Identification of Potential Predictors of Prognosis and Sorafenib-Associated Survival Benefits in Patients with Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Protocol
2.3. Outcomes
2.4. Radiographic Evaluation
2.5. Serological VEGF-A and Other Indicators Measurements
2.6. Statistical Analysis
3. Results
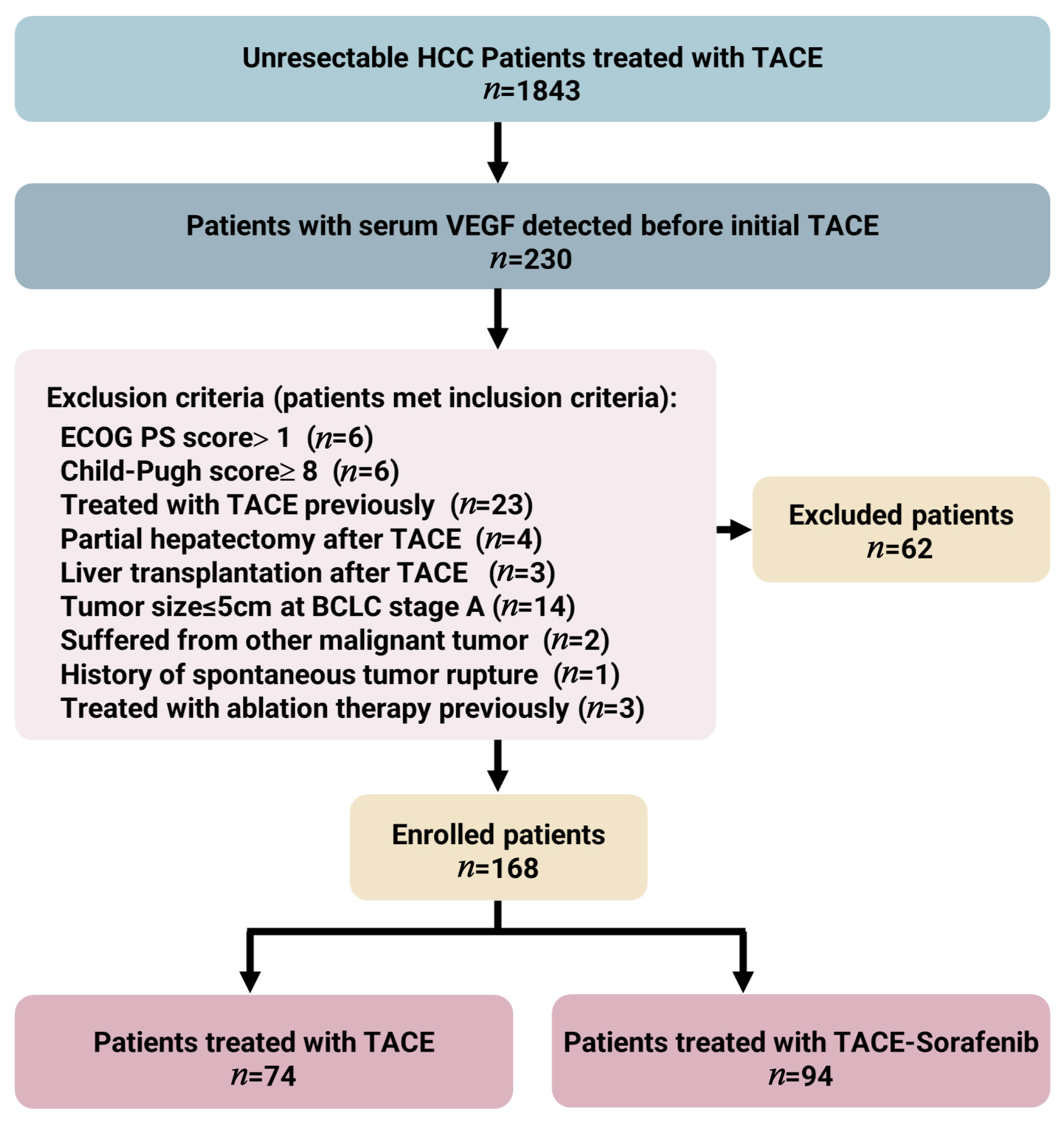
3.1. Baseline Characteristics and Follow-Up of Unresectable HCC Patients
3.2. Survival Analysis and Tumor Response
3.3. Adverse Events Attributed to Sorafenib
3.4. Prognostic Value of Serum VEGF
3.5. Nonlinear Association and Threshold Effect of Baseline VEGF on OS
3.6. Predictive Value of Serum VEGF and Other Clinical Characteristics
3.7. Comparison of Mortality Based on Different VEGF Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricke, J.; Klümpen, H.J.; Amthauer, H.; Bargellini, I.; Bartenstein, P.; de Toni, E.N.; Gasbarrini, A.; Pech, M.; Peck-Radosavljevic, M.; Popovič, P.; et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Xie, M.; Ji, X.; Luo, X.; Chen, X.; Zhang, B.; Liu, D.; Feng, Y.; Sun, M.; et al. HGF-mediated elevation of ETV1 facilitates hepatocellular carcinoma metastasis through upregulating PTK2 and c-MET. J. Exp. Clin. Cancer Res. CR 2022, 41, 275. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; An, J.; Lim, Y.S.; Han, S.; Lee, J.Y.; Byun, J.H.; Won, H.J.; Lee, S.J.; Lee, H.C.; Lee, Y.S. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017, 3, 456–463. [Google Scholar] [CrossRef]
- Ma, S.; Tang, K.H.; Chan, Y.P.; Lee, T.K.; Kwan, P.S.; Castilho, A.; Ng, I.; Man, K.; Wong, N.; To, K.F.; et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010, 7, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Shao, D.; Chang, Z.; Lu, M.; Wang, Y.; Yue, J.; Yang, D.; Li, M.; Xu, Q.; Dong, W.F. Janus Gold Nanoplatform for Synergetic Chemoradiotherapy and Computed Tomography Imaging of Hepatocellular Carcinoma. ACS Nano 2017, 11, 12732–12741. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Golfieri, R.; Bezzi, M.; Verset, G.; Fucilli, F.; Mosconi, C.; Cappelli, A.; Paccapelo, A.; Lucatelli, P.; Magand, N.; Rode, A.; et al. Balloon-Occluded Transarterial Chemoembolization: In Which Size Range Does It Perform Best? A Comparison of Its Efficacy versus Conventional Transarterial Chemoembolization, Using Propensity Score Matching. Liver Cancer 2021, 10, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Giannini, E.G.; Moscatelli, A.; Pellegatta, G.; Vitale, A.; Farinati, F.; Ciccarese, F.; Piscaglia, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Application of the Intermediate-Stage Subclassification to Patients With Untreated Hepatocellular Carcinoma. Am. J. Gastroenterol. 2016, 111, 70–77. [Google Scholar] [CrossRef]
- Sangro, B.; Salem, R. Transarterial chemoembolization and radioembolization. Semin. Liver Dis. 2014, 34, 435–443. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, M.J.; Sayed, A.A.; Sharma, R.; Siddique, A.; Pinato, D.J. Challenges and Opportunities in the Clinical Development of Immune Checkpoint Inhibitors for Hepatocellular Carcinoma. Hepatology 2019, 69, 2258–2270. [Google Scholar] [CrossRef] [Green Version]
- Flohr, F.; Harder, J.; Seufert, J.; Blum, H.E.; Spangenberg, H.C. Hypothyroidism in patients with hepatocellular carcinoma treated by transarterial chemoembolization. Hepatology 2008, 47, 2144. [Google Scholar] [CrossRef] [PubMed]
- Graepler, F.; Verbeek, B.; Graeter, T.; Smirnow, I.; Kong, H.L.; Schuppan, D.; Bauer, M.; Vonthein, R.; Gregor, M.; Lauer, U.M. Combined endostatin/sFlt-1 antiangiogenic gene therapy is highly effective in a rat model of HCC. Hepatology 2005, 41, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Duda, D.G.; Dima, S.O.; Cucu, D.; Sorop, A.; Klein, S.; Ancukiewicz, M.; Kitahara, S.; Iacob, S.; Bacalbasa, N.; Tomescu, D.; et al. Potential Circulating Biomarkers of Recurrence after Hepatic Resection or Liver Transplantation in Hepatocellular Carcinoma Patients. Cancers 2020, 12, 1275. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, J.; Lai, L.; Meng, X.; Zhou, B.; Huang, W.; Cai, M.; Shan, H. Hepatocellular carcinoma with portal vein tumor thrombus: Treatment with transarterial chemoembolization combined with sorafenib—A retrospective controlled study. Radiology 2014, 272, 284–293. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Yang, J.; Zhang, Y.; Wang, Y.; Fan, W.; Huang, Y.; Wang, W.; Ran, H.; Ke, S. Combination of individualized local control and target-specific agent to improve unresectable liver cancer managements: A matched case-control study. Target. Oncol. 2015, 10, 287–295. [Google Scholar] [CrossRef]
- Geschwind, J.F.; Kudo, M.; Marrero, J.A.; Venook, A.P.; Chen, X.P.; Bronowicki, J.P.; Dagher, L.; Furuse, J.; Ladrón de Guevara, L.; Papandreou, C.; et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology 2016, 279, 630–640. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Wang, M.; Yang, G.; Ye, X.; Wu, M.; Cheng, S. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: A systematic review and meta-analysis. Oncotarget 2017, 8, 29416–29427. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Song, R.; Pang, P.; Yan, Y.; Liao, Y.; Zhou, C.; Wang, S.; Zhou, X.; Wang, H.; Zhang, H.; et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: A meta-analysis. BMC Cancer 2017, 17, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Zhao, W.; Wang, M.; Hu, J.; Wang, E.; Zhao, Y.; Liu, L. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2018, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M.; Han, G.; Tak, W.Y.; Yang, J.; Guglielmi, A.; Paik, S.W.; Reig, M.; Kim, D.Y.; Chau, G.Y.; et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J. Hepatol. 2016, 64, 1090–1098. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Peña, C.E.; Lathia, C.D.; Shan, M.; Meinhardt, G.; Bruix, J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2290–2300. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Cheng, A.L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. Division of Cancer Treatment & Diagnosis. Cancer Therapy Evaluation Program. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 22 May 2022).
- Hothorn, T.; Zeileis, A. Generalized maximally selected statistics. Biometrics 2008, 64, 1263–1269. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Zhang, Y.; Zheng, P.; Wu, Z.; Wang, X.; Chen, Y.; Chen, X. Elevated Platelet Count is Associated with Poor Survival After Transarterial Chemoembolization Treatment in Patients with Hepatocellular Carcinoma: A Cohort Study. J. Hepatocell. Carcinoma 2020, 7, 191–199. [Google Scholar] [CrossRef]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M.; Imanaka, K.; Chida, N.; Nakachi, K.; Tak, W.Y.; Takayama, T.; Yoon, J.H.; Hori, T.; Kumada, H.; Hayashi, N.; et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Han, G.; Finn, R.S.; Poon, R.T.; Blanc, J.F.; Yan, L.; Yang, J.; Lu, L.; Tak, W.Y.; Yu, X.; et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology 2014, 60, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Cheng, A.L.; Park, J.W.; Park, J.H.; Liang, P.C.; Hidaka, H.; Izumi, N.; Heo, J.; Lee, Y.J.; Sheen, I.S.; et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): A randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol. Hepatol. 2018, 3, 37–46. [Google Scholar] [CrossRef]
- Luo, P.; Yan, H.; Chen, X.; Zhang, Y.; Zhao, Z.; Cao, J.; Zhu, Y.; Du, J.; Xu, Z.; Zhang, X.; et al. s-HBEGF/SIRT1 circuit-dictated crosstalk between vascular endothelial cells and keratinocytes mediates sorafenib-induced hand-foot skin reaction that can be reversed by nicotinamide. Cell Res. 2020, 30, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, H.; Bai, W.; Liu, J.; Lv, W.; Sahu, S.; Guan, S.; Qin, X.; Wang, W.; Ren, W.; et al. Early sorafenib-related adverse events predict therapy response of TACE plus sorafenib: A multicenter clinical study of 606 HCC patients. Int. J. Cancer 2016, 139, 928–937. [Google Scholar] [CrossRef]
- Zhong, B.Y.; Ni, C.F.; Chen, L.; Zhu, H.D.; Teng, G.J. Early Sorafenib-related Biomarkers for Combination Treatment with Transarterial Chemoembolization and Sorafenib in Patients with Hepatocellular Carcinoma. Radiology 2017, 284, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Himmelsbach, K.; Sauter, D.; Baumert, T.F.; Ludwig, L.; Blum, H.E.; Hildt, E. New aspects of an anti-tumour drug: Sorafenib efficiently inhibits HCV replication. Gut 2009, 58, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F. Editorial comment on: Large-scale real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: Identification of VEGFA as a major independent prognostic marker. Eur. Urol. 2009, 56, 688–689. [Google Scholar] [CrossRef]
- Lacin, S.; Yalcin, S. The Prognostic Value of Circulating VEGF-A Level in Patients with Hepatocellular Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820971677. [Google Scholar] [CrossRef]
- Jinno, K.; Tanimizu, M.; Hyodo, I.; Nishikawa, Y.; Hosokawa, Y.; Doi, T.; Endo, H.; Yamashita, T.; Okada, Y. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J. Gastroenterol. 1998, 33, 376–382. [Google Scholar] [CrossRef]
- Moon, W.S.; Rhyu, K.H.; Kang, M.J.; Lee, D.G.; Yu, H.C.; Yeum, J.H.; Koh, G.Y.; Tarnawski, A.S. Overexpression of VEGF and angiopoietin 2: A key to high vascularity of hepatocellular carcinoma? Mod. Pathol. Off. J. United States Can. Acad. Pathol. 2003, 16, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Godin, C.; Bodeau, S.; Saidak, Z.; Louandre, C.; François, C.; Barbare, J.C.; Coriat, R.; Galmiche, A.; Sauzay, C. Early decrease in serum amphiregulin or vascular endothelial growth factor levels predicts sorafenib efficacy in hepatocellular carcinoma. Oncol. Rep. 2019, 41, 2041–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, E.; Stein, I.; Andreozzi, M.; Nemeth, J.; Shoham, A.; Pappo, O.; Schweitzer, N.; Tornillo, L.; Kanarek, N.; Quagliata, L.; et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 2014, 4, 730–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Wang, Y.; Peng, H.; Chen, D.; Shen, S.; Peng, B.; Chen, M.; Lencioni, R.; Kuang, M. Autocrine vascular endothelial growth factor signaling promotes cell proliferation and modulates sorafenib treatment efficacy in hepatocellular carcinoma. Hepatology 2014, 60, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M. Focal gains of VEGFA: Candidate predictors of sorafenib response in hepatocellular carcinoma. Cancer Cell 2014, 25, 560–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, E.; Bai, W.; Xia, D.; Ding, R.; Li, J.; Wang, Q.; Liu, L.; Sun, J.; Mu, W.; et al. Exploratory Analysis to Identify Candidates Benefitting from Combination Therapy of Transarterial Chemoembolization and Sorafenib for First-Line Treatment of Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Observational Study. Liver Cancer 2020, 9, 308–325. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, W.; Han, G. Response assessment for HCC patients treated with repeated TACE: The optimal time-point is still an open issue. J. Hepatol. 2015, 63, 1530–1531. [Google Scholar] [CrossRef]
- Cheng, A.L.; Guan, Z.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Yang, T.S.; Tak, W.Y.; Pan, H.; Yu, S.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: Subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur. J. Cancer 2012, 48, 1452–1465. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Morimoto, M.; Rau, K.M.; Ikeda, M.; Yen, C.J.; Galle, P.R.; Llovet, J.M.; Daniele, B.; Lim, H.Y.; et al. Ramucirumab for Patients with Intermediate-Stage Hepatocellular Carcinoma and Elevated Alpha-Fetoprotein: Pooled Results from Two Phase 3 Studies (REACH and REACH-2). Liver Cancer 2021, 10, 451–460. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Zhao, Q.; Yan, D.; Lei, Z.; Hao, Y.; Chen, J.; Xue, Q.; Li, X.; Huang, Q.; Tang, D.; et al. Bilirubin inhibits the anticancer activity of sorafenib by blocking MCL-1 degradation in hepatocellular carcinoma cells. Cancer Biol. Med. 2022, 19, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Po Ching, L.; Morita, S.; Hu, F.C.; Cheng, A.L. Perspectives on the design of clinical trials combining transarterial chemoembolization and molecular targeted therapy. Liver Cancer 2012, 1, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Characteristic | TACE Cohort (n = 74) | TACE–Sorafenib Cohort (n = 94) | p-Value |
|---|---|---|---|
| Gender | 0.167 | ||
| Male | 63 (85.1) | 72 (76.6) | |
| Female | 11 (14.9) | 22 (23.4) | |
| Age (years) | 54.15 ± 12.03 | 53.68 ± 10.79 | 0.791 |
| ECOG PS | 0.996 | ||
| 0 | 63 (85.1) | 80 (85.1) | |
| 1 | 11 (14.9) | 14 (14.9) | |
| Etiologic cause | 0.406 | ||
| Hepatitis B | 61 (82.4) | 70 (74.5) | |
| Hepatitis C | 1 (1.4) | 4 (4.2) | |
| Other | 12 (16.2) | 20 (21.3) | |
| Child–Pugh score | 0.060 | ||
| 5 | 53 (71.6) | 81 (86.2) | |
| 6 | 18 (24.3) | 11 (11.7) | |
| 7 | 3 (4.1) | 2 (2.1) | |
| BCLC stage | 0.752 | ||
| A | 8 (10.8) | 13 (13.8) | |
| B | 36 (48.6) | 41 (43.6) | |
| C | 30 (40.6) | 40 (42.6) | |
| Tumor size (cm) | 0.745 | ||
| ≤5 cm | 15 (20.3) | 21 (22.3) | |
| >5 cm | 59 (79.7) | 73 (77.7) | |
| No. of tumors | 0.574 | ||
| 1 | 17 (23.0) | 28 (26.8) | |
| 2–3 | 10 (13.5) | 10 (11.9) | |
| ≥4 | 47 (63.5) | 56 (61.3) | |
| PVTT | 0.940 | ||
| No | 50 (67.6) | 63 (67.0) | |
| Yes | 24 (32.4) | 31 (33.0) | |
| EHS | 0.355 | ||
| No | 61 (82.4) | 72 (76.6) | |
| Yes | 13 (17.6) | 22 (23.4) | |
| Ascites | 0.590 | ||
| No | 65 (87.8) | 85 (90.4) | |
| Yes | 9 (12.2) | 9 (9.6) | |
| AFP (ng/mL) | 0.970 | ||
| ≤400 | 38 (51.4) | 48 (51.1) | |
| >400 | 36 (48.6) | 46 (48.9) | |
| Tbil (μmol/L) | 17.7 (13.4–25.4) | 16.5 (13.5–22.2) | 0.307 |
| ALP (U/L) | 140 (89–202) | 119 (93–154) | 0.223 |
| AST (U/L) | 51 (32–83) | 51 (32–74) | 0.506 |
| ALT (U/L) | 44 (26–72) | 41 (28–62) | 0.445 |
| NLR | 2.97 (2.03–4.09) | 2.98 (2.01–4.86) | 0.390 |
| VEGF (pg/mL) | 163.58 (119.68–239.82) | 154.62 (103.36–226.74) | 0.388 |
| All Patients (n = 168) | TACE Cohort (n = 74) | |||
|---|---|---|---|---|
| Baseline Factor | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Treatment (T + S vs. T) | 0.45 (0.30–0.69) | <0.001 | ||
| Age (>60 vs. ≤60) | 0.91 (0.58–1.43) | 0.686 | 0.97 (0.53–1.79) | 0.931 |
| Child–Pugh stage (B vs. A) | 1.74 (0.64–4.75) | 0.281 | 0.98 (0.24–4.07) | 0.977 |
| Tumor size (>5 vs. ≤5 cm) | 1.36 (0.81–2.29) | 0.244 | 1.98 (0.92–4.36) | 0.081 |
| No. of tumors | ||||
| 2–3 vs. 1 | 0.78 (0.33–1.85) | 0.571 | 1.29 (0.45–3.72) | 0.638 |
| ≥4 vs. 1 | 2.12 (1.26–3.55) | 0.004 | 2.68 (1.20–5.98) | 0.016 |
| PVTT (yes vs. no) | 3.14 (2.05–4.81) | <0.001 | 4.44 (2.27–8.71) | <0.001 |
| EHS (yes vs. no) | 3.79 (2.43–5.91) | <0.001 | 6.19 (2.97–12.89) | <0.001 |
| AFP (>400 vs. ≤400 ng/mL) | 2.06 (1.35–3.13) | 0.001 | 2.05 (1.13–3.71) | 0.018 |
| Tbil (>17.3 vs. ≤17.3 μmol/L) | 1.55 (1.02–2.35) | 0.041 | 0.92 (0.52–1.64) | 0.778 |
| ALP (>124 vs. ≤124.0 U/L) | 2.04 (1.34–3.10) | 0.001 | 1.87 (1.04–3.38) | 0.038 |
| NLR (>2.97 vs. ≤2.97) | 1.93 (1.27–2.94) | 0.002 | 2.44 (1.33–4.48) | 0.004 |
| VEGF (>131.09 vs. ≤131.09 pg/mL) | 2.99 (1.77–5.06) | <0.001 | 4.05 (1.70–9.65) | <0.001 |
| Cox Regression Model | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| The one-line Cox regression model | 1.003 (1.002–1.004) | 1.001 (1.000–1.002) |
| The two-piece-wise Cox regression model | ||
| ≤189.79 pg/mL | 1.009 (1.004–1.015) | 1.008 (1.002–1.014) |
| >189.79 pg/mL | 1.002 (1.000–1.003) | 1.000 (0.998–1.002) |
| p for log-likelihood ratio test | 0.016 | 0.014 |
| Baseline Factor | n (T + S/T) | HR (95% CI) | p for Inter-Action |
|---|---|---|---|
| Age | 0.587 | ||
| ≤60 | 119 (68/51) | 0.487 (0.296–0.801) | |
| >60 | 49 (26/23) | 0.397 (0.183–0.858) | |
| Hepatitis B * | 0.663 | ||
| No | 32 (20/12) | 0.312 (0.102–0.953) | |
| Yes | 131 (70/61) | 0.502 (0.319–0.790) | |
| BCLC stage | 0.620 | ||
| A # | 21 (13/8) | — | |
| B | 77 (41/36) | 0.303 (0.154–0.598) | |
| C | 70 (40/30) | 0.259 (0.141–0.474) | |
| Tumor size | 0.004 | ||
| ≤7 cm | 67 (38/29) | 0.965 (0.464–2.006) | |
| >7 cm | 101 (56/45) | 0.287 (0.170–0.484) | |
| No. of tumors | 0.281 | ||
| 1 | 45 (28/17) | 0.680 (0.273–1.693) | |
| 2–3 | 20(10/10) | 0.136 (0.016–1.142) | |
| ≥4 | 103(56/47) | 0.361 (0.214–0.608) | |
| PVTT | 0.208 | ||
| No | 113 (63/50) | 0.443 (0.253–0.775) | |
| Yes | 55 (31/24) | 0.260 (0.130–0.522) | |
| EHS | 0.150 | ||
| No | 133 (72/61) | 0.404 (0.241–0.678) | |
| Yes | 35 (22/13) | 0.200 (0.086–0.466) | |
| AFP (ng/mL) | 0.835 | ||
| ≤400 | 86 (48/38) | 0.392 (0.207–0.743) | |
| >400 | 82 (46/36) | 0.458 (0.261–0.801) | |
| Total bilirubin (μmol/L) | 0.031 | ||
| ≤17.3 | 84 (51/33) | 0.279 (0.147–0.527) | |
| >17.3 | 84 (43/41) | 0.716 (0.410–1.248) | |
| ALP (U/L) | 0.927 | ||
| ≤124 | 85 (51/34) | 0.425 (0.224–0.808) | |
| >124 | 83 (43/40) | 0.475 (0.273–0.826) | |
| NLR | 0.358 | ||
| ≤2.97 | 84 (47/37) | 0.498 (0.263–0.945) | |
| >2.97 | 84 (47/37) | 0.326 (0.183–0.580) | |
| VEGF (pg/mL) | 0.233 | ||
| ≤131.09 | 54 (34/20) | 0.833 (0.311–2.232) | |
| >131.09 | 114 (60/54) | 0.411 (0.257–0.657) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Yang, Z.; Liu, X.; Yang, Y.; Song, W.; Wang, S.; Chen, Y. Identification of Potential Predictors of Prognosis and Sorafenib-Associated Survival Benefits in Patients with Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization. Curr. Oncol. 2023, 30, 476-491. https://doi.org/10.3390/curroncol30010038
He K, Yang Z, Liu X, Yang Y, Song W, Wang S, Chen Y. Identification of Potential Predictors of Prognosis and Sorafenib-Associated Survival Benefits in Patients with Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization. Current Oncology. 2023; 30(1):476-491. https://doi.org/10.3390/curroncol30010038
Chicago/Turabian StyleHe, Kun, Zelong Yang, Xinyu Liu, Yanling Yang, Wenjie Song, Shangyu Wang, and Yong Chen. 2023. "Identification of Potential Predictors of Prognosis and Sorafenib-Associated Survival Benefits in Patients with Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization" Current Oncology 30, no. 1: 476-491. https://doi.org/10.3390/curroncol30010038





