Dose Volume and Liver Function Test Relationship following Radiotheraphy for Right Breast Cancer: A Multicenter Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. Radiation Therapy
2.2.1. Simulation
2.2.2. Contouring of Target Volumes
2.2.3. Contouring of the Liver
2.2.4. Radiotherapy Prescription and Planning
2.2.5. Liver Dose–Volume
2.3. Laboratory Tests
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data (accessed on 24 July 2023).
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, Y.; Debbi, K.; Loganadane, G.; Ghith, S.; Hadhri, A.; Hassani, W.; Cherif, M.A.; Coraggio, G.; To, N.H.; Colson-Durand, L.; et al. Radiothérapie Adjuvante et Néoadjuvante Des Cancers Du Sein: Mise Au Point Sur Les Données de La Littérature Disponibles En 2020. Cancer/Radiothérapie 2020, 24, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of Radiotherapy after Mastectomy and Axillary Surgery on 10-Year Recurrence and 20-Year Breast Cancer Mortality: Meta-Analysis of Individual Patient Data for 8135 Women in 22 Randomised Trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Gentilini, O.; et al. ESO-ESMO Fifth International Consensus Guidelines for Breast Cancer in Young Women (BCY5). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 1097–1118. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 24 July 2023).
- Debbi, K.; Janoray, G.; Scher, N.; Deutsch, É.; Mornex, F. Doses to Organs at Risk in Conformational and Stereotactic Body Radiation Therapy: Liver. Cancer Radiother. 2017, 21, 604–612. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y. Radiation-Induced Liver Disease: Current Understanding and Future Perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef]
- Lauffer, D.C.; Miglierini, P.; Kuhn, P.A.; Thalmann, S.U.; Gutierres-Demierre, N.; Khomsi, F.; Tercier, P.-A.; Allal, A.S. Impact of Adjuvant Radiotherapy on Biological and Clinical Parameters in Right-Sided Breast Cancer. Cancer Radiother. 2021, 25, 469–475. [Google Scholar] [CrossRef]
- Courtier, N.; Gambling, T.; Barrett-Lee, P.; Oliver, T.; Mason, M.D. The Volume of Liver Irradiated during Modern Free-Breathing Breast Radiotherapy: Implications for Theory and Practice. Radiography 2019, 25, 103–107. [Google Scholar] [CrossRef]
- Park, H.J.; Cheong, K.-H.; Koo, T.; Lee, M.Y.; Kim, K.J.; Park, S.; Han, T.; Kang, S.-K.; Ha, B.; Yoon, J.-W.; et al. Effects of Radiation Dose on Liver After Free-Breathing Volumetric Modulated Arc Therapy for Breast Cancer. In Vivo 2022, 36, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Quintin, K.; Loap, P.; Fourquet, A.; Kirova, Y. Late Hepatic Toxicity after Breast Cancer Intensity-Modulated Radiotherapy Using Helicoidal Tomotherapy. Cancer Radiother. 2023, 27, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Loganadane, G.; Truong, P.T.; Taghian, A.G.; Tešanović, D.; Jiang, M.; Geara, F.; Moran, M.S.; Belkacemi, Y. Comparison of Nodal Target Volume Definition in Breast Cancer Radiation Therapy According to RTOG Versus ESTRO Atlases: A Practical Review From the TransAtlantic Radiation Oncology Network (TRONE). Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 437–448. [Google Scholar] [CrossRef]
- Jabbour, S.K.; Hashem, S.A.; Bosch, W.; Kim, T.K.; Finkelstein, S.E.; Anderson, B.M.; Ben-Josef, E.; Crane, C.H.; Goodman, K.A.; Haddock, M.G.; et al. Upper Abdominal Normal Organ Contouring Guidelines and Atlas: A Radiation Therapy Oncology Group Consensus. Pract. Radiat. Oncol. 2014, 4, 82–89. [Google Scholar] [CrossRef]
- Alicikus, Z.A.; Aydin, B. Toxicity Management for Upper Abdomen Tumors in Radiation Oncology. In Prevention and Management of Acute and Late Toxicities in Radiation Oncology; Springer International Publishing: Cham, Germany, 2020; pp. 171–229. [Google Scholar]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of Normal Tissue Complication Probability Models in the Clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef]
- Robbins, M.E.C.; Zhao, W. Chronic Oxidative Stress and Radiation-Induced Late Normal Tissue Injury: A Review. Int. J. Radiat. Biol. 2004, 80, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.E.; Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Koay, E.J.; Owen, D.; Das, P. Radiation-Induced Liver Disease and Modern Radiotherapy. Semin. Radiat. Oncol. 2018, 28, 321–331. [Google Scholar] [CrossRef]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Ten Haken, R.K. Radiation-Associated Liver Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S94–S100. [Google Scholar] [CrossRef]
- Yamada, K.; Izaki, K.; Sugimoto, K.; Mayahara, H.; Morita, Y.; Yoden, E.; Matsumoto, S.; Soejima, T.; Sugimura, K. Prospective Trial of Combined Transcatheter Arterial Chemoembolization and Three-Dimensional Conformal Radiotherapy for Portal Vein Tumor Thrombus in Patients with Unresectable Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2003, 57, 113–119. [Google Scholar] [CrossRef]
- Liang, S.-X.; Zhu, X.-D.; Xu, Z.-Y.; Zhu, J.; Zhao, J.-D.; Lu, H.-J.; Yang, Y.-L.; Chen, L.; Wang, A.-Y.; Fu, X.-L.; et al. Radiation-Induced Liver Disease in Three-Dimensional Conformal Radiation Therapy for Primary Liver Carcinoma: The Risk Factors and Hepatic Radiation Tolerance. Int. J. Radiat. Oncol. 2006, 65, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, D.Y.; Park, J.-W.; Kim, S.H.; Choi, J.-I.; Kim, H.B.; Lee, W.J.; Park, S.J.; Hong, E.K.; Kim, C.-M. Dose–Volumetric Parameters Predicting Radiation-Induced Hepatic Toxicity in Unresectable Hepatocellular Carcinoma Patients Treated with Three-Dimensional Conformal Radiotherapy. Int. J. Radiat. Oncol. 2007, 67, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.; Ring, A.; Struycken, L.; Mack, W.; Koç, M.; Lang, J.E. Incidence of Radiation Induced Sarcoma Attributable to Radiotherapy in Adults: A Retrospective Cohort Study in the SEER Cancer Registries across 17 Primary Tumor Sites. Cancer Epidemiol. 2021, 70, 101857. [Google Scholar] [CrossRef]
- Huang, J.; Mackillop, W.J. Increased Risk of Soft Tissue Sarcoma after Radiotherapy in Women with Breast Carcinoma. Cancer 2001, 92, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Hiniker, S.M.; Donaldson, S.S. ALARA: In Radiation Oncology and Diagnostic Imaging Alike. Oncology 2014, 28, 247–248. [Google Scholar] [PubMed]
- Shi, J.; Liu, J.; Tian, G.; Li, D.; Liang, D.; Wang, J.; He, Y. Association of radiotherapy for stage I-III breast cancer survivors and second primary malignant cancers: A population-based study. Eur. J. Cancer Prev. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Buglione, M.; Guerini, A.E.; Filippi, A.R.; Spiazzi, L.; Pasinetti, N.; Magli, A.; Toraci, C.; Borghetti, P.; Triggiani, L.; Alghisi, A.; et al. A Systematic Review on Intensity Modulated Radiation Therapy for Mediastinal Hodgkin’s Lymphoma. Crit. Rev. Oncol. Hematol. 2021, 167, 103437. [Google Scholar] [CrossRef]
- Grantzau, T.; Overgaard, J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for pri-mary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 2016, 121, 402–413. [Google Scholar] [CrossRef]
- Donovan, E.M.; James, H.; Bonora, M.; Yarnold, J.R.; Evans, P.M. Second cancer incidence risk estimates using BEIR VII models for standard and complex external beam radiotherapy for early breast cancer. Med. Phys. 2012, 39, 5814–5824. [Google Scholar] [CrossRef]
- Santos, A.M.; Marcu, L.G.; Wong, C.M.; Bezak, E. Risk estimation of second primary cancers after breast radiotherapy. Acta Oncol. 2016, 55, 1331–1337. [Google Scholar] [CrossRef]
- Simonetto, C.; Eidemüller, M.; Gaasch, A.; Pazos, M.; Schönecker, S.; Reitz, D.; Kääb, S.; Braun, M.; Harbeck, N.; Niyazi, M.; et al. Does Deep Inspiration Breath-Hold Prolong Life? Individual Risk Estimates of Ischaemic Heart Disease after Breast Cancer Radiotherapy. Radiother. Oncol. 2019, 131, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Dumane, V.A.; Saksornchai, K.; Zhou, Y.; Hong, L.; Powell, S.; Ho, A.Y. Reduction in Low-Dose to Normal Tissue with the Addition of Deep Inspiration Breath Hold (DIBH) to Volumetric Modulated Arc Therapy (VMAT) in Breast Cancer Patients with Implant Reconstruction Receiving Regional Nodal Irradiation. Radiat. Oncol. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Swanson, T.; Grills, I.S.; Ye, H.; Entwistle, A.; Teahan, M.; Letts, N.; Yan, D.; Duquette, J.; Vicini, F.A. Six-Year Experience Routinely Using Moderate Deep Inspiration Breath-Hold for the Reduction of Cardiac Dose in Left-Sided Breast Irradiation for Patients with Early-Stage or Locally Advanced Breast Cancer. Am. J. Clin. Oncol. 2013, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Pandeli, C.; Smyth, L.M.L.; David, S.; See, A.W. Dose Reduction to Organs at Risk with Deep-Inspiration Breath-Hold during Right Breast Radiotherapy: A Treatment Planning Study. Radiat. Oncol. 2019, 14, 223. [Google Scholar] [CrossRef]
- Borgonovo, G.; Paulicelli, E.; Daniele, D.; Presilla, S.; Richetti, A.; Valli, M. Deep Inspiration Breath Hold in Post-Operative Radiotherapy for Right Breast Cancer: A Retrospective Analysis. Rep. Pract. Oncol. Radiother. 2022, 27, 717–723. [Google Scholar] [CrossRef]
- Loap, P.; Vu-Bezin, J.; Monceau, V.; Jacob, S.; Fourquet, A.; Kirova, Y. Dosimetric Evaluation of the Benefit of Deep Inspiration Breath Hold (DIBH) for Locoregional Irradiation of Right Breast Cancer with Volumetric Modulated Arctherapy (VMAT). Acta Oncol. 2023, 62, 150–158. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Nagarajan, D.; Vibhaa, K.K.; Anagha, V.; Joshua, P.P.; Tharani, P.T.; Chakraborty, R.; Renu, K.; Dey, A.; et al. Letrozole: Pharmacology, Toxicity and Potential Therapeutic Effects. Life Sci. 2022, 310, 121074. [Google Scholar] [CrossRef]
- Jannuzzo, M.G.; Poggesi, I.; Spinelli, R.; Rocchetti, M.; Cicioni, P.; Buchan, P. The Effects of Degree of Hepatic or Renal Impairment on the Pharmacokinetics of Exemestane in Postmenopausal Women. Cancer Chemother. Pharmacol. 2004, 53, 475–481. [Google Scholar] [CrossRef]

| Median Age | 56 (29–79) |
| Median CTV volume | 802 (214–2724) cc |
| Surgery modality | |
| breast conserving | 75% |
| mastectomy | 25% |
| T Stage | |
| T1 | 53% |
| T2 | 39% |
| T3 | - |
| T4 | - |
| Tx | 8% |
| N Stage | |
| N0 | 53% |
| N1 | 25% |
| N2 | - |
| N3 | - |
| Nx | 22% |
| RT technics | |
| FIF/IMRT | 67% |
| VMAT | 33% |
| Deep inspiration breath hold | 25% |
| RT boost dose (median) | 10 (10–16) Gy |
| RT boost | |
| Electron | 28% |
| IMRT | 31% |
| VMAT | 20% |
| Patient not received boost | 21% |
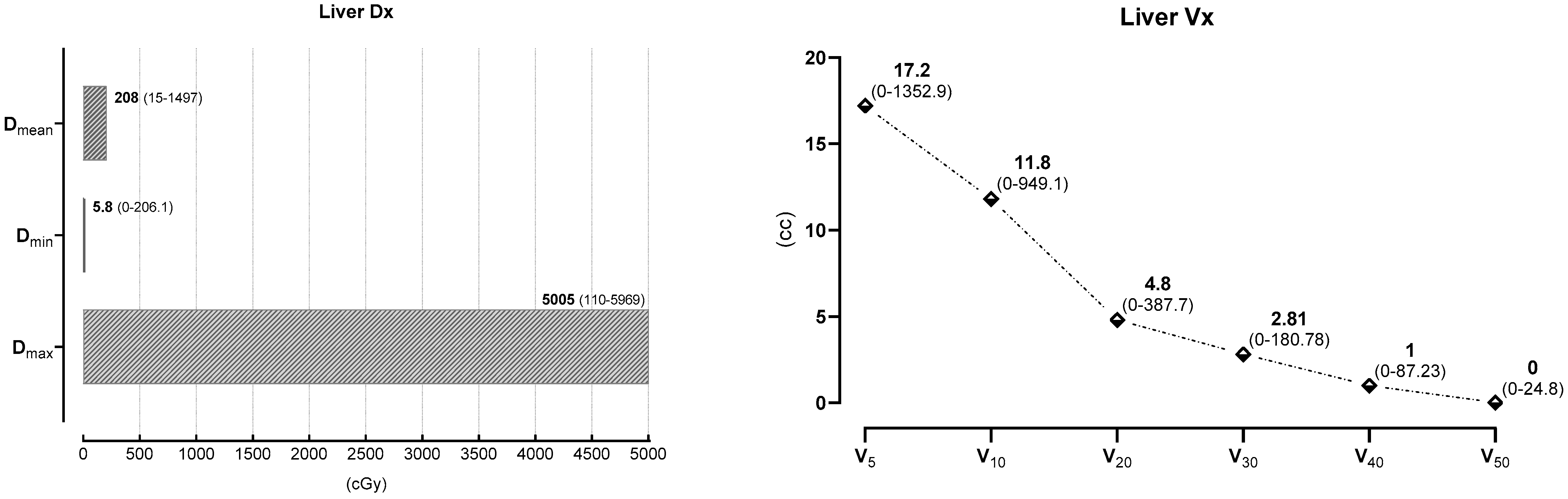
| Liver Dx | Dmax (cGy) | Dmin (cGy) | Dmean (cGy) |
|---|---|---|---|
| Dose (median) | 5005 (110–5969) | 5.8 (0–206.1) | 208 (15–1497) |
| Liver Vx | V5 | V10 | V20 | V30 | V40 | V50 |
|---|---|---|---|---|---|---|
| cc (median) | 17.2 | 11.8 | 4.8 | 2.81 | 1 | 0 |
| (0–1352.9) | (0–949.1) | (0–387.7) | (0–180.78) | (0–87.23) | (0–24.8) |
| Liver Test | Median (U/L) | Median Percentage Change (%) |
|---|---|---|
| AST | ||
| preRT | 19 (11–35) | 13% (−120 to 54.5) |
| postRT | 21 (10–52.32) | |
| ALT | ||
| preRT | 18 (1.97–39) | 3.03% (−292 to 46.1) |
| postRT | 20 (8- 55) | |
| GGT | ||
| preRT | 20 (12–44) | −6% (−93.18 to 42.86) |
| postRT | 19 (10–85) |
| Liver Test | Dose–Volume Parameters | p Value |
|---|---|---|
| ALT | Dmean | 0.03 |
| AST | Dmean | 0.023 |
| Dmin | 0.007 | |
| GGT | Dmean | 0.006 |
| Dmin | 0.014 | |
| Dmax | 0.023 | |
| V50 | 0.009 | |
| V40 | 0.03 | |
| V30 | 0.03 | |
| V20 | 0.01 | |
| V5 | 0.02 |
| The Number of Patients/RT Dose/Timing of Blood Test | Liver Dose | Hepatic Blood Test Results | |
|---|---|---|---|
| Lauffer et al. [11] | 34 right side 42.5 Gy/16 fr or 50 Gy/25 fr ±16 fr boosts Before and last week of RT | MLV: 1270.2 cc (918.5–2233.2) MLD: 1.94 Gy (0.2–9) | Correlation between irradiated liver volume and ALT (p = 0.05) and ALP (p = 0.006) |
| Courtier et al. [12] | 52 right side, 100 left side 40 Gy/15 fr Before and during 4 weeks after RT | Mean V10: 226 cm3 (19%) Mean V50: 92 cm3 (8%) Mean V90: 62 cm3 (5%) | V10 and IL-6 (p = 0.001) |
| Park et al. [13] | 47 right side, 78 left side 42.56–50 Gy/16–25 fr ± 10–14 Gy boost 1 week before vs. 6 months after | Dmean_right breast 434.1 cGy Dmean_left breast260.6 cGy V10 3% V20 1% V30 0% | ASTmedian: 23.2 ± 5.3 vs. 29.6 ± 14.6 ALTmedian: 20.2 ± 7.7 vs. 25.6 ± 20.0 |
| Quintin et al. [14] | 27 right side or bilateral, 29 left side Median follow-up 5.4 years | Dmean 2.8 Gy (0.3–16.6) Dmax 26.9Gy (0.7–51.7) | no grade 3 hepatotoxicity Three patients (6%) with grade 2 delayed hepatotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güzelöz, Z.; Ayrancıoğlu, O.; Aktürk, N.; Güneş, M.; Alıcıkuş, Z.A. Dose Volume and Liver Function Test Relationship following Radiotheraphy for Right Breast Cancer: A Multicenter Study. Curr. Oncol. 2023, 30, 8763-8773. https://doi.org/10.3390/curroncol30100632
Güzelöz Z, Ayrancıoğlu O, Aktürk N, Güneş M, Alıcıkuş ZA. Dose Volume and Liver Function Test Relationship following Radiotheraphy for Right Breast Cancer: A Multicenter Study. Current Oncology. 2023; 30(10):8763-8773. https://doi.org/10.3390/curroncol30100632
Chicago/Turabian StyleGüzelöz, Zeliha, Oğuzhan Ayrancıoğlu, Nesrin Aktürk, Merve Güneş, and Zümre Arıcan Alıcıkuş. 2023. "Dose Volume and Liver Function Test Relationship following Radiotheraphy for Right Breast Cancer: A Multicenter Study" Current Oncology 30, no. 10: 8763-8773. https://doi.org/10.3390/curroncol30100632
APA StyleGüzelöz, Z., Ayrancıoğlu, O., Aktürk, N., Güneş, M., & Alıcıkuş, Z. A. (2023). Dose Volume and Liver Function Test Relationship following Radiotheraphy for Right Breast Cancer: A Multicenter Study. Current Oncology, 30(10), 8763-8773. https://doi.org/10.3390/curroncol30100632





