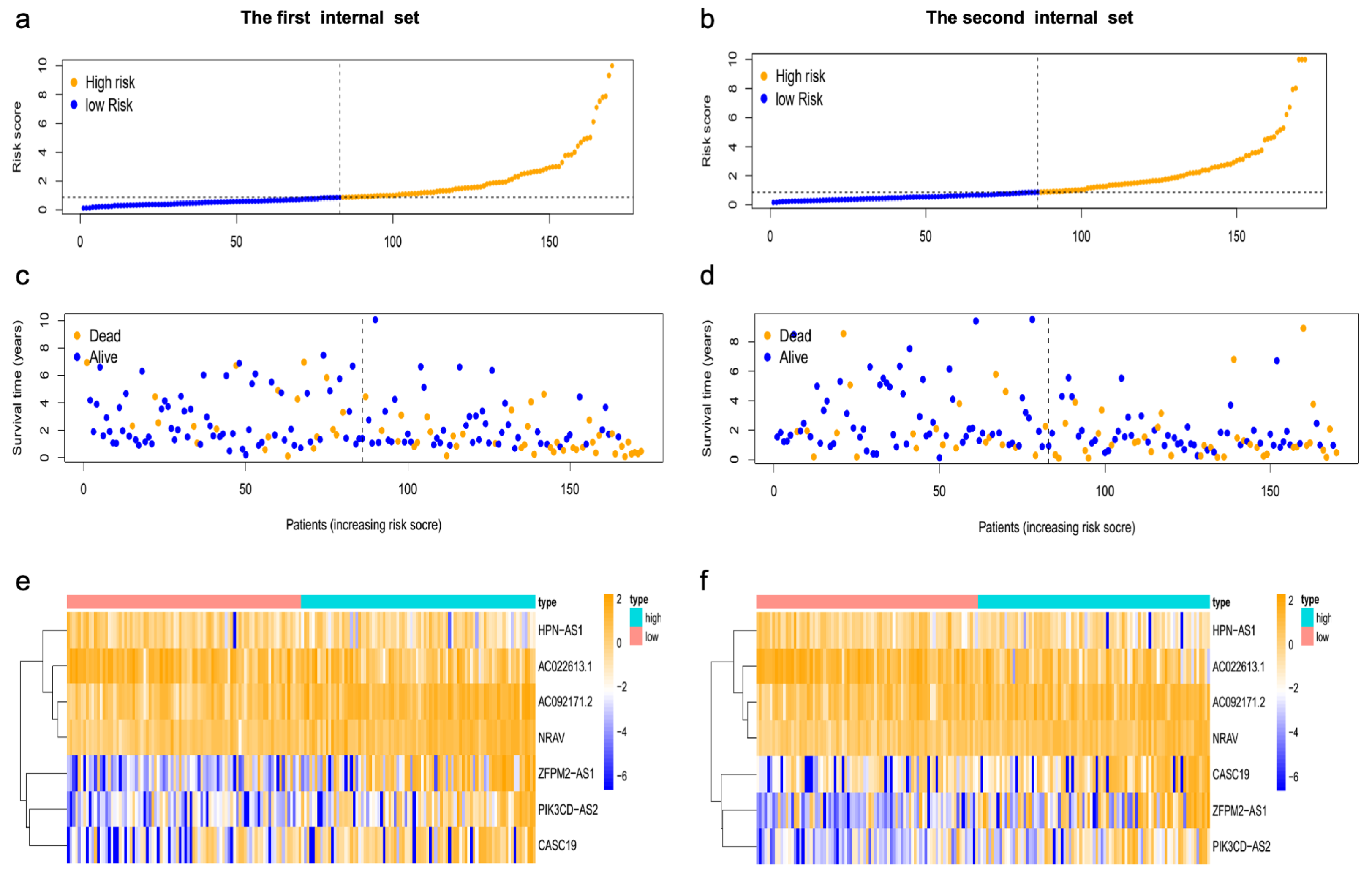
3.3. The Prognostic Influence of m7GRLSig
3.4. Independent Prognostic Analysis of m7GRLSig
3.5. Differential m7G Modification Statuses Stratified by m7GRLSig
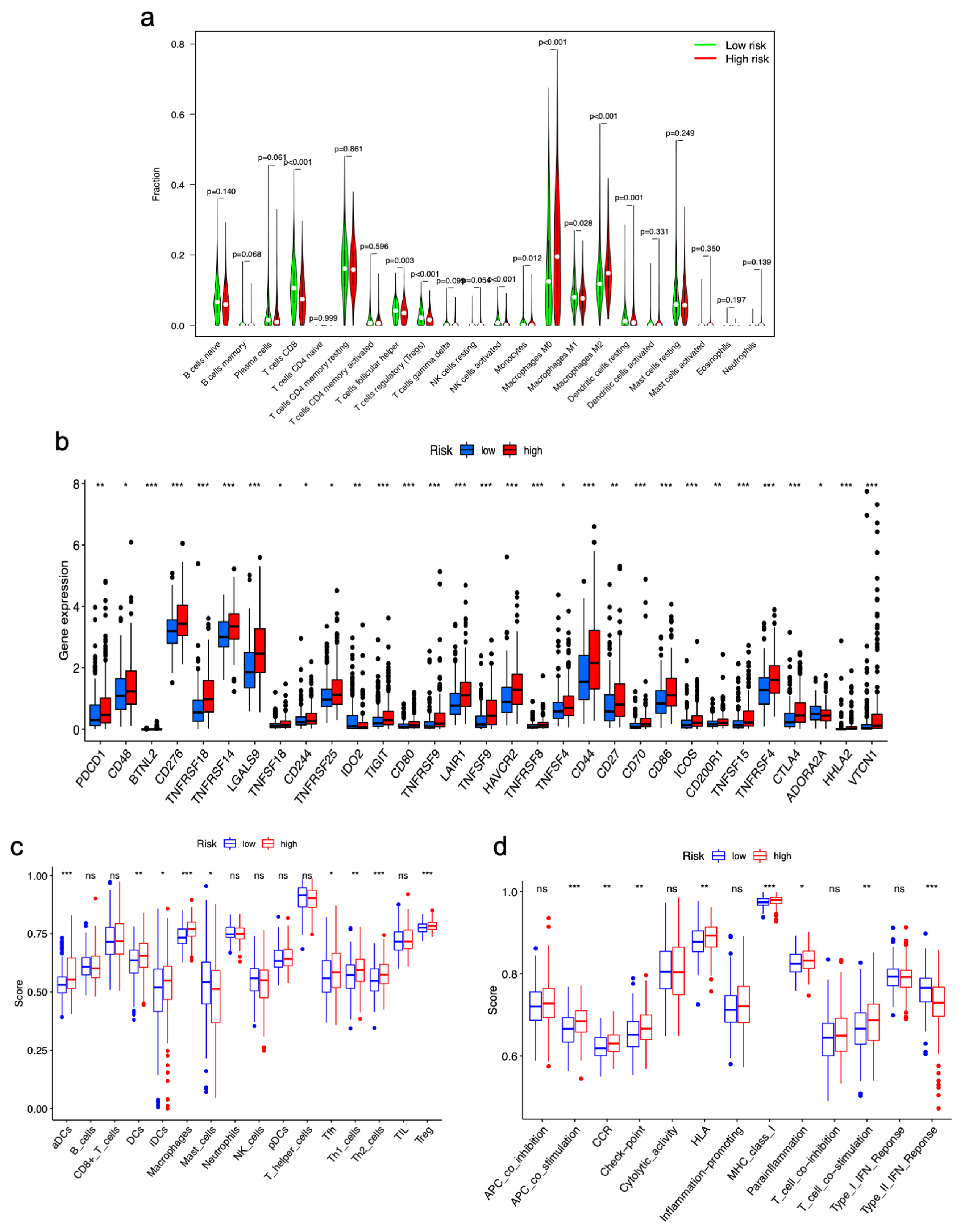
3.6. Correlations between Immune Landscape and m7GRLSig
3.7. Correlations between Drug Treatment and m7GRLSig
3.8. Knockdown of NRAV Inhibited Proliferation and Metastasis of HCC and Affected METTL1 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. A Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International trends in hepatocellular carcinoma incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Miller, K.; Fuchs, H.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Chlachterman, A.; Craft, W.W., Jr.; Hilgenfeldt, E.; Mitra, A.; Cabrera, R. Current and future treatments for hepatocellular. World J. Gastroenterol. 2015, 21, 8478–8491. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Su, C.W.; Huang, Y.H.; Lee, F.Y.; Lin, H.C.; Huo, T.I. Prognosis of Hepatocellular Carcinoma: Assessment of Eleven Staging Systems. J. Hepatol. 2016, 64, 601–608. [Google Scholar] [CrossRef]
- Ozen, C.; Yildiz, G.; Dagcan, A.T.; Cevik, D.; Ors, A.; Keles, U.; Topel, H.; Ozturk, M. Genetics and epigenetics of liver cancer. New Biotechnol. 2013, 30, 381–384. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef]
- Agris, P.F.; Narendran, A.; Sarachan, K.; Väre, V.Y.P.; Eruysal, E. The Importance of Being Modified: The Role of RNA Modifications in Translational Fidelity. Enzymes 2017, 41, 1–50. [Google Scholar] [CrossRef]
- Zhang, M.; Song, J.; Yuan, W.; Zhang, W.; Sun, Z. Roles of RNA Methylation on Tumor Immunity and Clinical. Front. Immunol. 2021, 2, 641507. [Google Scholar] [CrossRef]
- Furuichi, Y.; LaFiandra, A.; Shatkin, A.J. 5′-Terminal structure and mRNA stability. Nature 1977, 266, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Robb, G.B.; Chan, S.H. mRNA capping: Biological functions and applications. Nucleic Acids Res. 2016, 44, 7511–7526. [Google Scholar] [CrossRef] [PubMed]
- Song, B.W.; Tang, Y.J.; Chen, K.Q.; Wei, Z.; Rong, R.; Lu, Z.L.; Su, J.L.; Magalhães, J.; Rigden, D.J.; Meng, J. m7GHub: Deciphering the location, regulation and pathogenesis of internal mRNA N7-methylguanosine (m7G) sites in human. Bioinformatics 2020, 36, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, Q.; Lelyveld, V.S.; Choe, J.; Szostak, J.W.; Gregory, R.I. Mettl1/Wdr4-mediated m (7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell. 2018, 71, 244–255.e5. [Google Scholar] [CrossRef]
- Xie, S.; Chen, W.; Chen, K.; Chang, Y.; Yang, F.; Lin, A.; Shu, Q.; Zhou, T.; Yan, X. Emerging roles of RNA methylation in gastrointestinal cancers. Cancer Cell Int. 2020, 20, 585. [Google Scholar] [CrossRef]
- Osborne, M.J.; Volpon, L.; Memarpoor-Yazdi, M.; Pillay, S.; Thambipillai, A.; Czarnota, S.; Culjkovic-Kraljacic, B.; Trahan, C.; Oeffinger, M.; Cowling, V.H.; et al. Identification and characterization of the interaction between the methyl-7-guanosine cap maturation enzyme RNMT and the cap-binding protein eIF4E. J. Mol. Biol. 2022, 434, 167451. [Google Scholar] [CrossRef]
- Kumar, P.; Sweeney, T.R.; Skabkin, M.A.; Skabkina, O.V.; Hellen, C.U.T.; Pestova, T.V. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5′-terminal regions of cap0-, cap1-and 5′ppp-mRNAs. Nucleic Acids Res. 2014, 42, 3228–3245. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, X.; Carr-Schmid, A.; Kiledjian, M. The hDcp2 Protein Is a Mammalian mRNA Decapping Enzyme. Proc. Natl. Acad. Sci. USA 2022, 999, 12663–12668. [Google Scholar] [CrossRef]
- Han, H.; Yang, C.L.; Ma, J.Y.; Zhang, S.S.; Zheng, S.Y.; Ling, R.S.; Sun, K.Y.; Guo, S.Y.; Huang, B.X.; Liang, Y.; et al. N7-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat. Commun. 2022, 13, 1478. [Google Scholar] [CrossRef]
- Ying, X.; Liu, B.; Yuan, Z.; Huang, Y.; Chen, C.; Jiang, X.; Zhang, H.; Qi, D.; Yang, S.; Lin, S.; et al. METTL1-m (7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clin. Transl. Med. 2021, 11, e6752021. [Google Scholar] [CrossRef]
- Tian, Q.H.; Zhang, M.F.; Zeng, J.S.; Luo, R.G.; Wen, Y.; Chen, J.; Gan, L.G.; Xiong, J.P. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J. Mol. Med. 2019, 97, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Li, S.; Teng, K.W.; Foster, C.J.R.; Peng, D.; Ran, H.; Mita, P.; Geer, M.J.; Hattori, T.; Koide, A.; et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J. Exp. Med. 2021, 218, e202014142021. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique Features of Long Non-coding RNA Biogenesis and Function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Abbastabar, M.; Sarfi, M.; Golestani, A.; Ehsan, K. lncRNA Involvement in Hepatocellular Carcinoma Metastasis and Prognosis. EXCLI J. 2018, 17, 900–913. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Xi, X.; Zhang, M.; Liu, X.; Tang, W.; Cai, P.; Xing, S.; Bao, P.; Jin, Y.; et al. Integrative Analysis of Long Extracellular RNAs Reveals a Detection Panel of Noncoding RNAs for Liver Cancer. Theranostics 2021, 11, 181–193. [Google Scholar] [CrossRef]
- Tomikawa, C. 7-Methylguanosine Modifications in Transfer RNA (tRNA). Int. J. Mol. Sci. 2018, 19, 4080. [Google Scholar] [CrossRef]
- Geeleher, P.; Cox, N.J.; Huang, R.S. Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines. Genome Biol. 2014, 15, R472014. [Google Scholar] [CrossRef]
- Orellana, E.A.; Liu, Q.; Yankova, E.; Pirouz, M.; Braekeleer, E.D.; Zhang, W.; Lim, J.; Aspris, D.; Sendinc, E.; Garyfallos, D.A.; et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell. 2021, 81, 3323–3338.e14. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.; Zhao, Y.; Chi, Q.; Wang, Z.; Sun, B. Overexpressed methyltransferase-like 1 (METTL1) increased chemosensitivity of colon cancer cells to cisplatin by regulating miR-149-3p/S100A4/p53 axis. Aging 2019, 11, 12328–12344. [Google Scholar] [CrossRef]
- Luo, Y.J.; Yao, Y.X.; Wu, P.; Zi, X.H.; Sun, N.; He, J. The potential role of N7-methylguanosine (m7G) in cancer. J. Hematol. Oncol. 2022, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.L.; Niu, Y.; Cheng, S.J.; Li, Y.Y.; Bai, Z.F.; Yu, L.X.; Hong, Z.X.; Liu, H.; Liu, H.H.; Yan, J.; et al. Upregulation of long noncoding RNA W42 promotes tumor development by binding with DBN1 in hepatocellular carcinoma. World. J. Gastroentero. 2021, 27, 2586–2602. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Wang, F.F.; Wang, M.J.; Han, S.F.; Cui, Z.H.; Xi, S.M.; Xu, H.X.; Li, S.P. Downregulation of HULC Induces Ferroptosis in Hepatocellular Carcinoma via Targeting of the miR-3200-5p/ATF4 Axis. Oxid. Med. Cell Longev. 2022, 2022, 9613095. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wu, K.F.; Liu, K.L.; Gou, L.X.; Huang, A.L.; Tang, H. LINC02154 promotes the proliferation and metastasis of hepatocellular carcinoma by enhancing SPC24 promoter activity and activating the PI3K-AKT signaling pathway. Cell Oncol. 2022, 45, 447–462. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhao Zj Wang, J.B.; Shao, Y.H.; Liu, H.; You, J.X.; Yang, X.T. m7G Methylation-Related Genes as Biomarkers for Predicting Overall Survival Outcomes for Hepatocellular Carcinoma. Front. Bioeng. Biotechnol. 2022, 10, 849756. [Google Scholar] [CrossRef]
- Rong, J.; Wang, H.; Yao, Y.; Wu, Z.; Chen, L.; Jin, C.; Shi, Z.; Wu, C.; Hu, X. Identification of m7G-associated lncRNA prognostic signature for predicting the immune status in cutaneous melanoma. Aging 2022, 29, 5233–5249. [Google Scholar] [CrossRef]
- Chen, W.B.; Zhang, X.J.; Bi, K.F.; Zhou, H.T.; Xu, J.; Dai, Y.; Diao, H.Y. Comprehensive Study of Tumor Immune Microenvironment and Relevant Genes in Hepatocellular Carcinoma Identifies Potential Prognostic Significance. Front. Oncol. 2020, 10, 554165. [Google Scholar] [CrossRef]
- Giraud, J.; Chalopin, D.; Blanc, J.F.; Saleh, M. Hepatocellular Carcinoma Immune Landscape and the Potential of Immunotherapies. Front. Immunol. 2021, 12, 655697. [Google Scholar] [CrossRef]
- Huang, A.; Yang, X.R.; Chung, W.Y.; Dennison, A.R.; Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther. 2020, 11, 146. [Google Scholar] [CrossRef]
- Dai, Y.Z.; Liu, Y.D.; Li, J.; Chen, M.T.; Huang, M.; Wang, F.; Yang, Q.S.; Yuan, J.H.; Sun, S.H. METTL16 promotes hepatocellular carcinoma progression through downregulating RAB11B-AS1 in an m 6 A-dependent manner. Cell Mol. Biol. Lett. 2022, 20, 41. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, Z.; Gao, W.; Zhang, Y.; Wang, J.; Wang, J.; Cao, M.; Cai, J.; Wu, J.; Wang, X. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J. Hematol. Oncol. 2020, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.; Li, N.; He, L.; Zhang, S.; An, Y. Long non-coding RNA ILF3-AS1 facilitates hepatocellular carcinoma progression by stabilizing ILF3 mRNA in an m6A-dependent manner. Hum. Cell 2021, 34, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, M.; Wang, L. LncRNA CASC11 Promotes Hepatocellular Carcinoma Progression via Upregulation of UBE2T in a m6A-Dependent Manner. Front. Oncol. 2021, 11, 772671. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Sun, J.; Zhang, W.; Zhang, H.; Li, H. Long intergenic non-protein coding RNA 1273 confers sorafenib resistance in hepatocellular carcinoma via regulation of methyltransferase 3. Bioengineered 2022, 13, 3108–3121. [Google Scholar] [CrossRef] [PubMed]
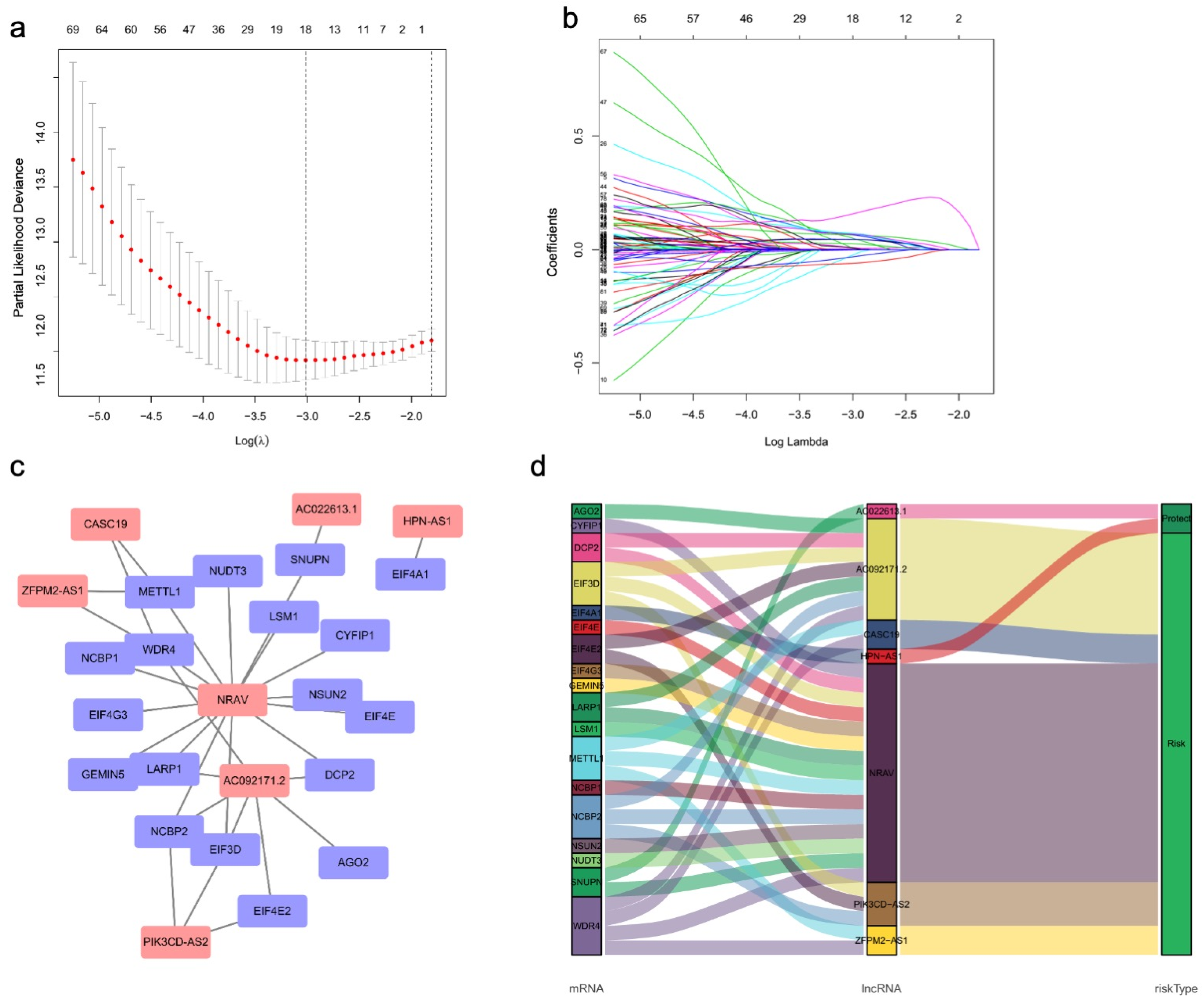

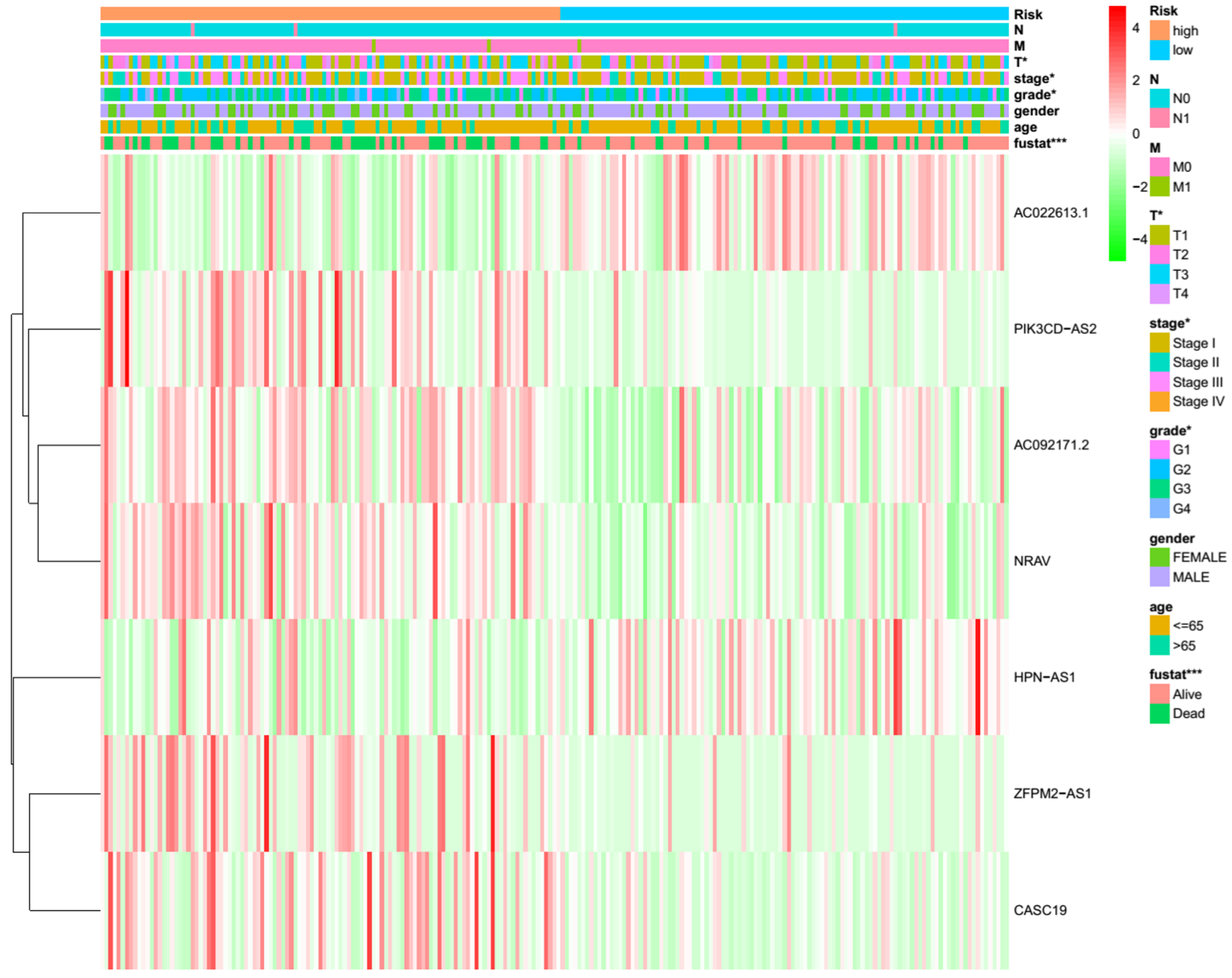




| LncRNAs | Coeffcient | HR | HR.95L | HR.95H |
|---|---|---|---|---|
| ZFPM2-AS1 | 0.181329605 | 1.198810248 | 0.946806641 | 1.517887549 |
| AC092171.2 | 0.360712851 | 1.434351529 | 0.977113068 | 2.105553981 |
| PIK3CD-AS2 | 0.340465024 | 1.405601077 | 0.932002427 | 2.119859704 |
| HPN-AS1 | −0.427741428 | 0.651979977 | 0.380372155 | 1.117531565 |
| AC022613.1 | −0.417716086 | 0.658549173 | 0.492770614 | 0.88009918 |
| NRAV | 0.488602295 | 1.630036318 | 0.929726329 | 2.85785001 |
| CASC19 | 0.342542856 | 1.408524715 | 0.973281485 | 2.038405029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhang, L.; Hao, X.; Tang, M.; Zhou, B.; Hou, J. Identification of a Novel N7-Methylguanosine-Related LncRNA Signature Predicts the Prognosis of Hepatocellular Carcinoma and Experiment Verification. Curr. Oncol. 2023, 30, 430-448. https://doi.org/10.3390/curroncol30010035
Yang C, Zhang L, Hao X, Tang M, Zhou B, Hou J. Identification of a Novel N7-Methylguanosine-Related LncRNA Signature Predicts the Prognosis of Hepatocellular Carcinoma and Experiment Verification. Current Oncology. 2023; 30(1):430-448. https://doi.org/10.3390/curroncol30010035
Chicago/Turabian StyleYang, Chou, Lingyan Zhang, Xin Hao, Mengdie Tang, Bin Zhou, and Jinlin Hou. 2023. "Identification of a Novel N7-Methylguanosine-Related LncRNA Signature Predicts the Prognosis of Hepatocellular Carcinoma and Experiment Verification" Current Oncology 30, no. 1: 430-448. https://doi.org/10.3390/curroncol30010035
APA StyleYang, C., Zhang, L., Hao, X., Tang, M., Zhou, B., & Hou, J. (2023). Identification of a Novel N7-Methylguanosine-Related LncRNA Signature Predicts the Prognosis of Hepatocellular Carcinoma and Experiment Verification. Current Oncology, 30(1), 430-448. https://doi.org/10.3390/curroncol30010035




