Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer
Abstract
1. Introduction
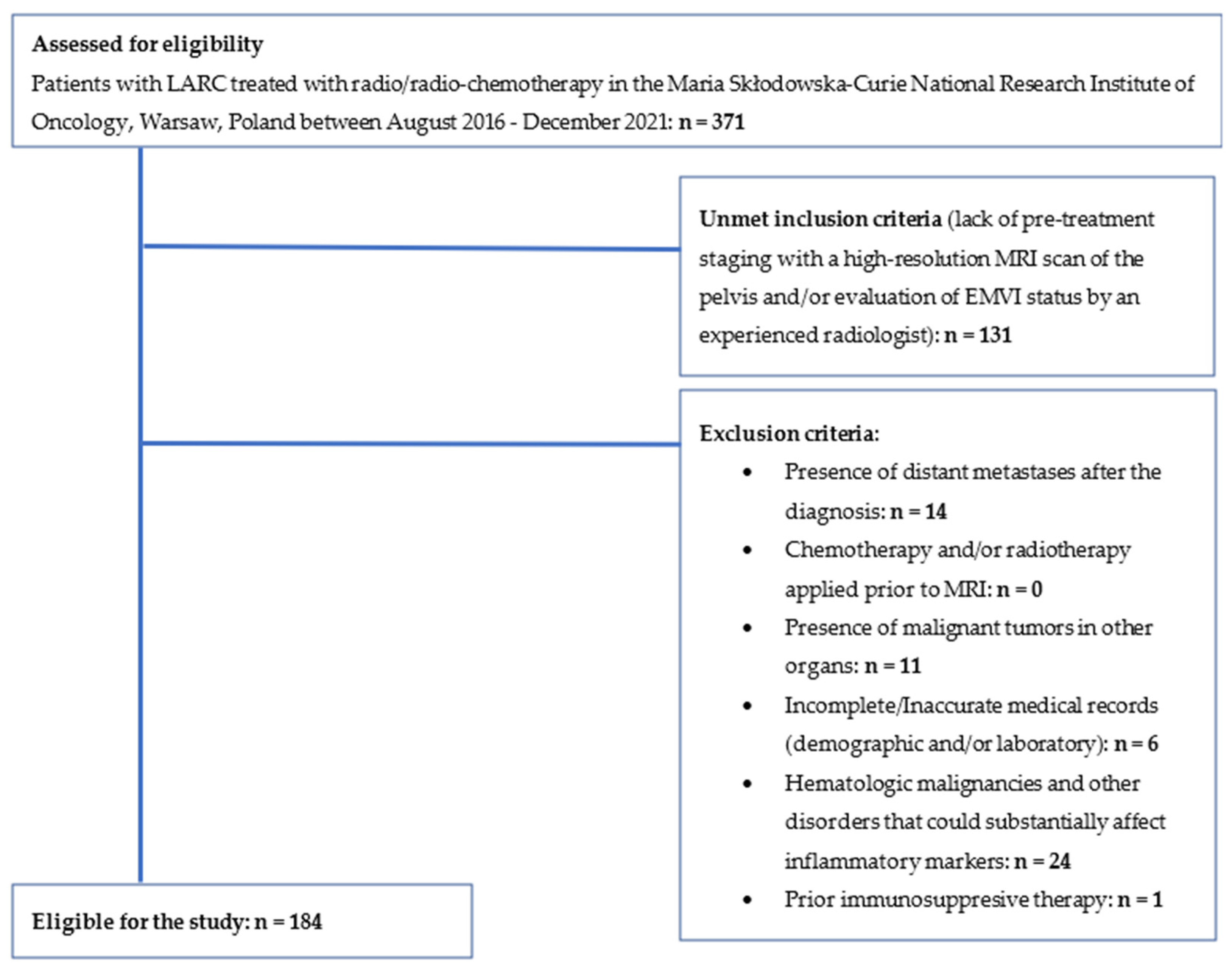
2. Materials and Methods
2.1. MRI Acquisition
2.2. Assessment of Status of EMVI
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. Union for International Cancer Control. TNM Classification of Malignant Tumours; John Wiley and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Torkzad, M.R.; Påhlman, L.; Glimelius, B. Magnetic resonance imaging (MRI) in rectal cancer: A comprehensive review. Insights Imaging 2010, 1, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Barbachano, Y.; Norman, A.R.; Swift, R.I.; Abulafi, A.M.; Brown, G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br. J. Surg. 2008, 95, 229–236. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sakurai, K.; Yamazoe, S.; Kimura, K.; Toyokawa, T.; Amano, R.; Tanaka, H.; et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 9966–9973. [Google Scholar] [CrossRef]
- Abe, S.; Kawai, K.; Nozawa, H.; Hata, K.; Kiyomatsu, T.; Morikawa, T.; Watanabe, T. LMR predicts outcome in patients after preoperative chemoradiotherapy for stage II–III rectal cancer. J. Surg. Res. 2018, 222, 122–131. [Google Scholar] [CrossRef]
- Dell’Aquila, E.; Cremolini, C.; Zeppola, T.; Lonardi, S.; Bergamo, F.; Masi, G.; Stellato, M.; Marmorino, F.; Schirripa, M.; Urbano, F.; et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: A retrospective analysis of the TRIBE study by GONO. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 924–930. [Google Scholar] [CrossRef]
- Sung, S.; Son, S.H.; Park, E.Y.; Kay, C.S. Prognosis of locally advanced rectal cancer can be predicted more accurately using pre- and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS ONE 2017, 12, e0173955. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Chen, W.J.; Zhang, X.; Wu, C.C.; Zhang, C.Y.; Sun, S.S.; Wu, J. An Elevated Platelet-to-Lymphocyte Ratio Predicts Poor Prognosis and Clinicopathological Characteristics in Patients with Colorectal Cancer: A Meta-Analysis. Dis. Markers 2017, 2017, 1053125. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Sun, H.F.; Zhang, Y.B.; Liao, Z.J.; Zhao, L.; Cui, J.; Wu, T.; Lu, J.-R.; Nan, K.-J.; Wang, S.-H. The clinical use of the platelet/lymphocyte ratio and lymphocyte/monocyte ratio as prognostic predictors in colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 20011–20024. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, H.; Yu, W.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int. J. Cancer 2016, 139, 220–231. [Google Scholar] [CrossRef]
- Koh, D.-M.; Smith, N.J.; Swift, R.I.; Brown, G. The Relationship between MR Demonstration of Extramural Venous Invasion and Nodal Disease in Rectal Cancer. Clin. Med. Oncol. 2008, 2, 267–273. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.; Yang, Z.; He, W.; Wang, Z.; Jin, E. Correlation of MRI-detected extramural vascular invasion with regional lymph node metastasis in rectal cancer. Clin. Imaging 2016, 40, 456–460. [Google Scholar] [CrossRef]
- Patel, U.B.; Brown, G.; Machado, I.; Santos-Cores, J.; Pericay, C.; Ballesteros, E.; Salud, A.; Isabel-Gil, M.; Montagut, C.; Maurel, J.; et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for primary rectal cancer: Long-term results from the GEMCAD 0801 trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 344–353. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Yang, Y.; Zhao, Z.; Shang, A.; Guo, Y.; Wang, M. To Explore the Effect of NLR and PLR on Prognosis of Rectal Cancer on the basis of T3 substage, and to draw the related nomogram. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Pine, J.K.; Morris, E.; Hutchins, G.; West, N.; Jayne, D.G.; Quirke, P.; Prasad, K.R. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: The relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br. J. Cancer 2015, 113, 204–211. [Google Scholar] [CrossRef]
- Ao, W.; Zhang, X.; Yao, X.; Zhu, X.; Deng, S.; Feng, J. Preoperative prediction of extramural venous invasion in rectal cancer by dynamic contrast-enhanced and diffusion weighted MRI: A preliminary study. BMC Med. Imaging 2022, 22, 78. [Google Scholar] [CrossRef]
- Yu, J.; Huang, D.Y.; Xu, H.X.; Li, Y.; Xu, Q. Correlation Between Magnetic Resonance Imaging-Based Evaluation of Extramural Vascular Invasion and Prognostic Parameters of T3 Stage Rectal Cancer. J. Comput. Assist. Tomogr. 2016, 40, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Talbot, I.C.; Ritchie, S.; Leighton, M.H.; Hughes, A.O.; Bussey, H.J.; Morson, B.C. The clinical significance of invasion of veins by rectal cancer. Br. J. Surg. 1980, 67, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Macaskill, P.; Smith, A.N. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet 1984, 2, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Messenger, D.E.; Driman, D.K.; Kirsch, R. Developments in the assessment of venous invasion in colorectal cancer: Implications for future practice and patient outcome. Hum. Pathol. 2012, 43, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; Kirsch, R.; Messenger, D.; Driman, D. A Review of Current Challenges in Colorectal Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 869–882. [Google Scholar] [CrossRef]
- Taylor, F.G.; Quirke, P.; Heald, R.J.; Moran, B.; Blomqvist, L.; Swift, I.; Sebag-Montefiore, D.; Tekkis, P.; Brown, G. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: A prospective, multicenter, European study. Ann. Surg. 2011, 253, 711–719. [Google Scholar] [CrossRef]
- Siddiqui, M.R.S.; Simillis, C.; Hunter, C.; Chand, M.; Bhoday, J.; Garant, A.; Vuong, T.; Artho, G.; Rasheed, S.; Tekkis, P.; et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br. J. Cancer 2017, 116, 1513–1519. [Google Scholar] [CrossRef]
- Chand, M.; Bhangu, A.; Wotherspoon, A.; Stamp, G.W.H.; Swift, R.I.; Chau, I.; Tekkis, P.; Brown, G. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 858–863. [Google Scholar] [CrossRef]
- Leijssen, L.G.J.; Dinaux, A.M.; Amri, R.; Taylor, M.S.; Deshpande, V.; Bordeianou, L.G.; Kunitake, H.; Berger, D.L. Impact of intramural and extramural vascular invasion on stage II–III colon cancer outcomes. J. Surg. Oncol. 2019, 119, 749–757. [Google Scholar] [CrossRef]
- Faiz, Z.; Huijgen, L.J.W.; Alqethami, H.J.; Burgerhof, J.G.M.; Kats-Ugurlu, G.; Plukker, J.T.M. Prevalence and Prognostic Significance of Extramural Venous Invasion in Patients with Locally Advanced Esophageal Cancer. Ann. Surg. Oncol. 2018, 25, 1588–1597. [Google Scholar] [CrossRef]
- Cheng, J.; Feng, C.; Zhang, Y.; Hong, N.; Ye, Y.; Wang, Y. CT-Detected Extramural Vessel Invasion and Regional Lymph Node Involvement in Stage T4a Gastric Cancer for Predicting Progression-Free Survival. AJR Am. J. Roentgenol. 2019, 212, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Antonio, N.; Bønnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P.M. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef]
- Tazzyman, S.; Lewis, C.E.; Murdoch, C. Neutrophils: Key mediators of tumour angiogenesis. Int. J. Exp. Pathol. 2009, 90, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W. Neutrophils diminish T-cell immunity to foster gastric cancer progression: The role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut 2017, 66, 1878–1880. [Google Scholar] [CrossRef]
- Bailey, S.E.; Ukoumunne, O.C.; Shephard, E.; Hamilton, W. Clinical relevance of thrombocytosis in primary care: A prospective cohort study of cancer incidence using English electronic medical records and cancer registry data. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2017, 67, e405–e413. [Google Scholar] [CrossRef]
- Kim, I.Y.; You, S.H.; Kim, Y.W. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014, 14, 94. [Google Scholar] [CrossRef]
- Ke, T.-M.; Lin, L.-C.; Huang, C.-C.; Chien, Y.-W.; Ting, W.-C.; Yang, C.-C. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict poor survival in rectal cancer patients receiving neoadjuvant concurrent chemoradiotherapy. Medicine 2020, 99, e19877. [Google Scholar] [CrossRef]
- Inoue, A.; Sheedy, S.P.; Heiken, J.P.; Mohammadinejad, P.; Graham, R.P.; Lee, H.E.; Kelley, S.R.; Hansel, S.L.; Bruining, D.H.; Fidler, J.L.; et al. MRI-detected extramural venous invasion of rectal cancer: Multimodality performance and implications at baseline imaging and after neoadjuvant therapy. Insights Imaging 2021, 12, 110. [Google Scholar] [CrossRef]
- Gawiński, C.; Hołdakowska, A.; Wyrwicz, L. P-207 Correlation between extramural vascular invasion (EMVI) and systemic inflammatory response markers in rectal cancer. Ann. Oncol. 2022, 33, S323. [Google Scholar] [CrossRef]





| Formulas: |
|---|
| LMR—absolute lymphocyte count (109/L)/absolute monocyte count (109/L) |
| NLR—absolute neutrophil count (109/L)/absolute lymphocyte count (109/L) |
| PLR—absolute platelet count (109/L)/absolute lymphocyte count (109/L) |
| Characteristics | Value |
|---|---|
| N | |
| EMVI +, n (%) | 78 (42.4) |
| EMVI −, n (%) | 106 (57.6) |
| Age (years), M ± SD, range (years)/ Me (Q1; Q3) | 65.55 ± 10.83, 36–87/65.69 (58.97; 72.32) |
| Sex (female), n (%) | 73 (39.9) |
| Tumor, n (%) | |
| T2-T3 | 146 (80.7) |
| T4 | 35 (19.3) |
| Lymph nodes, n (%) | |
| N0 | 68 (41.2) |
| N1 | 63 (38.2) |
| N2 | 34 (26.0) |
| Stage, n (%) | |
| II-III A | 84 (47.2) |
| III B | 65 (36.5) |
| III C | 29 (16.3) |
| ALC (109/L), M ± SD | 1.77 (1.38; 2.24) |
| AMC (109/L), Me (Q1; Q3) | 0.60 (0.50; 0.76) |
| ANC (109/L), Me (Q1; Q3) | 4.77 (3.77; 5.99) |
| Platelets (109/L), Me (Q1; Q3) | 274.50 (230.75; 337.25) |
| LMR, Me (Q1; Q3) | 2.87 (2.25; 3.81) |
| NLR, Me (Q1; Q3) | 2.62 (2.09; 3.42) |
| PLR, Me (Q1; Q3) | 153.11 (117.98; 204.10) |
| CEA (ng/mL), Me (Q1; Q3) | 3.88 (2.33; 9.06) |
| CA19-9 (U/mL), Me (Q1; Q3) | 10.04 (4.64; 17.73) |
| Characteristics | EMVI − | EMVI + | p |
|---|---|---|---|
| Age (years), Me (Q1; Q3) | 65.90 (58.75; 74.00) | 64.90 (59.48; 71.76) | 0.667 |
| Sex (female), n (%) | 43 (41.0) | 30 (38.5) | 0.8512 |
| Tumor, n (%) | |||
| T2-T3 | 96 (91.4) | 50 (65.8) | <0.0012 |
| T4 | 9 (8.6) | 26 (34.2) | |
| Lymph nodes, n (%) | |||
| N0 | 49 (52.7) | 19 (26.4) | <0.0012 |
| N1 | 35 (37.6) | 28 (38.9) | |
| N2 | 9 (9.7) | 25 (34.7) | |
| Stage, n (%) | |||
| II-III A | 59 (57.8) | 25 (32.9) | <0.0012 |
| III B | 38 (37.3) | 27 (35.5) | |
| III C | 5 (4.9) | 24 (31.6) | |
| ALC (109/L), Me (Q1; Q3) | 1.70 (1.33; 2.24) | 1.90 (1.45; 2.26) | 0.217 |
| AMC (109/L), Me (Q1; Q3) | 0.58 (0.46; 0.80) | 0.63 (0.53; 0.75) | 0.181 |
| ANC (109/L), Me (Q1; Q3) | 4.49 (3.62; 5.81) | 5.10 (4.07; 6.16) | 0.041 |
| Platelets (109/L), Me (Q1; Q3) | 253.50 (217.00; 313.25) | 303.00 (249.50; 361.75) | 0.001 |
| LMR, Me (Q1; Q3) | 2.90 (2.30; 3.83) | 2.86 (2.20; 3.79) | 0.885 |
| NLR, Me (Q1; Q3) | 2.54 (2.05; 3.31) | 2.82 (2.18; 3.43) | 0.189 |
| PLR, Me (Q1; Q3) | 150.70 (115.97; 200.00) | 167.19 (119.95; 218.05) | 0.293 |
| LMR above median, n (%) | 53 (50.0) | 38 (48.7) | 0.9822 |
| NLR above median, n (%) | 49 (46.2) | 43 (55.1) | 0.2962 |
| PLR above median, n (%) | 49 (46.2) | 43 (55.1) | 0.2962 |
| CEA, Me (Q1; Q3) | 3.62 (2.24; 6.97) | 5.63 (3.07; 9.68) | 0.027 |
| CA19-9, Me (Q1; Q3) | 9.87 (5.36; 15.76) | 11.71 (2.77; 18.29) | 0.741 |
| Characteristics | EMVI − | EMVI + | p |
|---|---|---|---|
| Stage, n (%) | |||
| pCR | 12 (13.0) | 5 (8.2) | 0.350 |
| I | 25 (27.2) | 8 (13.1) | 0.002 |
| II | 33 (35.9) | 15 (24.6) | |
| III | 22 (23.9) | 33 (54.1) | |
| pT, n (%) | |||
| 0 | 10 (11.2) | 4 (6.7) | 0.078 |
| 1 and 2 | 30 (33.7) | 12 (20.0) | |
| 3 and 4 | 49 (55.1) | 44 (73.3) | |
| pN, n (%) | |||
| 0 | 67 (75.3) | 27 (45.0) | <0.001 |
| 1 | 15 (16.9) | 22 (36.7) | |
| 2 | 7 (7.9) | 11 (18.3) |
| Stage, n (%) | EMVI − | EMVI + | p |
|---|---|---|---|
| LMR ≤ median | |||
| II–III A | 29 (58.0) | 15 (38.5) | 0.002 |
| III B | 19 (38.0) | 11 (28.2) | |
| III C | 2 (4.0) | 13 (33.3) | |
| LMR > median | |||
| II–III A | 30 (57.7) | 10 (27.0) | 0.002 |
| III B | 19 (36.5) | 16 (43.2) | |
| III C | 3 (5.8) | 11 (29.7) | |
| NLR ≤ median | |||
| II–III A | 30 (55.6) | 12 (34.3) | 0.008 |
| III B | 21 (38.9) | 13 (37.1) | |
| III C | 3 (5.6) | 10 (28.6) | |
| NLR > median | |||
| II–III A | 29 (60.4) | 13 (31.7) | 0.001 |
| III B | 17 (35.4) | 14 (34.1) | |
| III C | 2 (4.2) | 14 (34.1) | |
| PLR ≤ median | |||
| II–III A | 31 (56.4) | 10 (29.4) | 0.010 |
| III B | 21 (38.2) | 16 (47.1) | |
| III C | 3 (5.5) | 8 (23.5) | |
| PLR > median | |||
| II–III A | 28 (59.6) | 15 (35.7) | <0.001 |
| III B | 17 (36.2) | 11 (26.2) | |
| III C | 2 (4.3) | 16 (38.1) | |
| Variables | CEA | CA19-9 | ||
|---|---|---|---|---|
| tau-b | p | tau-b | p | |
| LMR | 0.03 | 0.634 | −0.06 | 0.306 |
| NLR | <0.01 | 0.964 | 0.04 | 0.526 |
| PLR | 0.04 | 0.506 | −0.07 | 0.226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawiński, C.; Hołdakowska, A.; Wyrwicz, L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer. Curr. Oncol. 2023, 30, 545-558. https://doi.org/10.3390/curroncol30010043
Gawiński C, Hołdakowska A, Wyrwicz L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer. Current Oncology. 2023; 30(1):545-558. https://doi.org/10.3390/curroncol30010043
Chicago/Turabian StyleGawiński, Cieszymierz, Anna Hołdakowska, and Lucjan Wyrwicz. 2023. "Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer" Current Oncology 30, no. 1: 545-558. https://doi.org/10.3390/curroncol30010043
APA StyleGawiński, C., Hołdakowska, A., & Wyrwicz, L. (2023). Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer. Current Oncology, 30(1), 545-558. https://doi.org/10.3390/curroncol30010043




