Abstract
Lung cancer is the second most common cancer and the leading cause of cancer-related deaths in 2022. The majority (80%) of lung cancer cases belong to the non-small cell lung carcinoma (NSCLC) subtype. Despite the increased screening efforts, the median five-year survival of metastatic NSCLC remains low at approximately 3%. Common treatment approaches for NSCLC include surgery, multimodal chemotherapy, and concurrent radio and chemotherapy. NSCLC exhibits high rates of resistance to treatment, driven by its heterogeneity and the plasticity of cancer stem cells (CSCs). Drug repurposing offers a faster and cheaper way to develop new antineoplastic purposes for existing drugs, to help overcome therapy resistance. The decrease in time and funds needed stems from the availability of the pharmacokinetic and pharmacodynamic profiles of the Food and Drug Administration (FDA)-approved drugs to be repurposed. This review provides a synopsis of the drug-repurposing approaches and mechanisms of action of potential candidate drugs used in treating NSCLC, including but not limited to antihypertensives, anti-hyperlipidemics, anti-inflammatory drugs, anti-diabetics, and anti-microbials.
1. Introduction
Lung cancer is the leading cause of cancer-related deaths in 2022, with 350 deaths per day [1]. It has the second-highest incidence rate among all cancers, with 236,740 projected new cases in 2022, second only to breast cancer in females and prostate cancer in males [1]. Cigarette smoking is still a major culprit behind lung cancer, as it directly caused 81% of lung cancer death in 2022 [2]. This relationship is reflected in global incidence and mortality trends; regions with lower human development index have higher incidence and mortality from lung cancer [3]. Lung cancer incidence declined during the last decade by 3% in men and 1% in women [1]. This decline narrowed the historic gender gap in lung cancer incidence, with the men to women ratio once reaching three-fold in the 1970s, caused by the disproportionate smoking rates among men and women [1]. Despite having the highest mortality, lung cancer survival is more promising during all stages. The percentage of people living at least three years after diagnosis increased from 19% in 2001 to 31% in 2017, with a median survival increase from 8 to 13 months [1]. The survival gains can be attributed to advanced diagnostic procedures and treatments such as enhanced pathological staging and video-assisted thoracoscopic surgery [4,5].
Lung cancer is broadly classified into small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC), with NSCLC accounting for 80% of the cases. Within NSCLC, adenocarcinoma is the most common subtype, accounting for 60% of the cases. Other NSCLCs include squamous cell, large cell, adenosquamous, pleomorphic, spindle cell, and giant cell carcinomas [6].
When assessing the cell of origin of NSCLC, a group of distal adult lung epithelial stem cells called the bronchioalveolar stem cells (BASCs) were identified at the bronchioalveolar duct junction [7]. Even though it was later shown that BASCs do not act similar to tissue stem cells, these cells are pluripotent as they proliferate when a KRAS mutant is induced [8]. The expansion of the BASC population in KRAS-mutant mice was correlated with tumor progression and an increase in cell size and number [8].
Multiple molecular pathways affecting oncogenes and tumor suppressor genes have been implicated in the pathobiology of the NSCLC [9]. One of the key cancer-related genes affecting NSCLC is the epidermal growth factor receptor (EGFR) gene. Mutations in EGFR genes are typically diagnosed in adenocarcinoma patients; they are ubiquitous among those with never-smoking status, female gender, and East Asian ethnicity [10]. Another gene commonly identified in adenocarcinoma is the anaplastic lymphoma kinase [11] gene [11], specifically the EML4-ALK fusion in young, never-smoker patients [12]. Another common gene detected in adenocarcinoma among smokers is the Kirsten rat sarcoma viral oncogene homolog gene (KRAS). The KRAS and EGFR pathways in adenocarcinoma are mutually exclusive, which suggests different molecular pathways being implicated in the development of adenocarcinoma between smokers and non-smokers [13]. The MET gene, which occurs in 7% of NSCLC, encodes for a receptor tyrosine kinase that activates multiple signaling pathways involved in lung cancer pathogenesis and metastasis [14]. The fibroblast growth factor receptor type 1 (FGFR1) gene encodes for a cell surface tyrosine kinase receptor of the FGFR tyrosine kinase family. FGFR1 has been reported to be amplified in 20% of squamous cell carcinomas and 1–3% of adenocarcinomas [11,15]. Finally, mutations in the discoidin domain receptor 2 (DDR2) gene, a receptor of tyrosine kinase, were found in 4% of squamous cell carcinomas [16].
The stage of NSCLC at the time of diagnosis is critical in determining survival; supported by the fact that the 5-year relative survival rate for localized NSCLC is 64%, as opposed to 8% for metastatic NSCLC [17]. Staging also directs treatment with the standard of care for stage I cancers being surgery with either lobectomy or pneumonectomy as the procedure of choice [18]. The treatment of stage II cancers includes surgical resection along with cisplatin-based adjuvant systematic therapy [18,19]. The standard of care therapy for unresectable stage III NSCLC since 2017 has been concurrent chemoradiation followed by durvalumab [20,21]. This combination therapy offers a 24-month overall survival rate of 66.3% and a median progression free survival of 17.2 months [21]. Patients with stage III NSCLC are managed with multimodal treatment regimens consisting of concurrent or sequential chemotherapy and radiotherapy [18,19]. The five-year survival in stage IV disease is only 1–3%, so therapy is aimed at alleviating symptoms with single- or double-based chemotherapy [18]. More recent drugs treating NSCLC include targeted therapy, specifically tyrosine kinase inhibitors erlotinib, gefitinib, and afatinib targeting EGFR [22]. Another newly utilized therapy avenue is immunotherapy, which includes IgG4 monoclonal antibodies against programmed cell death protein 1 (PD-1), such as Nivolumab and Pembrolizumab [18].
Cancer stem cells (CSCs), the underlying subpopulations of tumor cells that drive tumor growth and progression, cause NSCLC to be a heterogenous tumor [23]. Cancer stem cells are a subpopulation of cells residing within the tumor bulk that are believed to be responsible for therapy resistance and recurrence [24,25,26,27,28,29,30,31]. This heterogeneity drives drug resistance in NSCLC, leading to therapeutic failure [23]. The plasticity of CSCs in NSCLC allows them to reverse differentiate into different cell types [32]. CSCs play a major role in driving resistance to multiple chemotherapy agents by releasing multidrug ATP-binding cassette (ABC)-transporters [33]. Some of the cell-intrinsic treatment resistance mechanisms in NSCLC include activating pro-survival and anti-apoptotic pathways and signaling and expressing drug transporters [23]. Moreover, the resistance can be classified as on-target resistance, affecting oncogenes such as ROS1 and RET rearrangements and BRAF mutations. Resistance can also be off target, affecting signaling pathways via epigenetic modifications [32].
Even though the treatment of NSCLC is advancing, the treatment resistance, plasticity of the tumor, and heterogeneity cause treatment to be challenging. So, in addition to exploring novel agents for treating NSCLC, we should turn our attention to repurposing some of the readily available agents to help combat the aggressive disease (Table 1).

Table 1.
Summary table of the drugs that have been repurposed to be used in NSCLC in pre-clinical models.
2. Repurposing Approved Drugs in Cancer
The substantial cost, slow pace, and high attrition rates of new drug discoveries are driving many researchers toward drug repurposing, also known as drug repositioning [60]. Drug repurposing is the use of existing drugs to treat a new medical condition, which was not the intended indication of the drug [61]. The new indication is built upon the established safety, pharmacokinetics, and manufacturing data of existing drugs, which includes approved, discontinued, shelved, and investigational therapeutics [27,28,30,31,61].
Repurposed drugs fall into various classes; first, drugs that are no longer used because of toxicity or side effects but are not toxic if used in lower doses or a different population [62]. An excellent example from this class is thalidomide, which was teratogenic to pregnant women when used for morning sickness but was later found to be safe and efficacious in treating refractory multiple myeloma. Another class of repurposed drugs is the ones in which their off-target effect became the primary desired outcome, such as sildenafil, which was developed as an anti-anginal medication but is now used to treat erectile dysfunction. An additional class includes medications that are efficacious in treating multiple etiologies but are only used to treat one. A final class involves medications that exhibit better effects when combined with the conventional medication regimen, known as the synergic effect [62] (Figure 1).
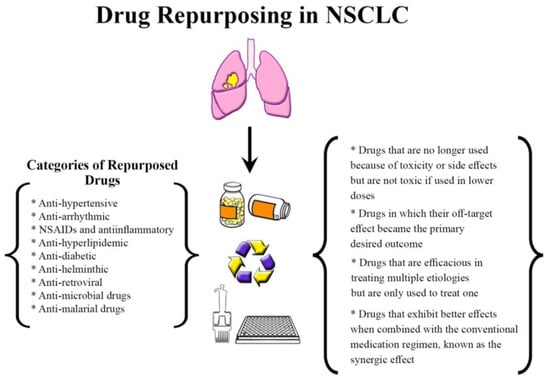
Figure 1.
Drug repurposing in NSCLC. Food and Drug Administration (FDA)-approved drugs identified and repurposed to treat patients with NSCLC. Abbreviations: NSAIDs: non-steroidal anti-inflammatory drugs.
2.1. Computational-Based Approach to Drug Repurposing
Since there are multiple classes of repurposed drugs, there are also multiple approaches to repurposing these drugs. The computational method, also known as in silico drug repurposing, involves the collection and analysis of diverse types of data from different sources, mainly from databases, including chemical structure, gene expression, proteomics, or electronic health records (EHRs) [63]. This approach combines data to locate and explore potential medications for repurposing and involves different techniques.
The first technique of the computational approach is network-based drug repurposing, which utilizes the advances in genotyping technology by using genome-wide association studies (GWAS) to find genetic variants that affect common diseases [64]. GWAS identifies new targets shared by multiple disease phenotypes; however, these targets might not be druggable. Using network-based algorithms allows researchers to find druggable genes upstream or downstream of the target gene [65,66]. Examples of drugs repurposed using this technique include vismodegib, an inhibitor of the Hedgehog signaling pathway, to treat Gorlin syndrome [67], and iloperidone, an antipsychotic for the treatment of schizophrenia, to treat hypertension [68]. Another example includes the random walk propagation algorithm, which expands a set of disease-associated genes to genes sharing neighbors in a gene–gene or protein–protein network. This algorithm was used to identify novel indications for diseases such as gabapentin for anxiety disorder, cisplatin for breast cancer, donepezil for Parkinson’s disease, and methotrexate for Crohn’s [69].
Another technique in the computational approach is profile-based drug repurposing, which compares different drug profiles with another drug, disease, or clinical phenotype [70]. An example would be comparing the differential gene expression in a cell or tissue before and after therapy and contrasting it to the expression profile associated with the disease [71,72]. For example, suppose a drug is shown to reverse the transcription of a certain gene related to the pathophysiology of a disease. In that case, the drug can reverse the disease and should be a candidate for repurposing [71,72]. Another profile-based technique compares the chemical structure of different drugs, which can lead to new drug-target associations [73]. This can be performed via molecular docking, which evaluates multiple ligands against a receptor [60]. Mebendazole, an antiparasitic medication, was found to have, using a computational docking technique, the structural capacity to block vascular endothelial growth factor receptor 2 (VEGFR2) [74].
Moreover, retrospective clinical data can be used as a tool in drug repurposing, especially with the abundance of data extracted from EHRs [75]. Raloxifene for breast cancer, propranolol for osteoporosis [76], aspirin for colorectal cancer [77], and sildenafil for erectile dysfunction [78] were all repurposed based on simple clinical studies rather than complex network analyses. Text-mining tools accelerate the data-based drug repurposing approach by decreasing the time needed to go through the complex scientific literature. Text-mining was used to create Alzheimer-specific drug–protein connectivity maps, which found diltiazem and quinidine as possible therapeutic candidates [79].
2.2. Pros and Cons of Drug Repurposing
The main advantages of repurposing drugs in treating cancer revolve around time and cost. It was shown that the average time from filing the investigational drug application to the first new drug application is 8.3 years for novel antineoplastics and from 3 to 4 years for repurposed drugs [78,80]. This improved timeline stems from the fact the pharmacokinetics and pharmacodynamics of the repurposed drug are already established, which sometimes overcomes the need for a phase I clinical trial [60]. Another advantage of repurposing drugs is cost. It costs USD 300 million to bring a repurposed drug to the market, compared to the USD 2–3 billion it requires to develop a novel drug [81].
Nevertheless, repurposing drugs has some disadvantages; for example, redirecting an established drug toward new usage is a high-risk investment as the new direction might not be as successful as planned. Additionally, some repurposed drugs might undergo similar processes in terms of time and cost as novel drugs would, to ensure their safety in the new population [60].
3. Repurposing Approved Drugs in NSCLC
Oncology has greatly benefited from the recent movement toward drug repurposing, given that only 5% of antineoplastic drugs that enter a phase I trial will eventually be approved [82]. This is driving research groups to repurpose known drugs and use them in the battle against highly resistant cancers, such as colon and prostate cancers [30,31].
Despite improved screening efforts and declining incidence rates, NSCLC still poses many therapeutic challenges. These challenges can be summarized by high resistance to conventional chemotherapy, which causes single-drug therapy to be useless, necessitating double therapy or a combination of chemotherapy and radiotherapy [12,32]. The need for more therapy options causes NSCLC to be a suitable candidate for drug repurposing.
3.1. Anti-Hypertensives and Anti-Arrhythmic Drugs
Beta-blockers are a class of drugs known to be useful in the management of cardiovascular diseases, hyperthyroidism, migraines, and glaucoma. With the increasing popularity of drug repurposing, the anticancer effects of beta adreno-blockers are now being studied more extensively. The exact mechanism for their anti-NSCLC effects is still unknown, but many speculations exist. A study by Sidorova et al., which studied the effects of beta blockers on the viability and cell colony formation of NSCLC, showed that propranolol and betaxolol are the most effective in inhibiting lung cancer cell colony formation at 90% of the EC50 value [83]. Propranolol decreases tumor angiogenesis and can stimulate the immune system [84]. Beta blockers may help treat cancer via desensitization of beta receptors to chronic beta-agonist use. This increases IL-6 expression stimulating cell proliferation in lung cancer and inhibits tumor suppressor liver kinase B1 (LKB1) in EGFR-positive lung adenocarcinoma tumors [34]. Chronic adrenergic stimulation impairs the response to chemotherapy. Thus, using beta blockers could help enhance the effectiveness of chemotherapy [35]. Oh et al. found that patients treated with immune checkpoint inhibitors and beta-blockers had increased progression-free survival [36]. However, there has been no correlation between using beta-blockers alone and improved overall survival in lung cancer [85]. Another study by Sidora et al. proved the effect of beta-blockers on NSCLC cell apoptosis with no significant difference between selective and non-selective beta-blockers in vitro. However, further investigation is needed concerning their anti-tumor role in vivo [83].
Angiotensin I converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), known to have life-prolonging effects, are frequently used drugs to treat numerous diseases, including hypertension and heart failure [30]. The renin-angiotensin-aldosterone system has been linked to many of the hallmarks of cancer, which is one rationale behind repurposing ARBs and ACEIs to treat NSLC [86]. The interaction between angiotensin II and angiotensin II receptor 1 stimulates vascular smooth muscle cells to produce VEGF, which aids tumor angiogenesis [87]. Telmisartan is an ARB clinically indicated for hypertension therapy and has also proved to have anti-cancer effects by activating PPARγ, which halts tumor metastases [37]. Based on a study by Godugu et al., in vitro experiments with losartan and telmisartan nanoparticles proved to be both effective against lung cancer and well tolerated by human normal fibroblast cells. Due to their adequate aerosol performance, both drugs could be used as inhalation aerosols for lung cancer treatment. However, the intratumoral distribution of telmisartan was around 2.7 times higher than losartan. Hence, telmisartan exhibits a significantly higher anticancer and antifibrotic role in lung tumor models [38].
3.2. NSAIDs, Anti-Inflammatory Drugs and Aspirin
Nonsteroidal anti-inflammatory drugs (NSAIDs) have anti-inflammatory, analgesic, and antipyretic properties by inhibiting cyclooxygenase (COX) enzymes [88]. According to recent data, COX-2 is upregulated in lung adenocarcinomas. COX-2 is associated with enhanced cell proliferation and reduced apoptosis, two factors necessary for invasive tumor growth and metastasis. Therefore, COX-2 inhibition forms an important checkpoint [89]. Several NSCLC cases have been associated with the overexpression of COX-2 proteins in lesions of human lung adenocarcinoma and hint at a worse prognosis [90]. Celecoxib is a specific inhibitor of COX-2 widely used initially as an anti-analgesic for osteoarthritis and rheumatoid arthritis pain [91]. The anti-cancer role of celecoxib is through various intrinsic and extrinsic pathways associated with apoptosis and the downregulation of NF-kB, caspase-9, BAX, and BCL-xL [92].
Furthermore, celecoxib binds to 3-phosphoinositide-dependent protein kinase-1 (PDK-1) to inhibit the PDK1/Akt pathway via a COX-independent mechanism. PDK1/Akt controls nucleus–centrosome coupling and regulates microtubules and MAP binding to microtubules. Therefore, by suppressing the PDK1/Akt pathway, the aerosolized formulation of celecoxib should be effective in treating NSCLC [39]. Based on an intervention performed by Haynes et al., inhaled celecoxib resulted in in vitro cytotoxic and apoptotic responses against human NSCLC, demonstrated by the increase in PPAR-γ and p53 expression [41]. In addition, evidence suggests the possible involvement of a COX-2-independent pathway of celecoxib against NSCLC. Under physiological conditions, cPLA2 and 5-LOX regulate the arachidonic acid pathway. By releasing arachidonic acid from membrane lipids, cPLA2 and 5-LOX may promote lung mouse tumorigenesis. However, in the presence of aerosolized celecoxib, cPLA2 and 5-LOX expression in NSCLC cells decreased substantially [40]. In addition, indomethacin [36] is another NSAID that was proven to aid in NSCLC. A few clinical trials have combined this drug with other cytotoxic drugs. IND was noted to induce apoptosis in the cancer cells by initiating the caspase-3 enzymatic activity through upregulating the Bax, Bak, and PPAR-ɣ pathway [42,43]. Sarvepalli et al. prepared a liposomal formulation of IND to increase its anticancer potential. The significant inhibition of COX-2 and induction of caspase in all the IND-treated groups was observed. Sarvepalli et al. concluded that liposomes are more efficacious compared to plain IND in treating NSCLC [89]. However, additional clinical studies and in vivo experiments are required to attain a complete view of this approach and to validate this therapy.
3.3. Anti-Hyperlipidemic Drugs
Statins are the drugs of choice for treating elevated cholesterol levels in the blood [93]. Many pre-clinical and clinical findings have proven the anti-neoplastic effects of statins [31]. Thus, drug repurposing studies have focused on statins as an off-label drug for cancer treatment [94]. By inhibiting β-hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase, an enzyme essential for synthesizing cholesterol [95], statins decrease the production of mevalonate derivatives that are essential for many growth regulatory processes such as proliferation, apoptosis, and differentiation [25]. Atorvastatin is a statin with an antitumor mechanism that inhibits Akt/mTOR and activates the MAPK pathway [84]. Atorvastatin induces apoptosis of tumor cells by depleting the isoprenoid driven growth proteins necessary for the function of cell-growth-stimulating proteins Ras, Rac, and Rho. In addition, by stimulating autophagy and ferroptosis, a type of programmed cell death associated with excess iron overload and accumulating lipid reactive oxygen species, atorvastatin acts as an anti-tumor agent [96]. Hosseinimehr et al. studied the effects of atorvastatin on lung cancer cells. In this study, the apoptosis rate of A-549 lung cancer cells that were both treated with atorvastatin and irradiated was higher than the apoptosis rate of cells that were only irradiated; this difference was statistically significant. This was correlated to atorvastatin’s role in producing ROS, which aids in the apoptosis of NSCLC [44,45].
Lovastatin is another statin that exhibits a hypolipidemic effect by competing with HMG-CoA reductase. Lovastatin also induces apoptosis by inhibiting cell proliferation and regulating cancer cell signaling pathways [97]. To study lovastatin efficiency in NSCLC, Walther et al. found that lovastatin increases COX2 expression and subsequently activates PPAR-γ, which induces the cytotoxicity of lung cancer cells [98].
Simvastatin is another anti-hyperlipidemic being repurposed in the treatment of NSCLC. A phase II trial showed that treating NSCLC with gefitinib plus simvastatin instead of gefitinib showed a higher response rate and prolonged progression-free survival [99]. On a molecular level, Simvastatin was shown to induce apoptosis in p53 mutated cells, in addition to inhibiting cell growth and proliferation [48].
Furthermore, Pitavastatin can also be used in the treatment of NSCLC because of its proven potential in in vitro studies [47]. Using a combination of Pitavastatin and erlotinib on an EGFR-TKI-resistant human lung adenocarcinoma cell line showed a promising synergic cytotoxic effect. The proposed mechanism involves the induction of alternative regulated cell death pathways by inhibiting mevalonic acid (Mev) and the pan-caspase inhibitor zVAD [47]. Its anti-neoplastic effects were also explained by downregulating the MVA pathway and inhibiting the expression of EGFR, hence suppressing the Ras/Raf/MEK/ERK signal cascade and inducing the apoptosis of lung cancer cells [100].
To conclude, even though more research is needed to better explain and affirm the effectiveness of statins in treating NSCLC, much research has already correlated the role of statins in ameliorating the treatment outcomes of lung cancer.
3.4. Anti-Diabetic Drugs
Biguanides and thiazolidinediones are the drug of choice for treating diabetes mellitus. To start with, metformin, a biguanide, has been used as an antidiabetic drug by a direct and indirect mechanism [101]. Metformin acts directly by inhibiting mitochondrial ETC/OxPhos and the consequent activation of AMPK and it has an indirect role by inhibiting hepatic gluconeogenesis, hence lowering systemic insulin levels [102]. Metformin also has an antitumor effect. Knowing that insulin stimulates cellular proliferation and signaling pathways, decreasing insulin levels will indirectly suppress the tumor-stimulating pathways [103]. Additionally, metformin induces cancer cell apoptosis by halting mTOR activity and stimulating the AMPK/LKB1/TORC1 signaling pathway. Further mTOR inhibition, cell cycle arrest, and repression of colony formation ability are achieved by metformin via increasing tumor radiosensitivity through the downregulation of the EGFR/PI3K/Akt signaling pathway [50,104]. The experiments performed to study the effect of metformin on treating lung cancer concluded that metformin was tolerated but did not provide any oncological benefit [51]. In a lung cancer trial where 167 non-diabetic patients with unresectable stage 3 NSCLC maintained on carboplatin and paclitaxel-based chemoradiation either alone or with metformin, the metformin arm failed to provide any significant differences in the rates of survival or distant metastasis [84]. Another drug used to treat diabetes is thiazolidinediones, which functions by activating PPAR-γ (a nuclear receptor), which increases insulin sensitivity and levels of adiponectin resulting in the regulation of glucose metabolism and fatty acid storage [105]. In lung cancer, studies have shown that TZDs prevent the growth of NSCLC cells in vitro [106]. In a xenograft model, TZD blocked tumor progression and, in samples from human lung tumors, decreased PPAR-γ expression worsened the prognosis [105]. Pioglitazone is a PPARγ agonist used commonly for the treatment of type II diabetes. Seabloom et al. recently thought of repurposing pioglitazone for lung cancer treatment via the inhalation route. The authors administered mutated mice with NSCLC cells with aerosolized pioglitazone and the reduction in the size of the adenoma was remarkable [52]. However, insignificant results in terms of inhibiting tumor load and multiplicity were obtained by Zhang et al. when they previously studied the effect of oral pioglitazone in combination with aerosolized budesonide on a lung carcinogenesis mouse model [37]. To conclude, the evidence is inconclusive concerning thiazolidinediones’ role in treating NSCLC and more studies are imperative to determine the downstream effectors of PPAR-γ that mediate the antitumorigenic effects in NSCLC.
3.5. Anti-Microbial Drugs
Bedaquiline, initially FDA-approved as an anti-tuberculosis medication, has shown promising anti-cancer effects. Patil et al. studied the delivery of bedaquiline via inhalation, which demonstrated enhanced anti-NSCLC activity, circumventing the issue of the drug’s poor lung solubility in the aqueous form [107]. These results were also demonstrated by Parvathaneni et al., who showed repurposing bedaquiline as an inhalable cyclodextrin complex is a promising NSCLC treatment [108]. Another antimicrobial medication, Tigecycline, was shown to target NSCLC cells via inhibiting proliferation, inducing the apoptosis of cell lines derived from NSCLC subtypes, and dose-dependently inhibiting the colony formation of highly proliferative and invasive NSCLC subgroups [109]. Tigecycline, through the inhibition of mitochondrial function, may be repurposed as a new targeted NSCLC therapy [109].
Similarly, minocycline may be a repurposed target to be included in NSCLC treatment protocols because it reduces side effects associated with chemoradiation. The local application of minocycline was found to prevent and repair afatinib-induced skin disorders in NSCLC patients [53]. The mechanism was unraveled by histological staining, which showed that minocycline maintained similar EGFR status on the skin of mice treated with anti-EGFR (afatinib) compared to mice not treated with afatinib [53].
Itraconazole is an antifungal shown to inhibit the proliferation, migration, and tube formation of endothelial cells [110]. In a phase I trial in patients with advanced lung cancer, itraconazole was well tolerated in combination with pemetrexed. The overall median progression-free survival was longer in the patients taking itraconazole than in the controls. Even though the mechanism of action of itraconazole as an antineoplastic is not clear yet, it is suggested to be the inhibition of dirtier and the activity against multiple angiogenic stimuli [110]. Furthermore, oral itraconazole’s efficacy in suppressing tumor growth was similar to that of cisplatin in multiple xenograft models of NSCLC [54].
3.6. Anti-Helminthic Drugs
Mebendazole, an anti-helminthic that inhibits microtubule synthesis by blocking tubulin polymerization, was shown to have a cytotoxic effect on NSCLC cell lines A549, H1299, and H460 [55]. In vivo, mice treated with mebendazole showed no side effects and had an 80% lower mean colony count as compared to controls [55]. The molecular mechanism involves the stabilization of post-translational p53 and the downstream expression of p21 and MDM2 [55]. Mebendazole can also induce mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells [111]. Another popular anti-helminthic is niclosamide, which can inhibit glucose uptake and the anaerobic metabolism of cells [112]. Niclosamide has been shown to have an anti-proliferative effect on human lung cancer cells in vivo [113]. Similarly, Piperazine hybrids were also found to have anti-prolific properties against human lung cancer cells [113]. Ivermectin is another anti-helminthic that could be effective in the treatment of NSCLC. Ivermectin’s antineoplastic properties are from its ability to block the canonical WNT (wingless-related integration site) signaling pathway that influences a transcriptional factor of the T-cell factor (TCF) family [114]. Levamisole was shown to arrest the cell in the G0/G1 phase and thus reduce the proliferation of lung cancer cells. Through the restriction of the phosphorylation of c-Jun N-terminal kinase (JNK), levamisole increased the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced death receptor 4 (DR4)-independent apoptosis rate [56].
3.7. Anti-Retroviral Drugs
Lopinavir, a protease inhibitor, is an FDA-approved antiretroviral drug for treating HIV, in combination with Ritonavir [57]. This combination is being tested in the treatment of lung cancer. It was shown that Lopinavir and Ritonavir exposure causes cell cycle arrest, decreased cell viability, and apoptosis in lung cancer cells [115]. Efavirenz is another antiretroviral drug that downregulates the synthesis of viral DNA by binding the viral reverse transcriptase [116]. Efavirenz is a candidate for repurposing because it was shown not only to inhibit the progression of lung cancer but also to have a synergic effect in combination with radiation therapy [115,117]. Nelfinavir is another HIV protease inhibitor. It has the potential to be repurposed for NSCLC treatment since it inhibits the proteasome activity in lung cancer cells [118]. Nelfinavir also has a synergic effect with other chemotherapy agents [118].
3.8. Anti-Malarial Drugs
Atovaquone, an antimalarial drug known to inhibit mitochondrial oxygen consumption, has been shown to reduce tumor hypoxia in patients with NSCLC [58]. Repurposing atovaquone may provide a new radiosensitizer that improves radiation outcomes in NSCLC patients, seeing that tumor hypoxia is a common obstacle in radiation, chemotherapy, and immunotherapy [58]. Pretreatment with chloroquine can oppose nonsmall adenocarcinoma resistance via autophagy inhibition mediated through ROS modulation of the β-catenin pathway [119]. Dihydroartemisinin can lead to the inhibition of NSCLC metastasis via glucose metabolism modulation, specifically by inhibiting the NF-κB pathway, causing dihydroartemisinin to be a promising NSCLC treatment [120]. Quinacrine is an antimalarial that inhibits the FACT (facilitates chromatin transcription) complex, which may be involved in TKI (tyrosine kinase inhibitor) resistance. A phase I clinical trial showed that a combination of erlotinib and quinacrine was well tolerated; however, its efficacy was minimal in advanced NSCLC [121].
3.9. Other Drugs
Sertraline, an FDA-approved antidepressant, was found to sensitize NSCLC to erlotinib by inducing autophagic flux confirmed by the accumulation of LC3-II and autolysosome formation [59]. The effect of the dual therapy, sertraline, and erlotinib, stems from the regulation of the AMPK/mTOR pathway in NSCLC cells [59].
Nitroglycerin is a nitric oxide (NO) donner used to treat angina; however, nitroglycerin can also induce apoptosis, downregulate HIF1alpha, and inhibit angiogenesis [122]. A phase II clinical trial showed that nitroglycerin could improve the response rate to vinorelbine with tolerable toxicity, warranting a phase III trial [123]. The same trial demonstrated that combining vinorelbine and cisplatin with nitroglycerin improved the survival of patients with untreated stage IIIB/IV non-squamous cell lung cancer [123].
4. Clinical Trials
While using the multiple repurposing approaches mentioned earlier, scientists were able to identify potential targets for repurposing based on the mechanism of action. However, only a few drugs reached the clinical trial stage. Glucocorticoid is the most represented class of medications in the undergoing clinical trials investigating candidate drugs for repurposing in the treatment of NSCLC (Table 2). A phase II trial aims at investigating the combination of prednisone with Afatinib in advanced NSCLC. The study’s outcomes include progression-free survival, overall survival, and response rate (ClinicalTrials.gov; NCT04497584).

Table 2.
Current clinical trials investigating repurposed drugs for the treatment of NSCLC.
A phase 1/2 study explores the effect of Dexamethasone, a popular glucocorticoid, in reducing the FLT-PET signal in NSCLC (ClinicalTrials.gov; NCT04037462). Similarly, another phase 2/3 trial explores the synergic effect of multiple drugs, including dexamethasone, on the overall survival of advanced stages of NSCLC (ClinicalTrials.gov; NCT05096663).
Metformin, used in the treatment of type 2 diabetes, is explored in a phase 3 trial. The trial assesses the progression-free survival, overall survival, and overall response rate in patients with EGFR mutation NSCLC (ClinicalTrials.gov; NCT05445791).
A phase 1 trial aims to assess the safety of the combination of Statins with PD-1/PD-L1 inhibitors (ClinicalTrials.gov; NC T05636592). Furthermore, the Th1 immune response of the Vancomycin and Stereotactic Body Radiation Therapy combination is explored in an early phase I trial (ClinicalTrials.gov; NCT03546829). Antimalarial drugs are also being investigated in clinical trials. Hence, a phase I trial in locally advanced NSCLC is exploring the safety of Atovaquone by measuring the dose-limiting toxicity (DLT) rate and maximum tolerated dose (MTD) (ClinicalTrials.gov; NCT04648033). Finally, Hydroxychloroquine added to Binimetinib in advanced KRAS mutant NSCLC is being considered in a phase II trial by measuring the objective response rate (ClinicalTrials.gov; NCT04735068).
5. Conclusions and Future Directions
Lung cancer remains the main culprit behind cancer-related deaths worldwide, which is why expanding treatment options is paramount. Despite high mortality rates, survival rates are looking more promising. Drugs initially designed for other diseases share similar channels with many lung cancer subtypes. As such, drug repurposing may serve as a fast and cost-effective method of providing new treatment options for lung cancer patients, further boosting survival rates. Drug repurposing bypasses Phase I and II of clinical trials, rendering the drug approval process much swifter than usual. With the ever-growing advances in precision medicine and artificial intelligence, a wider array of drugs can be repurposed in a more directed and strategic manner. This is in hopes of ultimately accounting for patient-specific tumor characteristics and improving survival rates, qualities of life, and overall costs via true personalized medicine. Further studies should be conducted, with in vivo experiments and clinical trials whenever possible, to evaluate potential avenues of drug repurposing within lung cancer treatment.
Author Contributions
Conceptualization, G.D.; methodology, G.D., D.D., M.B.Z. and N.N.; validation, H.F.B., M.R. and R.P.; investigation, H.F.B., M.R. and R.P.; resources, G.D., D.D., M.B.Z. and N.N.; data curation, G.D., D.D., M.B.Z. and N.N.; writing—original draft preparation, G.D., D.D., M.B.Z. and N.N.; writing—review and editing, H.F.B., M.R. and R.P.; visualization, H.F.B.; supervision, H.F.B.; project administration, H.F.B. and G.D.; funding acquisition, H.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank all members of the Faculty of Medicine, American University of Beirut (Beirut, Lebanon) and the Arkadi M. Rywlin. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center (Miami Beach, FL, USA) for their help with this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Gonzalez-Cao, M. Ablating lung cancer, knowing the tumor better. Lancet Reg. Health Eur. 2022, 22, 100494. [Google Scholar] [CrossRef]
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18, s41–s46. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Call, S.; Dooms, C.; Obiols, C.; Sanchez, M.; Travis, W.D.; Vollmer, I. Lung cancer staging: A concise update. Eur. Respir. J. 2018, 51, 1800190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 447–468. [Google Scholar] [CrossRef]
- Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005, 121, 823–835. [Google Scholar] [CrossRef]
- Hanna, J.M.; Onaitis, M.W. Cell of origin of lung cancer. J. Carcinog. 2013, 12, 6. [Google Scholar] [CrossRef]
- Li, T.; Kung, H.J.; Mack, P.C.; Gandara, D.R. Genotyping and genomic profiling of non-small-cell lung cancer: Implications for current and future therapies. J. Clin. Oncol. 2013, 31, 1039–1049. [Google Scholar] [CrossRef]
- Sos, M.L.; Koker, M.; Weir, B.A.; Heynck, S.; Rabinovsky, R.; Zander, T.; Seeger, J.M.; Weiss, J.; Fischer, F.; Frommolt, P.; et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009, 69, 3256–3261. [Google Scholar] [CrossRef]
- Weiss, J.; Sos, M.L.; Seidel, D.; Peifer, M.; Zander, T.; Heuckmann, J.M.; Ullrich, R.T.; Menon, R.; Maier, S.; Soltermann, A.; et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci. Transl. Med. 2010, 2, 62ra93. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef]
- Roberts, P.J.; Stinchcombe, T.E.; Der, C.J.; Socinski, M.A. Personalized medicine in non-small-cell lung cancer: Is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J. Clin. Oncol. 2010, 28, 4769–4777. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzo, F.; Marchetti, A.; Skokan, M.; Rossi, E.; Gajapathy, S.; Felicioni, L.; Del Grammastro, M.; Sciarrotta, M.G.; Buttitta, F.; Incarbone, M.; et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J. Clin. Oncol. 2009, 27, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Dutt, A.; Ramos, A.H.; Hammerman, P.S.; Mermel, C.; Cho, J.; Sharifnia, T.; Chande, A.; Tanaka, K.E.; Stransky, N.; Greulich, H.; et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PLoS ONE 2011, 6, e20351. [Google Scholar] [CrossRef]
- Ford, C.E.; Lau, S.K.; Zhu, C.Q.; Andersson, T.; Tsao, M.S.; Vogel, W.F. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br. J. Cancer 2007, 96, 808–814. [Google Scholar] [CrossRef]
- SEER Surveillance, Epidemiology, and End Results Program. SEER*Stat Database: Incidence—SEER Research Data, 18 Registries (2000–2018). National Cancer Institute DCCPS, Surveillance Research Program. 2021. Available online: www.seer.cancer.gov (accessed on 19 December 2022).
- Siddiqui, F.V.S.; Siddiqui, A. Lung Cancer; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Crequit, P.; Chaimani, A.; Yavchitz, A.; Attiche, N.; Cadranel, J.; Trinquart, L.; Ravaud, P. Comparative efficacy and safety of second-line treatments for advanced non-small cell lung cancer with wild-type or unknown status for epidermal growth factor receptor: A systematic review and network meta-analysis. BMC Med. 2017, 15, 193. [Google Scholar] [CrossRef]
- Sosa Iglesias, V.; Giuranno, L.; Dubois, L.J.; Theys, J.; Vooijs, M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting? Front. Oncol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Chamaa, F.; Assi, S.; Chalhoub, R.M.; Abou-Antoun, T.; Abou-Kheir, W. Cancer Stem Cells in Neuroblastoma: Expanding the Therapeutic Frontier. Front. Mol. Neurosci. 2019, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, H.; Saker, Z.; Harati, H.; Fares, Y.; Bahmad, H.F.; Nabha, S. Drug Repurposing in Medulloblastoma: Challenges and Recommendations. Curr. Treat. Options Oncol. 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Poppiti, R.J. Medulloblastoma cancer stem cells: Molecular signatures and therapeutic targets. J. Clin. Pathol. 2020, 73, 243–249. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Elajami, M.K.; El Zarif, T.; Bou-Gharios, J.; Abou-Antoun, T.; Abou-Kheir, W. Drug repurposing towards targeting cancer stem cells in pediatric brain tumors. Cancer Metastasis Rev. 2020, 39, 127–148. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daher, D.; Aljamal, A.A.; Elajami, M.K.; Oh, K.S.; Alvarez Moreno, J.C.; Delgado, R.; Suarez, R.; Zaldivar, A.; Azimi, R.; et al. Repurposing of Anticancer Stem Cell Drugs in Brain Tumors. J. Histochem. Cytochem. 2021, 69, 749–773. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Elajami, M.K.; Daouk, R.; Jalloul, H.; Darwish, B.; Chalhoub, R.M.; Assi, S.; Chamaa, F.; Abou-Kheir, W. Stem Cells: In Sickness and in Health. Curr. Stem Cell Res. Ther. 2021, 16, 262–276. [Google Scholar] [CrossRef]
- El Zarif, T.; Yibirin, M.; De Oliveira-Gomes, D.; Machaalani, M.; Nawfal, R.; Bittar, G.; Bahmad, H.F.; Bitar, N. Overcoming Therapy Resistance in Colon Cancer by Drug Repurposing. Cancers 2022, 14, 2105. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Demus, T.; Moubarak, M.M.; Daher, D.; Alvarez Moreno, J.C.; Polit, F.; Lopez, O.; Merhe, A.; Abou-Kheir, W.; Nieder, A.M.; et al. Overcoming Drug Resistance in Advanced Prostate Cancer by Drug Repurposing. Med. Sci. 2022, 10, 15. [Google Scholar] [CrossRef]
- Liu, W.J.; Du, Y.; Wen, R.; Yang, M.; Xu, J. Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacol. Ther. 2020, 206, 107438. [Google Scholar] [CrossRef]
- Ho, M.M.; Ng, A.V.; Lam, S.; Hung, J.Y. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007, 67, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Park, P.G.; Merryman, J.; Orloff, M.; Schuller, H.M. Beta-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: Implications for individuals with preexisting chronic lung disease. Cancer Res. 1995, 55, 3504–3508. [Google Scholar] [PubMed]
- Kang, Y.; Nagaraja, A.S.; Armaiz-Pena, G.N.; Dorniak, P.L.; Hu, W.; Rupaimoole, R.; Liu, T.; Gharpure, K.M.; Previs, R.A.; Hansen, J.M.; et al. Adrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Guzner, A.; Wainwright, D.A.; Mohindra, N.A.; Chae, Y.K.; Behdad, A.; Villaflor, V.M. The Impact of Beta Blockers on Survival Outcomes in Patients With Non-small-cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, e57–e62. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Ghadiri, M.; Leong, C.R.; Young, P.M.; Traini, D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv. Drug Deliv. Rev. 2018, 133, 107–130. [Google Scholar] [CrossRef]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Marepally, S.; Jackson, T.; Singh, M. Inhalation delivery of Telmisartan enhances intratumoral distribution of nanoparticles in lung cancer models. J. Control. Release 2013, 172, 86–95. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Qu, L.; Zhu, J. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int. 2017, 17, 1. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.M.; Chen, Z.F.; Ji, R.; Guo, Q.H.; Li, Q.; Zhang, H.L.; Zhou, Y.N. Celecoxib regulates apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells. Int. J. Mol. Med. 2014, 33, 1451–1458. [Google Scholar] [CrossRef]
- Haynes, A.; Shaik, M.S.; Chatterjee, A.; Singh, M. Formulation and evaluation of aerosolized celecoxib for the treatment of lung cancer. Pharm. Res. 2005, 22, 427–439. [Google Scholar] [CrossRef]
- Petkova, D.K.; Clelland, C.; Ronan, J.; Pang, L.; Coulson, J.M.; Lewis, S.; Knox, A.J. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 2004, 98, 164–172. [Google Scholar] [CrossRef]
- Zhou, X.M.; Wong, B.C.; Fan, X.M.; Zhang, H.B.; Lin, M.C.; Kung, H.F.; Fan, D.M.; Lam, S.K. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis 2001, 22, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Ghasemi, F.; Flahatgar, F.; Rahmanian, N.; Ghasemi, A.; Asgarian-Omran, H. Atorvastatin Sensitizes Breast and Lung Cancer Cells to Ionizing Radiation. Iran. J. Pharm. Res. 2020, 19, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J.; Izakmehri, M.; Ghasemi, A. In vitro protective effect of atorvastatin against ionizing radiation induced genotoxicity in human lymphocytes. Cell Mol. Biol. 2015, 61, 68–71. [Google Scholar] [PubMed]
- Sanli, T.; Liu, C.; Rashid, A.; Hopmans, S.N.; Tsiani, E.; Schultz, C.; Farrell, T.; Singh, G.; Wright, J.; Tsakiridis, T. Lovastatin sensitizes lung cancer cells to ionizing radiation: Modulation of molecular pathways of radioresistance and tumor suppression. J. Thorac. Oncol. 2011, 6, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Otahal, A.; Aydemir, D.; Tomasich, E.; Minichsdorfer, C. Delineation of cell death mechanisms induced by synergistic effects of statins and erlotinib in non-small cell lung cancer cell (NSCLC) lines. Sci. Rep. 2020, 10, 959. [Google Scholar] [CrossRef]
- Chou, C.W.; Lin, C.H.; Hsiao, T.H.; Lo, C.C.; Hsieh, C.Y.; Huang, C.C.; Sher, Y.P. Therapeutic effects of statins against lung adenocarcinoma via p53 mutant-mediated apoptosis. Sci. Rep. 2019, 9, 20403. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Li, Y.; Li, W.; Chen, Y. Simvastatin prevents proliferation and bone metastases of lung adenocarcinoma in vitro and in vivo. Neoplasma 2013, 60, 240–246. [Google Scholar] [CrossRef]
- Zannella, V.E.; Dal Pra, A.; Muaddi, H.; McKee, T.D.; Stapleton, S.; Sykes, J.; Glicksman, R.; Chaib, S.; Zamiara, P.; Milosevic, M.; et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin. Cancer Res. 2013, 19, 6741–6750. [Google Scholar] [CrossRef]
- Skinner, H.; Hu, C.; Tsakiridis, T.; Santana-Davila, R.; Lu, B.; Erasmus, J.J.; Doemer, A.J.; Videtic, G.M.M.; Coster, J.; Yang, A.X.; et al. Addition of Metformin to Concurrent Chemoradiation in Patients With Locally Advanced Non-Small Cell Lung Cancer: The NRG-LU001 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1324–1332. [Google Scholar] [CrossRef]
- Seabloom, D.E.; Galbraith, A.R.; Haynes, A.M.; Antonides, J.D.; Wuertz, B.R.; Miller, W.A.; Miller, K.A.; Steele, V.E.; Suen, C.S.; O’Sullivan, M.G.; et al. Safety and Preclinical Efficacy of Aerosol Pioglitazone on Lung Adenoma Prevention in A/J Mice. Cancer Prev. Res. 2017, 10, 124–132. [Google Scholar] [CrossRef]
- Sano, K.; Nakadate, K.; Hanada, K. Minocycline prevents and repairs the skin disorder associated with afatinib, one of the epidermal growth factor receptor-tyrosine kinase inhibitors for non-small cell lung cancer. BMC Cancer 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Aftab, B.T.; Dobromilskaya, I.; Liu, J.O.; Rudin, C.M. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011, 71, 6764–6772. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Sasaki, J.; Ramesh, R.; Roth, J. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 2002, 8, 2963–2969. [Google Scholar] [PubMed]
- Laudisi, F.; Maronek, M.; Di Grazia, A.; Monteleone, G.; Stolfi, C. Repositioning of Anthelmintic Drugs for the Treatment of Cancers of the Digestive System. Int. J. Mol. Sci. 2020, 21, 4957. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, R.S.; Goa, K.L. Lopinavir/ritonavir: A review of its use in the management of HIV infection. Drugs 2003, 63, 769–802. [Google Scholar] [CrossRef]
- Skwarski, M.; McGowan, D.R.; Belcher, E.; Di Chiara, F.; Stavroulias, D.; McCole, M.; Derham, J.L.; Chu, K.Y.; Teoh, E.; Chauhan, J.; et al. Mitochondrial Inhibitor Atovaquone Increases Tumor Oxygenation and Inhibits Hypoxic Gene Expression in Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 2459–2469. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.; Shen, X.; Wang, Q.; Lv, J.; Liu, M.; Cheng, F.; Zhao, Z.; Pang, X. Repurposing sertraline sensitizes non-small cell lung cancer cells to erlotinib by inducing autophagy. JCI Insight 2018, 3, e98921. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Talevi, A. Drug Repurposing; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Scapozza, L.; Ruiz i Altaba, A. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Shim, J.S.; Liu, J.O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef]
- Sanseau, P.; Agarwal, P.; Barnes, M.R.; Pastinen, T.; Richards, J.B.; Cardon, L.R.; Mooser, V. Use of genome-wide association studies for drug repositioning. Nat. Biotechnol. 2012, 30, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.S.; Voight, B.F. Pathway and network-based strategies to translate genetic discoveries into effective therapies. Hum. Mol. Genet. 2016, 25, R94–R98. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, L.; Yin, J.; Huang, T.; Bi, Y.; Kong, X.; Zheng, M.; Cai, Y.D. Identification of new candidate drugs for lung cancer using chemical-chemical interactions, chemical-protein interactions and a K-means clustering algorithm. J. Biomol. Struct. Dyn. 2016, 34, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Subelj, L.; Bajec, M. Unfolding communities in large complex networks: Combining defensive and offensive label propagation for core extraction. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2011, 83, 036103. [Google Scholar] [CrossRef]
- Yu, L.; Huang, J.; Ma, Z.; Zhang, J.; Zou, Y.; Gao, L. Inferring drug-disease associations based on known protein complexes. BMC Med. Genom. 2015, 8 (Suppl. S2), S2. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Navarro, C.; Cano, C.; Fajardo, W.; Blanco, A. DrugNet: Network-based drug-disease prioritization by integrating heterogeneous data. Artif. Intell. Med. 2015, 63, 41–49. [Google Scholar] [CrossRef]
- Hieronymus, H.; Lamb, J.; Ross, K.N.; Peng, X.P.; Clement, C.; Rodina, A.; Nieto, M.; Du, J.; Stegmaier, K.; Raj, S.M.; et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 2006, 10, 321–330. [Google Scholar] [CrossRef]
- Dudley, J.T.; Sirota, M.; Shenoy, M.; Pai, R.K.; Roedder, S.; Chiang, A.P.; Morgan, A.A.; Sarwal, M.M.; Pasricha, P.J.; Butte, A.J. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci. Transl. Med. 2011, 3, 96ra76. [Google Scholar] [CrossRef]
- Sirota, M.; Dudley, J.T.; Kim, J.; Chiang, A.P.; Morgan, A.A.; Sweet-Cordero, A.; Sage, J.; Butte, A.J. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med. 2011, 3, 96ra77. [Google Scholar] [CrossRef]
- Oprea, T.I.; Tropsha, A.; Faulon, J.L.; Rintoul, M.D. Systems chemical biology. Nat. Chem. Biol. 2007, 3, 447–450. [Google Scholar] [CrossRef]
- Dakshanamurthy, S.; Issa, N.T.; Assefnia, S.; Seshasayee, A.; Peters, O.J.; Madhavan, S.; Uren, A.; Brown, M.L.; Byers, S.W. Predicting new indications for approved drugs using a proteochemometric method. J. Med. Chem. 2012, 55, 6832–6848. [Google Scholar] [CrossRef]
- Jensen, P.B.; Jensen, L.J.; Brunak, S. Mining electronic health records: Towards better research applications and clinical care. Nat. Rev. Genet. 2012, 13, 395–405. [Google Scholar] [CrossRef]
- Cavalla, D.; Singal, C. Retrospective clinical analysis for drug rescue: For new indications or stratified patient groups. Drug Discov. Today 2012, 17, 104–109. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Force, U.S.P.S.T. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, X.; Chen, J.Y. Building disease-specific drug-protein connectivity maps from molecular interaction networks and PubMed abstracts. PLoS Comput. Biol. 2009, 5, e1000450. [Google Scholar] [CrossRef] [PubMed]
- Kaitin, K.I.; DiMasi, J.A. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000-2009. Clin. Pharmacol. Ther. 2011, 89, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Nosengo, N. Can you teach old drugs new tricks? Nature 2016, 534, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Sidorova, M.; Petrikaitė, V. The Effect of Beta Adrenoreceptor Blockers on Viability and Cell Colony Formation of Non-Small Cell Lung Cancer Cell Lines A549 and H1299. Molecules 2022, 27, 1938. [Google Scholar] [CrossRef]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, W.; Zuo, Y. Beta-blocker and survival in patients with lung cancer: A meta-analysis. PLoS ONE 2021, 16, e0245773. [Google Scholar] [CrossRef] [PubMed]
- Wegman-Ostrosky, T.; Soto-Reyes, E.; Vidal-Millán, S.; Sánchez-Corona, J. The renin-angiotensin system meets the hallmarks of cancer. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Egami, K.; Murohara, T.; Shimada, T.; Sasaki, K.; Shintani, S.; Sugaya, T.; Ishii, M.; Akagi, T.; Ikeda, H.; Matsuishi, T.; et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J. Clin. Investig. 2003, 112, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Sarvepalli, S.; Parvathaneni, V.; Chauhan, G.; Shukla, S.K.; Gupta, V. Inhaled Indomethacin-Loaded Liposomes as Potential Therapeutics against Non-Small Cell Lung Cancer (NSCLC). Pharm. Res. 2022, 39, 2801–2815. [Google Scholar] [CrossRef] [PubMed]
- Brabender, J.; Park, J.; Metzger, R.; Schneider, P.M.; Lord, R.V.; Hölscher, A.H.; Danenberg, K.D.; Danenberg, P.V. Prognostic significance of cyclooxygenase 2 mRNA expression in non-small cell lung cancer. Ann. Surg. 2002, 235, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Lynch, P.M.; Burke, C.A.; Phillips, R.; Morris, J.S.; Slack, R.; Wang, X.; Liu, J.; Patterson, S.; Sinicrope, F.A.; Rodriguez-Bigas, M.A.; et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut 2016, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, K.P.; Tan, G.S. Cyclooxygenase-2 inhibitors in lung cancer treatment: Bench to bed. Eur. J. Pharmacol. 2015, 769, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tatsuno, I.; Uchida, D.; Moroo, I.; Morio, H.; Nakamura, S.; Noguchi, Y.; Yasuda, T.; Kitagawa, M.; Saito, Y.; et al. Geranylgeranyl-pyrophosphate, an isoprenoid of mevalonate cascade, is a critical compound for rat primary cultured cortical neurons to protect the cell death induced by 3-hydroxy-3-methylglutaryl-CoA reductase inhibition. J. Neurosci. 2000, 20, 2852–2859. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, C.W.; Ku, J.H.; Kwak, C.; Kim, H.H. Inhibition of autophagy potentiates atorvastatin-induced apoptotic cell death in human bladder cancer cells in vitro. Int. J. Mol. Sci. 2014, 15, 8106–8121. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]
- Walther, U.; Emmrich, K.; Ramer, R.; Mittag, N.; Hinz, B. Lovastatin lactone elicits human lung cancer cell apoptosis via a COX-2/PPARγ-dependent pathway. Oncotarget 2016, 7, 10345–10362. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Lee, S.H.; Yoo, N.J.; Hyung, L.S.; Moon, Y.J.; Yun, T.; Kim, H.T.; Lee, J.S. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 1553–1560. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhang, R.; Xia, Y.; Shao, Z.; Mei, Z. Effects of statin exposure and lung cancer survival: A meta-analysis of observational studies. Pharmacol. Res. 2019, 141, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Wagner, M.J.; Cranmer, L.D.; Loggers, E.T.; Pollack, S.M. Propranolol for the treatment of vascular sarcomas. J. Exp. Pharmacol. 2018, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Kazazis, C. Metformin and cancer. Rev. Diabet. Stud. 2013, 10, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Nemenoff, R.A. Peroxisome proliferator-activated receptor-gamma in lung cancer: Defining specific versus “off-target” effectors. J. Thorac. Oncol. 2007, 2, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.; Hurteau, G.; Dessev, C.; Chan, D.; Geraci, M.W.; Winn, R.A.; Heasley, L.E.; Nemenoff, R.A. Peroxisome proliferator-activated receptor-gamma is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol. Pharmacol. 2002, 62, 1207–1214. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Inhalable bedaquiline-loaded cubosomes for the treatment of non-small cell lung cancer (NSCLC). Int. J. Pharm. 2021, 607, 121046. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Elbatanony, R.S.; Goyal, M.; Chavan, T.; Vega, N.; Kolluru, S.; Muth, A.; Gupta, V.; Kunda, N.K. Repurposing Bedaquiline for Effective Non-Small Cell Lung Cancer (NSCLC) Therapy as Inhalable Cyclodextrin-Based Molecular Inclusion Complexes. Int. J. Mol. Sci. 2021, 22, 4783. [Google Scholar] [CrossRef]
- Jia, X.; Gu, Z.; Chen, W.; Jiao, J. Tigecycline targets nonsmall cell lung cancer through inhibition of mitochondrial function. Fundam Clin. Pharmacol. 2016, 30, 297–306. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brahmer, J.R.; Juergens, R.A.; Hann, C.L.; Ettinger, D.S.; Sebree, R.; Smith, R.; Aftab, B.T.; Huang, P.; Liu, J.O. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 619–623. [Google Scholar] [CrossRef]
- Sasaki, J.; Ramesh, R.; Chada, S.; Gomyo, Y.; Roth, J.A.; Mukhopadhyay, T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol. Cancer Ther. 2002, 1, 1201–1209. [Google Scholar]
- Pampori, N.A.; Singh, G.; Srivastava, V.M. Cotugnia digonopora: Carbohydrate metabolism and effect of anthelmintics on immature worms. J. Helminthol. 1984, 58, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, A.; Turanli, S.; Çalişkan, B.; Arka, M.; Banoglu, E. Evaluation of Cytotoxic Activity of New Benzimidazole-Piperazine Hybrids Against Human MCF-7 and A549 Cancer Cells. Pharm. Chem. J. 2020, 53, 1036–1046. [Google Scholar] [CrossRef]
- Armando, R.G.; Mengual Gomez, D.L.; Gomez, D.E. New drugs are not enough-drug repositioning in oncology: An update. Int. J. Oncol. 2020, 56, 651–684. [Google Scholar] [CrossRef] [PubMed]
- Marima, R.; Hull, R.; Dlamini, Z.; Penny, C. Efavirenz and Lopinavir/Ritonavir Alter Cell Cycle Regulation in Lung Cancer. Front. Oncol. 2020, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Adkins, J.C.; Noble, S. Efavirenz. Drugs 1998, 56, 1055–1064, discussion 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Harrer, T.; Korber, V.; Sarpong, E.O.; Moser, F.; Fiebig, N.; Schwegler, M.; Sturzl, M.; Fietkau, R.; Distel, L.V. Cytotoxic effect of Efavirenz in BxPC-3 pancreatic cancer cells is based on oxidative stress and is synergistic with ionizing radiation. Oncol. Lett. 2018, 15, 1728–1736. [Google Scholar] [CrossRef]
- Kawabata, S.; Gills, J.J.; Mercado-Matos, J.R.; Lopiccolo, J.; Wilson, W., 3rd; Hollander, M.C.; Dennis, P.A. Synergistic effects of nelfinavir and bortezomib on proteotoxic death of NSCLC and multiple myeloma cells. Cell Death Dis. 2012, 3, e353. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Choudhury, D.; Das, A.; Mukherjee, D.D.; Dasgupta, M.; Bandopadhyay, S.; Chakrabarti, G. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of beta-catenin pathway. Apoptosis 2019, 24, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Geng, G.; Yu, X.; Liu, H.; Gao, J.; An, H.; Cai, C.; Li, N.; Shen, D.; Wu, X.; et al. Repurposing the anti-malarial drug dihydroartemisinin suppresses metastasis of non-small-cell lung cancer via inhibiting NF-kappaB/GLUT1 axis. Oncotarget 2016, 7, 87271–87283. [Google Scholar] [CrossRef] [PubMed]
- Bhateja, P.; Dowlati, A.; Sharma, N. Phase I study of the combination of quinacrine and erlotinib in patients with locally advanced or metastatic non small cell lung cancer. Investig. New Drugs 2018, 36, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Nitroglycerin enhances vascular blood flow and drug delivery in hypoxic tumor tissues: Analogy between angina pectoris and solid tumors and enhancement of the EPR effect. J. Control. Release 2010, 142, 296–298. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).