A Case of Sporadic Multiple Colonic Polyps in a Young Woman
Abstract
:1. Introduction
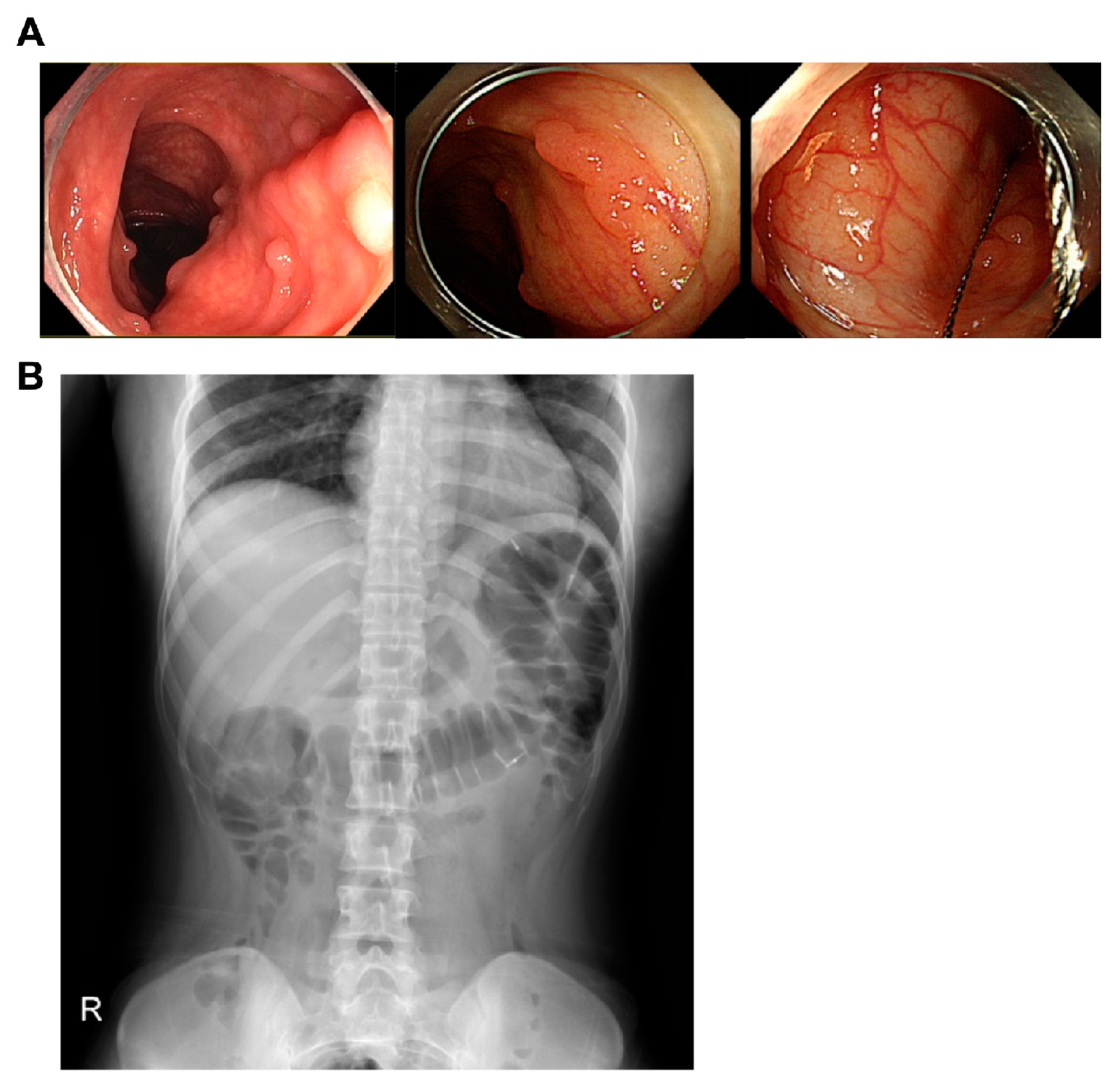
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Lal, S.K.; Farraye, F.A. Colorectal cancer screening in average risk individuals. Cancer Causes Control. 2005, 16, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The ABC of APC. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelps, R.A.; Chidester, S.; Dehghanizadeh, S.; Phelps, J.; Sandoval, I.T.; Rai, K.; Broadbent, T.; Sarkar, S.; Burt, R.W.; Jones, D.A. A Two-Step Model for Colon Adenoma Initiation and Progression Caused by APC Loss. Cell 2009, 137, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Sparks, A.B.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998, 58, 1130–1134. [Google Scholar]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef] [Green Version]
- Ichii, S.; Horii, A.; Nakatsuru, S.; Furuyama, J.; Utsunomiya, J.; Nakamura, Y. Inactivation of both APC alleles in an early stage of colon adenomas in a patient with familial adenomatous polyposis (FAP). Hum. Mol. Genet. 1992, 1, 387–390. [Google Scholar] [CrossRef]
- Levy, D.B.; Smith, K.J.; Beazer-Barclay, Y.; Hamilton, S.R.; Vogelstein, B.; Kinzler, K.W. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994, 54, 5953–5958. [Google Scholar] [PubMed]
- Mori, Y.; Nagse, H.; Ando, H.; Horii, A.; Ichii, S.; Nakatsuru, S.; Aoki, T.; Miki, Y.; Mori, T.; Nakamura, Y. Somatic mutations of the APC gene in colorectal tumors: Mutation cluster region in the APC gene. Hum. Mol. Genet. 1992, 1, 229–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaki, M.; Konishi, M.; Kikuchi-Yanoshita, R.; Enomoto, M.; Igari, T.; Tanaka, K.; Muraoka, M.; Takahashi, H.; Amada, Y.; Fukayama, M.; et al. Characteristics of somatic mutation of the adenomatous polyposis coli gene in colorectal tumors. Cancer Res. 1994, 54, 3011–3020. [Google Scholar] [PubMed]
- Powell, S.M.; Zilz, N.; Beazer-Barclay, Y.; Bryan, T.M.; Hamilton, S.R.; Thibodeau, S.N.; Vogelstein, B.; Kinzler, K.W. APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, S.; Bodmer, W.; Bicknell, D.; Kaklamanis, L. Molecular analysis of APC mutations in familial adenomatous polyposis and sporadic colon carcinomas. Lancet 1992, 340, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Kikuchi-Yanoshita, R.; Tanaka, K.; Muraoka, M.; Onda, A.; Okumura, Y.; Kishi, N.; Iwama, T.; Mori, T.; Koike, M.; et al. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology 1996, 111, 307–317. [Google Scholar] [CrossRef]
- Béroud, C.; Soussi, T. APC gene: Database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1996, 24, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, H.; Nakamura, Y. Mutations of theAPC adenomatous polyposis coli) gene. Hum. Mutat. 1993, 2, 425–434. [Google Scholar] [CrossRef]
- Polakis, P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta (BBA)-Rev. Cancer 1997, 1332, F127–F147. [Google Scholar] [CrossRef]
- Cha, J.M.; La Selva, D.; Kozarek, R.A.; Gluck, M.; Ross, A.; Lin, O.S. Young patients with sporadic colorectal adenomas: Current endoscopic surveillance practices and outcomes. Gastrointest. Endosc. 2018, 88, 818–825.e1. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Livingston, E.H.; Yo, C.K. Colorectal cancer in the young. Am. J. Surg. 2004, 187, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; De La Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. Gynecol. Oncol. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Kinzler, K.W.; Vogelstein, B. Lessons from Hereditary Colorectal Cancer. Cell 1996, 87, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boman, B.M.; Fields, J.Z. An APC:WNT Counter-Current-Like Mechanism Regulates Cell Division Along the Human Colonic Crypt Axis: A Mechanism That Explains How APC Mutations Induce Proliferative Abnormalities That Drive Colon Cancer Development. Front. Oncol. 2013, 3, 244. [Google Scholar] [CrossRef] [Green Version]
- Rowan, A.J.; Lamlum, H.; Ilyas, M.; Wheeler, J.; Straub, J.; Papadopoulou, A.; Bicknell, D.; Bodmer, W.F.; Tomlinson, I.P.M. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interde-pendence of the “two hits”. Proc. Natl. Acad. Sci. USA 2000, 97, 3352–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüchtenborg, M.; Weijenberg, M.P.; Roemen, G.M.J.M.; de Bruïne, A.P.; Brandt, P.A.V.D.; Lentjes, M.H.F.M.; Brink, M.; van Engeland, M.; Goldbohm, R.A.; de Goeij, A.F.P.M. APC mutations in sporadic colorectal carcinomas from The Netherlands Cohort Study. Carcinogenesis 2004, 25, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, M.J.; Jorissen, R.N.; Mouradov, D.; Sakthianandeswaren, A.; Li, S.; Day, F.L.; Tsui, C.; Lipton, L.; Desai, J.P.; Jones, I.T.; et al. Different APC genotypes in proximal and distal sporadic colorectal cancers suggest distinct WNT/β-catenin signalling thresholds for tumourigenesis. Oncogene 2013, 32, 4675–4682. [Google Scholar] [CrossRef]
- Bodmer, W. Familial adenomatous polyposis (FAP) and its gene, APC. Cytogenet. Cell Genet. 1999, 86, 99–104. [Google Scholar] [CrossRef]
- Lamlum, H.; Papadopoulou, A.; Ilyas, M.; Rowan, A.; Gillet, C.; Hanby, A.; Talbot, I.; Bodmer, W.; Tomlinson, I. APC mutations are sufficient for the growth of early colorectal adenomas. Proc. Natl. Acad. Sci. USA 2000, 97, 2225–2228. [Google Scholar] [CrossRef] [Green Version]
- Preisler, L.; Habib, A.; Shapira, G.; Kuznitsov-Yanovsky, L.; Mayshar, Y.; Carmel-Gross, I.; Malcov, M.; Azem, F.; Shomron, N.; Kariv, R.; et al. Heterozygous APC germline mutations impart predisposition to colorectal cancer. Sci. Rep. 2021, 11, 5113. [Google Scholar] [CrossRef]
- Goss, K.H.; Groden, J. Biology of the Adenomatous Polyposis Coli Tumor Suppressor. J. Clin. Oncol. 2000, 18, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Dihlmann, S.; Gebert, J.; Siermann, A.; Herfarth, C.; Doeberitz, M.V.K. Dominant negative effect of the APC1309 mutation: A possible explanation for genotype-phenotype correlations in familial adenomatous polyposis. Cancer Res. 1999, 59, 1857–1860. [Google Scholar] [PubMed]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Bushyhead, D.; Lin, O.S.T.; Kozarek, R.A. A Review of the Management of Sporadic Colorectal Adenomas in Young People: Is Surveillance Wasted on the Young? Dig. Dis. Sci. 2019, 64, 2107–2112. [Google Scholar] [CrossRef]
- Gayther, S.A.; Wells, D.; Sengupta, S.B.; Chapman, P.; Neale, K.; Tsioupra, K.; Delhanty, J.D.A. Regionally clustered APC mutations are associated with a severe phenotype and occur at a high frequency in new mutation cases of adenomatous polyposis coli. Hum. Mol. Genet. 1994, 3, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, M.L.; Fenger, K.; Bülow, S.; Niebuhr, E.; Mohr, J. Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Hum. Mutat. 1994, 3, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ripa, R.S.; Bisgaard, M.L.; Bülow, S.; Nielsen, F.C. De novo mutations in familial adenomatous polyposis (FAP). Eur. J. Hum. Genet. 2002, 10, 631–637. [Google Scholar] [CrossRef]


| Chrom | Pos | Ref | Alt | Effect | Gene | Feature ID | HGVS.c |
|---|---|---|---|---|---|---|---|
| Chr1 | 94,473,263 | TGCCGGCACCATTCA | T | Frameshift _variant | ABCA4 | NM_000350.3 | c.5918_5931delTGAATGGTGCCGG |
| Chr2 | 42,936,061 | G | GC | Frameshift _variant | MTA3 | NM_001330442.2 | c.1352dupC |
| Chr4 | 9,270,328 | G | A | Stop _gained | USP17L20 | NM_001256861.1.3 | c.984G>A |
| Chr4 | 48,037,939 | AGTGGTATGGCATGACAGCTG | A | Frameshift _variant | NIPAL1 | NM_207330.3 | c.985_1004delTGGTATGGCATGAC |
| Chr5 | 112,157,610 | CAT | C | Frameshift _variant | APC | NM_001354896.2 | c.1331_1332delAT |
| Chr12 | 117,685,302 | G | A | Stop _gained | NOS1 | NM_001204218.1 | c.2776C>T |
| Chr14 | 45,605,385 | G | T | Stop _gained | FANCM | NM_020937.4 | c.151G>T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sin, S.H.; Yoon, J.H.; Kim, S.W.; Park, W.S.; Chae, H.S. A Case of Sporadic Multiple Colonic Polyps in a Young Woman. Curr. Oncol. 2023, 30, 1293-1299. https://doi.org/10.3390/curroncol30020100
Sin SH, Yoon JH, Kim SW, Park WS, Chae HS. A Case of Sporadic Multiple Colonic Polyps in a Young Woman. Current Oncology. 2023; 30(2):1293-1299. https://doi.org/10.3390/curroncol30020100
Chicago/Turabian StyleSin, Seung Ho, Jung Hwan Yoon, Sang Woo Kim, Won Sang Park, and Hiun Suk Chae. 2023. "A Case of Sporadic Multiple Colonic Polyps in a Young Woman" Current Oncology 30, no. 2: 1293-1299. https://doi.org/10.3390/curroncol30020100




