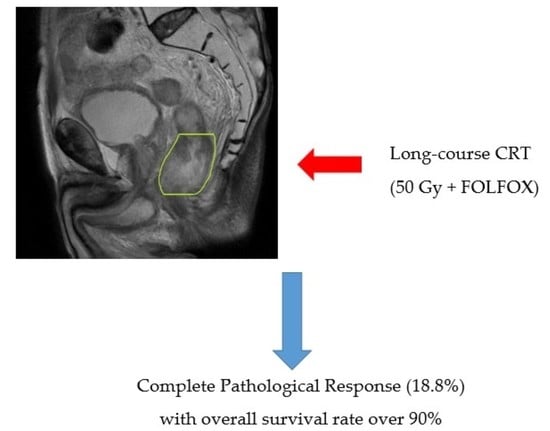
Analysis of Survival in Complete Pathological Response after Long-Course Chemoradiotherapy in Patients with Advanced Rectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paquette, I.M.; Atkinson, S.J. The Epidemiology of Rectal Cancer. In Comprehensive Rectal Cancer Care; Springer: Cham, Switzerland, 2019; pp. 3–20. [Google Scholar]
- Rouleau-Fournier, F.; Brown, C.J. Can less be more? Organ preservation strategies in the management of rectal cancer. Curr. Oncol. 2019, 26, S16–S23. [Google Scholar] [CrossRef] [Green Version]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, e42, Erratum in Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rodriguez, R.M.; Quezada-Diaz, F.; Hameed, I.; Kalabin, A.; Patil, S.; Smith, J.J.; Garcia-Aguilar, J. Organ Preservation in Patients with Rectal Cancer Treated with Total Neoadjuvant Therapy. Dis. Colon Rectum 2021, 64, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Li, D.G.; Li, L.; Kong, F.B.; Pang, L.M.; Mai, W. Meta-Analysis of Oncological Outcome After Abdominoperineal Resection or Low Anterior Resection for Lower Rectal Cancer. Pathol. Oncol. Res. 2015, 21, 19–27. [Google Scholar] [CrossRef] [Green Version]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Tseng, M.S.F.; Zheng, H.; Ng, I.W.S.; Leong, Y.H.; Leong, C.N.; Yong, W.P.; Cheong, W.K.; Tey, J.C.S. Outcomes of neoadjuvant chemoradiotherapy followed by total mesorectal excision surgery for locally advanced rectal cancer: A single-institution experience. Singapore Med. J. 2018, 59, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.S.; Colangelo, L.H.; O’Connell, M.J.; Yothers, G.; Deutsch, M.; Allegra, C.J.; Kahlenberg, M.S.; Baez-Diaz, L.; Ursiny, C.S.; Petrelli, N.J.; et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J. Clin. Oncol. 2009, 27, 5124–5130. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, T.; Hayakawa, K.; Koizumi, W.; Kumagai, Y.; Watanabe, M. Strategy to avoid local recurrence in patients with locally advanced rectal cancer. Radiat. Oncol. 2019, 14, 53. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef]
- Marchiò, C.; Sapino, A. The pathologic complete response open question in primary therapy. J. Natl. Cancer Inst. Monogr. 2011, 2011, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Color. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; Mulcahy, H.E.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
- Chen, H.Y.; Feng, L.L.; Li, M.; Ju, H.Q.; Ding, Y.; Lan, M.; Song, S.M.; Han, W.D.; Yu, L.; Wei, M.B.; et al. College of American Pathologists Tumor Regression Grading System for Long-Term Outcome in Patients with Locally Advanced Rectal Cancer. Oncologist 2021, 26, e780–e793. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Beets-Tan, R.G.; Lambregts, D.M.; Lammering, G.; Nelemans, P.J.; Engelen, S.M.; van Dam, R.M.; Jansen, R.L.; Sosef, M.; Leijtens, J.W.; et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 2011, 29, 4633–4640. [Google Scholar] [CrossRef]
- Park, I.J.; You, Y.N.; Agarwal, A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Eng, C.; Feig, B.W.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J. Clin. Oncol. 2012, 30, 1770–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalilian, M.; Davis, S.; Mohebbi, M.; Sugamaran, B.; Porter, I.W.; Bell, S.; Warrier, S.K.; Wale, R. Pathologic response to neoadjuvant treatment in locally advanced rectal cancer and impact on outcome. J. Gastrointest. Oncol. 2016, 7, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Amaral, E.; Bernardes, M.; Ribeiro, S.; Rosa, B.; Pereira, A. Predictors of pathological response and clinical outcome following chemoradiation for locally advanced rectal cancer—A systematic review. JCOL 2020, 40, 278–299. [Google Scholar] [CrossRef]
- de Campos-Lobato, L.F.; Stocchi, L.; da Luz Moreira, A.; Geisler, D.; Dietz, D.W.; Lavery, I.C.; Fazio, V.W.; Kalady, M.F. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann. Surg. Oncol. 2011, 18, 1590–1598. [Google Scholar] [CrossRef]
- Belluco, C.; De Paoli, A.; Canzonieri, V.; Sigon, R.; Fornasarig, M.; Buonadonna, A.; Boz, G.; Innocente, R.; Perin, T.; Cossaro, M.; et al. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: Implications for local excision surgical strategies. Ann. Surg. Oncol. 2011, 18, 3686–3693. [Google Scholar] [CrossRef]
- Pucciarelli, S.; Toppan, P.; Friso, M.L.; Russo, V.; Pasetto, L.; Urso, E.; Marino, F.; Ambrosi, A.; Lise, M. Complete pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis. Colon Rectum 2004, 47, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Huang, X.Z.; Gao, P.; Song, Y.X.; Chen, X.W.; Lv, X.E.; Fu, Y.; Xiao, Q.; Ye, S.Y.; Wang, Z.N. Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data. BMC Cancer 2021, 21, 1214. [Google Scholar] [CrossRef]
- Shin, J.K.; Huh, J.W.; Lee, W.Y.; Yun, S.H.; Kim, H.C. Clinical prediction model of pathological response following neoadjuvant chemoradiotherapy for rectal cancer. Sci. Rep. 2022, 12, 7145. [Google Scholar] [CrossRef] [PubMed]
- Jayanand, S.B.; Seshadri, R.A.; Tapkire, R. Signet ring cell histology and non-circumferential tumors predict pathological complete response following neoadjuvant chemoradiation in rectal cancers. Int. J. Color. Dis. 2011, 26, 23–27. [Google Scholar] [CrossRef]
- Garland, M.L.; Vather, R.; Bunkley, N.; Pearse, M.; Bissett, I.P. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int. J. Color. Dis. 2014, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Sgroi, G.; Sarti, E.; Barni, S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann. Surg. 2016, 263, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, Y.; Liu, K.; Pei, Q.; Zhu, H. Comparing neoadjuvant long-course chemoradiotherapy with short-course radiotherapy in rectal cancer. BMC Gastroenterol. 2021, 21, 227. [Google Scholar] [CrossRef]
- Huang, C.M.; Huang, M.Y.; Ma, C.J.; Yeh, Y.S.; Tsai, H.L.; Huang, C.W.; Huang, C.J.; Wang, J.Y. Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer. Radiat. Oncol. 2017, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.M.; Huang, C.W.; Ma, C.J.; Yeh, Y.S.; Su, W.C.; Chang, T.K.; Tsai, H.L.; Juo, S.H.; Huang, M.Y.; Wang, J.Y. Predictive Value of FOLFOX-Based Regimen, Long Interval, Hemoglobin Levels and Clinical Negative Nodal Status, and Postchemoradiotherapy CEA Levels for Pathological Complete Response in Patients with Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy. J. Oncol. 2020, 2020, 9437684. [Google Scholar] [CrossRef]
- Fernández-Martos, C.; Pericay, C.; Aparicio, J.; Salud, A.; Safont, M.; Massuti, B.; Vera, R.; Escudero, P.; Maurel, J.; Marcuello, E.; et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J. Clin. Oncol. 2010, 28, 859–865. [Google Scholar] [CrossRef]
- Chau, I.; Brown, G.; Cunningham, D.; Tait, D.; Wotherspoon, A.; Norman, A.R.; Tebbutt, N.; Hill, M.; Ross, P.J.; Massey, A.; et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J. Clin. Oncol. 2006, 24, 668–674. [Google Scholar] [CrossRef]
- De Felice, F.; D’Ambrosio, G.; Musio, D.; Iafrate, F.; Benevento, I.; Marzo, M.; Mancini, M.; Urbano, F.; Iannitti, M.; Marampon, F.; et al. Induction chemotherapy followed by neoadjuvant chemoradiotherapy and surgery in locally advanced rectal cancer: Preliminary results of a phase II study. Oncotarget 2018, 9, 33702–33709. [Google Scholar] [CrossRef] [Green Version]
- Sell, N.M.; Qwaider, Y.Z.; Goldstone, R.N.; Cauley, C.E.; Cusack, J.C.; Ricciardi, R.; Bordeianou, L.G.; Berger, D.L.; Kunitake, H. Ten-year survival after pathologic complete response in rectal adenocarcinoma. J. Surg. Oncol. 2021, 123, 293–298. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Chen, G.; Pan, Y. Stratified Prognostic Value of Pathological Response to Preoperative Treatment in yp II/III Rectal Cancer. Front. Oncol. 2021, 11, 795137. [Google Scholar] [CrossRef]
- Sakin, A.; Sahin, S.; Sengul Samanci, N.; Yasar, N.; Demir, C.; Geredeli, C.; Erhan, S.S.; Akboru, M.H.; Cihan, S. The impact of tumor regression grade on long-term survival in locally advanced rectal cancer treated with preoperative chemoradiotherapy. J. Oncol. Pharm. Pract. 2020, 26, 1611–1620. [Google Scholar] [CrossRef]
- Fokas, E.; Ströbel, P.; Fietkau, R.; Ghadimi, M.; Liersch, T.; Grabenbauer, G.G.; Hartmann, A.; Kaufmann, M.; Sauer, R.; Graeven, U.; et al. German Rectal Cancer Study Group. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. J. Natl. Cancer Inst. 2017, 109, djx095. [Google Scholar] [CrossRef] [Green Version]
- Rödel, C.; Martus, P.; Papadoupolos, T.; Füzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, J.L.; Zhu, X.G.; He, J.Y.; Lin, L.M.; Lin, X.Y.; Tang, L.R.; Cai, Y. Associations of tumor regression grade with outcomes in patients with locally advanced rectal cancer treated with preoperative two-week course of radiotherapy. Oncotarget 2017, 8, 100165–100175. [Google Scholar] [CrossRef]
- Chen, P.J.; Su, W.C.; Chang, T.K.; Chen, Y.C.; Li, C.C.; Yin, T.C.; Tsai, H.L.; Ma, C.J.; Huang, C.W.; Wang, J.Y. Oncological outcomes of robotic-assisted total mesorectal excision after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Asian J. Surg. 2021, 44, 957–963. [Google Scholar] [CrossRef]
- Trakarnsanga, A.; Gönen, M.; Shia, J.; Nash, G.M.; Temple, L.K.; Guillem, J.G.; Paty, P.B.; Goodman, K.A.; Wu, A.; Gollub, M.; et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J. Natl. Cancer Inst. 2014, 106, dju248. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Gavioli, M.; Luppi, G.; Losi, L.; Bertolini, F.; Santantonio, M.; Falchi, A.M.; D’Amico, R.; Conte, P.F.; Natalini, G. Incidence and clinical impact of sterilized disease and minimal residual disease after preoperative radiochemotherapy for rectal cancer. Dis. Colon Rectum 2005, 48, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Leow, Y.C.; Roslani, A.C.; Xavier, R.G.; Lee, F.Y. Pathological Complete Response After Neoadjuvant Therapy in Rectal Adenocarcinoma: A 5-Year Follow-up. Indian J. Surg. 2021, 83 (Suppl. S3), 768–775. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Fuenmayor, L.J.; Naranjo-Isaza, A.M.; Serna-Ortiz, C.A.; Mosquera-Castro, D.A.; Arbeláez-Leon, L.M.; Gómez-Wolff, L.R.; García-García, H.I.; Sánchez-Patiño, L.A. Evaluation of the pathologic response to neoadjuvant treatment in rectal cancer. Experience at the Instituto de Cancerología de Medellín (Colombia, 2011–2017). Rev. Gastroenterol. Mex. (Engl. Ed.) 2021, 86, 13–20. [Google Scholar] [CrossRef] [PubMed]






| Total | Male | Female | p Value | |
|---|---|---|---|---|
| Age, years (mean) | 61.1 | 60.8 | 61.9 | – |
| Type of surgery | ||||
| LAR 1 | 105 | 72 | 33 | 0.87 a |
| APR/ELAPE 2 | 49 | 33 | 16 | |
| Tumor stage | ||||
| II | 65 | 46 | 19 | 0.55 a |
| III | 89 | 59 | 30 | |
| Lymph node status | ||||
| N0 | 98 | 64 | 34 | 0.43 a |
| N1, N2 | 56 | 40 | 16 | |
| LVI 3 presence (%) | 30 (19.5%) | 22 | 8 | 0.48 a |
| PNI 4 presence (%) | 47 (30.5%) | 35 | 12 | 0.32 a |
| Tumor Regression Grade Score | ||||||
|---|---|---|---|---|---|---|
| Variable | TRG0 | TRG1 | TRG2 | TRG3 | Total | p Value |
| Gender | ||||||
| Male | 18 | 23 | 41 | 23 | 105 | 0.57 a |
| Female | 11 | 9 | 15 | 14 | 49 | |
| Total (%) | 29 (18.8) | 32 (20.7) | 56 (36.3) | 37 (24.2) | ||
| Type of surgery | ||||||
| LAR | 24 | 20 | 36 | 25 | 105 | 0.29 a |
| APR | 5 | 12 | 20 | 12 | 49 | |
| Neoadjuvant response according to stage | ||||||
| Stage II | 20 | 16 | 20 | 9 | 65 | 0.002 a |
| Stage III | 9 | 16 | 36 | 28 | 89 | |
| Mean number of harvested lymph nodes | 16.4 | 17.7 | 16.4 | 16.3 | 16.7 | 0.93 b |
| Mean survival (months) | 43.8 | 40.1 | 37.5 | 29.4 | 37.2 | 0.006 b |
| Variables | Sig. | Hazard Ratio | 95.0% CI 1 for Exp(B) | Omnibus Tests of Model Coefficients | ||||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | −2 Log Likelihood | Chi-Squared | df | Sig. | |||
| LVI 2 | 0.542 | 0.740 | 0.281 | 1.946 | 263.394 | 21.871 | 5 | 0.001 |
| PNI 3 | 0.041 | 2.042 | 1.031 | 4.046 | ||||
| Stage | 0.550 | 1.324 | 0.528 | 3.322 | ||||
| MetLymph | 0.940 | 0.996 | 0.907 | 1.095 | ||||
| Pathologic response grade | 0.001 | 2.231 | 1.362 | 3.655 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulusoy, C.; Kamalı, G.H.; Nikolovski, A. Analysis of Survival in Complete Pathological Response after Long-Course Chemoradiotherapy in Patients with Advanced Rectal Cancer. Curr. Oncol. 2023, 30, 1054-1064. https://doi.org/10.3390/curroncol30010081
Ulusoy C, Kamalı GH, Nikolovski A. Analysis of Survival in Complete Pathological Response after Long-Course Chemoradiotherapy in Patients with Advanced Rectal Cancer. Current Oncology. 2023; 30(1):1054-1064. https://doi.org/10.3390/curroncol30010081
Chicago/Turabian StyleUlusoy, Cemal, Gülçin Harman Kamalı, and Andrej Nikolovski. 2023. "Analysis of Survival in Complete Pathological Response after Long-Course Chemoradiotherapy in Patients with Advanced Rectal Cancer" Current Oncology 30, no. 1: 1054-1064. https://doi.org/10.3390/curroncol30010081