Five-Fraction Stereotactic Radiotherapy for Brain Metastases—A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Accrual
2.3. Fractionated Stereotactic Radiotherapy (FSRT)
2.4. Follow-Up
2.5. Study Endpoints
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Treatment and Dosimetry
3.3. Toxicity
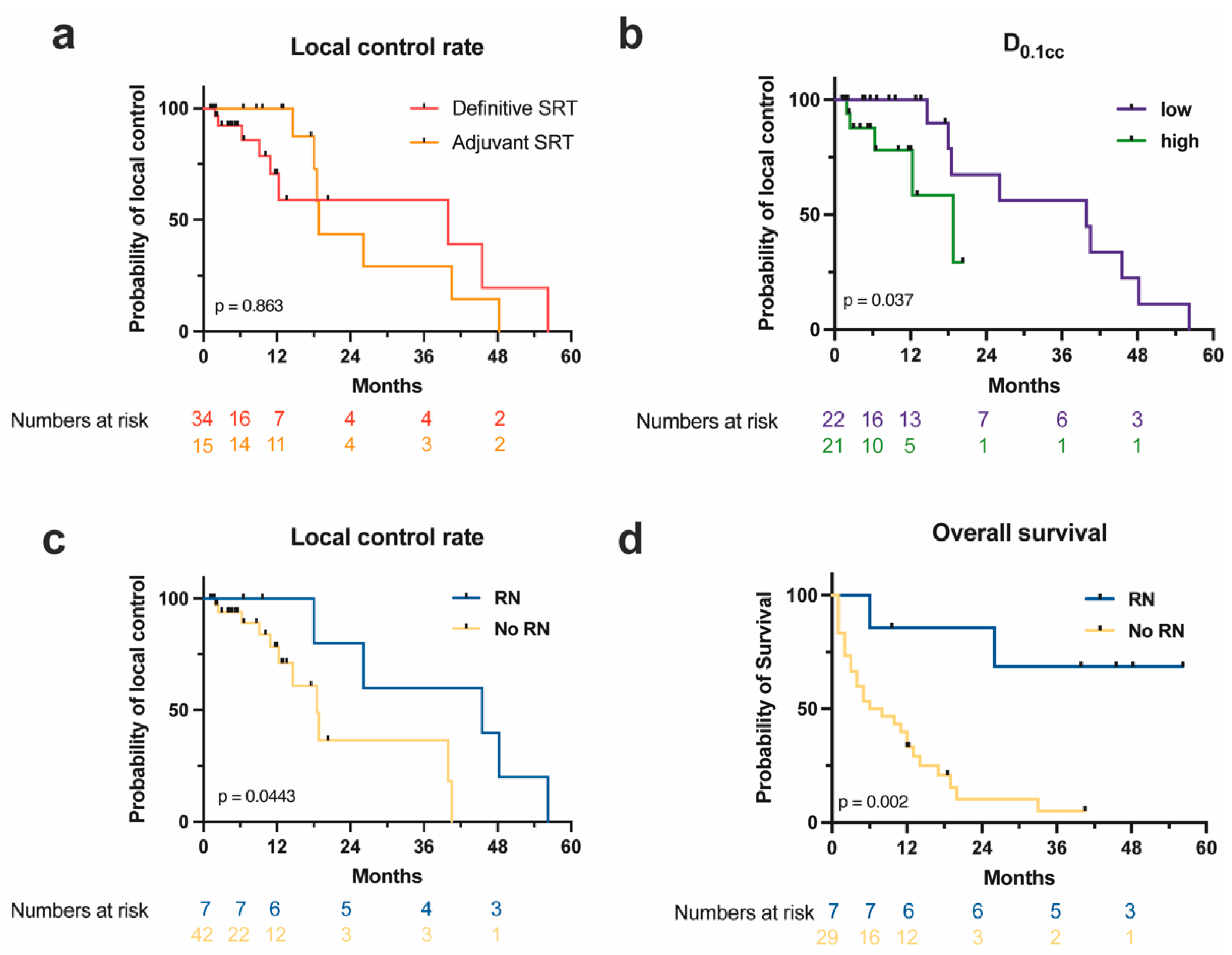
3.4. Survival and Control Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and Prognosis of Patients with Brain Metastases at Diagnosis of Systemic Malignancy: A Population-Based Study. Neuro Oncol. 2017, 19, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.D.; Young, B. Demographics of Brain Metastasis. Neurosurg. Clin. N. Am. 1996, 7, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Sundermeyer, M.L.; Meropol, N.J.; Rogatko, A.; Wang, H.; Cohen, S.J. Changing Patterns of Bone and Brain Metastases in Patients with Colorectal Cancer. Clin. Color. Cancer 2005, 5, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The Incidence of Brain Metastases among Patients with Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Neuro Oncol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Lamba, N.; Wen, P.Y.; Aizer, A.A. Epidemiology of Brain Metastases and Leptomeningeal Disease. Neuro Oncol. 2021, 23, 1447–1456. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, Y.; Serizawa, T.; Kawabe, T.; Higuchi, Y.; Nagano, O.; Barfod, B.E.; Ono, J.; Kasuya, H.; Urakawa, Y. Subclassification of Recursive Partitioning Analysis Class II Patients With Brain Metastases Treated Radiosurgically. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1399–1405. [Google Scholar] [CrossRef]
- Nieder, C.; Stanisavljevic, L.; Aanes, S.G.; Mannsåker, B.; Haukland, E.C. 30-Day Mortality in Patients Treated for Brain Metastases: Extracranial Causes Dominate. Radiat. Oncol. 2022, 17, 92. [Google Scholar] [CrossRef]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-Operative Stereotactic Radiosurgery versus Observation for Completely Resected Brain Metastases: A Single-Centre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in Patients with Brain Metastases Treated with Radiosurgery or Radiosurgery plus Whole-Brain Irradiation: A Randomised Controlled Trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.-D.; Carrie, C.; et al. Adjuvant Whole-Brain Radiotherapy Versus Observation After Radiosurgery or Surgical Resection of One to Three Cerebral Metastases: Results of the EORTC 22952-26001 Study. JCO J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic Radiosurgery for Brain Metastases: Analysis of Outcome and Risk of Brain Radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901): A Multi-Institutional Prospective Observational Study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Cho, K.H.; Kim, J.-Y.; Lim, Y.K.; Min, H.S.; Lee, S.H.; Kim, H.J.; Gwak, H.S.; Yoo, H.; Lee, S.H. Single-Dose Versus Fractionated Stereotactic Radiotherapy for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 483–489. [Google Scholar] [CrossRef]
- Baliga, S.; Garg, M.K.; Fox, J.; Kalnicki, S.; Lasala, P.A.; Welch, M.R.; Tomé, W.A.; Ohri, N. Fractionated Stereotactic Radiation Therapy for Brain Metastases: A Systematic Review with Tumour Control Probability Modelling. Br. J. Radiol. 2017, 90, 20160666. [Google Scholar] [CrossRef] [Green Version]
- Kohutek, Z.A.; Yamada, Y.; Chan, T.A.; Brennan, C.W.; Tabar, V.; Gutin, P.H.; Jonathan Yang, T.; Rosenblum, M.K.; Ballangrud, Å.; Young, R.J.; et al. Long-Term Risk of Radionecrosis and Imaging Changes after Stereotactic Radiosurgery for Brain Metastases. J. Neurooncol. 2015, 125, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 Cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Henzel, M.; Surber, G.; Kleinert, G.; Hamm, K.; Engenhart-Cabillic, R. Stereotactic Radiosurgery and Fractionated Stereotactic Radiotherapy: Comparison of Efficacy and Toxicity in 260 Patients with Brain Metastases. J. Neurooncol. 2012, 109, 91–98. [Google Scholar] [CrossRef]
- Putz, F.; Pirschel, W.; Fietkau, R. FSRT-Trial: Erste Phase-III-Studie zum Vergleich fraktionierte stereotaktische Radiotherapie (FSRT) versus Einzeitradiochirurgie (SRS) bei Hirnmetastasen. Forum 2022, 37, 241–245. [Google Scholar] [CrossRef]
- Putz, F.; Weissmann, T.; Oft, D.; Schmidt, M.A.; Roesch, J.; Siavooshhaghighi, H.; Filimonova, I.; Schmitter, C.; Mengling, V.; Bert, C.; et al. FSRT vs. SRS in Brain Metastases-Differences in Local Control and Radiation Necrosis-A Volumetric Study. Front. Oncol. 2020, 10, 559193. [Google Scholar] [CrossRef]
- Lutz, S.T.; Chow, E.L.; Hartsell, W.F.; Konski, A.A. A Review of Hypofractionated Palliative Radiotherapy. Cancer 2007, 109, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Gripp, S.; Mjartan, S.; Boelke, E.; Willers, R. Palliative Radiotherapy Tailored to Life Expectancy in End-Stage Cancer Patients: Reality or Myth? Cancer 2010, 116, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef]
- Ernst-Stecken, A.; Ganslandt, O.; Lambrecht, U.; Sauer, R.; Grabenbauer, G. Phase II Trial of Hypofractionated Stereotactic Radiotherapy for Brain Metastases: Results and Toxicity. Radiother. Oncol. 2006, 81, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vergalasova, I.; Liu, H.; Alonso-Basanta, M.; Dong, L.; Li, J.; Nie, K.; Shi, W.; Teo, B.-K.K.; Yu, Y.; Yue, N.J.; et al. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front. Oncol. 2019, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Musunuru, H.B.; Quon, H.; Davidson, M.; Cheung, P.; Zhang, L.; D’Alimonte, L.; Deabreu, A.; Mamedov, A.; Loblaw, A. Dose-Escalation of Five-Fraction SABR in Prostate Cancer: Toxicity Comparison of Two Prospective Trials. Radiother. Oncol. 2016, 118, 112–117. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. JCO J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 n.d. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 10 December 2021).
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response Assessment Criteria for Brain Metastases: Proposal from the RANO Group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Steinmann, D.; Vordermark, D.; Gerstenberg, W.; Aschoff, R.; Gharbi, N.; Müller, A.; Schäfer, C.; Theodorou, M.; Wypior, H.-J.; Geinitz, H.; et al. Quality of Life in Patients with Limited (1–3) Brain Metastases Undergoing Stereotactic or Whole Brain Radiotherapy: A Prospective Study of the DEGRO QoL Working Group. Strahlenther. Onkol. 2020, 196, 48–57. [Google Scholar] [CrossRef]
- Park, K.; Bae, G.H.; Kim, W.K.; Yoo, C.-J.; Park, C.W.; Kim, S.-K.; Cha, J.; Kim, J.W.; Jung, J. Radiotherapy for Brain Metastasis and Long-Term Survival. Sci. Rep. 2021, 11, 8046. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-Analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Wiggenraad, R.; Kanter, A.V.; Kal, H.B.; Taphoorn, M.; Vissers, T.; Struikmans, H. Dose–Effect Relation in Stereotactic Radiotherapy for Brain Metastases. A Systematic Review. Radiother. Oncol. 2011, 98, 292–297. [Google Scholar] [CrossRef]
- Mulvenna, P.; Nankivell, M.; Barton, R.; Faivre-Finn, C.; Wilson, P.; McColl, E.; Moore, B.; Brisbane, I.; Ardron, D.; Holt, T.; et al. Dexamethasone and Supportive Care with or without Whole Brain Radiotherapy in Treating Patients with Non-Small Cell Lung Cancer with Brain Metastases Unsuitable for Resection or Stereotactic Radiotherapy (QUARTZ): Results from a Phase 3, Non-Inferiority, Randomised Trial. Lancet 2016, 388, 2004–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, M.A.; Cardinale, R.M.; Benedict, S.H.; Kavanagh, B.D.; Zwicker, R.D.; Amir, C.; Broaddus, W.C. Hypofractionated Stereotactic Radiotherapy as an Alternative to Radiosurgery for the Treatment of Patients with Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Mauldon, E.; Anderson, N. Cost-Containment in Hypofractionated Radiation Therapy: A Literature Review. J. Med. Radiat. Sci. 2018, 65, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Scarpelli, D.B.; Fatheree, S.; Jaboin, J.J. Cost-Effectiveness of Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy in Treating Brain Metastases. Pract. Radiat. Oncol. 2021, 11, 488–490. [Google Scholar] [CrossRef]
- Medenwald, D.; Brunner, T.; Christiansen, H.; Kisser, U.; Mansoorian, S.; Vordermark, D.; Prokosch, H.-U.; Seuchter, S.A.; Kapsner, L.A.; Our MII research group. Shift of Radiotherapy Use during the First Wave of the COVID-19 Pandemic? An Analysis of German Inpatient Data. Strahlenther. Onkol. 2022, 198, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Akuamoa-Boateng, D.; Wegen, S.; Ferdinandus, J.; Marksteder, R.; Baues, C.; Marnitz, S. Managing Patient Flows in Radiation Oncology during the COVID-19 Pandemic: Reworking Existing Treatment Designs to Prevent Infections at a German Hot Spot Area University Hospital. Strahlenther. Onkol. 2020, 196, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Di Perri, D.; Tanguy, R.; Malet, C.; Robert, A.; Sunyach, M.-P. Risk of Radiation Necrosis after Hypofractionated Stereotactic Radiotherapy (HFSRT) for Brain Metastases: A Single Center Retrospective Study. J. Neurooncol. 2020, 149, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Park, J.H.; Lee, E.J.; Kim, J.H.; Kim, C.J.; Cho, Y.H. Efficacy and Safety of Fractionated Stereotactic Radiosurgery for Large Brain Metastases. J. Korean Neurosurg. Soc. 2015, 58, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Tomita, N.; Adachi, S.; Tanaka, H.; Tachibana, H.; Kodaira, T. Retrospective Analysis of Hypofractionated Stereotactic Radiotherapy for Tumors Larger than 2 Cm. Nagoya J. Med. Sci. 2019, 81, 397–406. [Google Scholar] [CrossRef]
- Mengue, L.; Bertaut, A.; Ngo Mbus, L.; Doré, M.; Ayadi, M.; Clément-Colmou, K.; Claude, L.; Carrie, C.; Laude, C.; Tanguy, R.; et al. Brain Metastases Treated with Hypofractionated Stereotactic Radiotherapy: 8 Years Experience after Cyberknife Installation. Radiat. Oncol. 2020, 15, 82. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative Stereotactic Radiosurgery Compared with Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC·3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hong, A.; Wang, T.; Lo, S.; Chen, B.; Silva, I.; Kapoor, R.; Hsiao, E.; Fogarty, G.B.; Carlino, M.S.; et al. Risk of Radiation Necrosis after Stereotactic Radiosurgery for Melanoma Brain Metastasis by Anatomical Location. Strahlenther. Onkol. 2021, 197, 1104–1112. [Google Scholar] [CrossRef]
- Doré, M.; Martin, S.; Delpon, G.; Clément, K.; Campion, L.; Thillays, F. Stereotactic Radiotherapy Following Surgery for Brain Metastasis: Predictive Factors for Local Control and Radionecrosis. Cancer/Radiothérapie 2017, 21, 4–9. [Google Scholar] [CrossRef]
- Eitz, K.A.; Lo, S.S.; Soliman, H.; Sahgal, A.; Theriault, A.; Pinkham, M.B.; Foote, M.C.; Song, A.J.; Shi, W.; Redmond, K.J.; et al. Multi-Institutional Analysis of Prognostic Factors and Outcomes After Hypofractionated Stereotactic Radiotherapy to the Resection Cavity in Patients With Brain Metastases. JAMA Oncol. 2020, 6, 1901. [Google Scholar] [CrossRef]
- Jhaveri, J.; Chowdhary, M.; Zhang, X.; Press, R.H.; Switchenko, J.M.; Ferris, M.J.; Morgan, T.M.; Roper, J.; Dhabaan, A.; Elder, E.; et al. Does Size Matter? Investigating the Optimal Planning Target Volume Margin for Postoperative Stereotactic Radiosurgery to Resected Brain Metastases. J. Neurosurg. 2019, 130, 797–803. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Ahluwalia, M.S.; Gurewitz, J.; Bernstein, K.; Kondziolka, D.; Niranjan, A.; Wei, Z.; Lunsford, L.D.; Fakhoury, K.R.; Rusthoven, C.G.; et al. Imaging-Defined Necrosis after Treatment with Single-Fraction Stereotactic Radiosurgery and Immune Checkpoint Inhibitors and Its Potential Association with Improved Outcomes in Patients with Brain Metastases: An International Multicenter Study of 697 Patients. J. Neurosurg. 2022. publish before print. [Google Scholar] [CrossRef]
- Lischalk, J.W.; Oermann, E.; Collins, S.P.; Nair, M.N.; Nayar, V.V.; Bhasin, R.; Voyadzis, J.-M.; Rudra, S.; Unger, K.; Collins, B.T. Five-Fraction Stereotactic Radiosurgery (SRS) for Single Inoperable High-Risk Non-Small Cell Lung Cancer (NSCLC) Brain Metastases. Radiat. Oncol. 2015, 10, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, G.; D’Angelillo, R.M.; Scaringi, C.; Trodella, L.E.; Clarke, E.; Matteucci, P.; Osti, M.F.; Ramella, S.; Enrici, R.M.; Trodella, L. Fractionated Stereotactic Radiosurgery for Patients with Brain Metastases. J. Neurooncol. 2014, 117, 295–301. [Google Scholar] [CrossRef]
- Piras, A.; Boldrini, L.; Menna, S.; Sanfratello, A.; D’Aviero, A.; Cusumano, D.; Di Cristina, L.; Messina, M.; Spada, M.; Angileri, T.; et al. Five-Fraction Stereotactic Radiotherapy for Brain Metastases: A Single-Institution Experience on Different Dose Schedules. Oncol. Res. Treat. 2022, 45, 408–414. [Google Scholar] [CrossRef]
- Wegner, R.E.; Leeman, J.E.; Kabolizadeh, P.; Rwigema, J.-C.; Mintz, A.H.; Burton, S.A.; Heron, D.E. Fractionated Stereotactic Radiosurgery for Large Brain Metastases. Am. J. Clin. Oncol. 2015, 38, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Kahl, K.-H.; Shiban, E.; Gutser, S.; Maurer, C.J.; Sommer, B.; Müller, H.; Konietzko, I.; Grossert, U.; Berlis, A.; Janzen, T.; et al. Focal Cavity Radiotherapy after Neurosurgical Resection of Brain Metastases: Sparing Neurotoxicity without Compromising Locoregional Control. Strahlenther. Onkol. 2022, 198, 1105–1111. [Google Scholar] [CrossRef]
- Korytko, T.; Radivoyevitch, T.; Colussi, V.; Wessels, B.W.; Pillai, K.; Maciunas, R.J.; Einstein, D.B. 12 Gy Gamma Knife Radiosurgical Volume Is a Predictor for Radiation Necrosis in Non-AVM Intracranial Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 419–424. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory Microenvironment Remodelling by Tumour Cells after Radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Constanzo, J.; Midavaine, É.; Fouquet, J.; Lepage, M.; Descoteaux, M.; Kirby, K.; Tremblay, L.; Masson-Côté, L.; Geha, S.; Longpré, J.-M.; et al. Brain Irradiation Leads to Persistent Neuroinflammation and Long-Term Neurocognitive Dysfunction in a Region-Specific Manner. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109954. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect When Combined with Anti-CTLA-4 Antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA Exonuclease Trex1 Regulates Radiotherapy-Induced Tumour Immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristic | n (%) | Median (Range)/Mean (±SD) |
|---|---|---|
| Total number | 36 (100) | |
| Male | 20 (55.6) | |
| Female | 16 (44.4) | |
| Age (years) | 64.5 (34–92) | |
| Total number of brain lesions | 3 (1–10) | |
| ECOG performance score | 1 (0–2) | |
| 0 | 16 (44.4) | |
| 1 | 11 (30.6) | |
| 2 | 9 (25) | |
| DS-GPA | 2 (0–4)/2.1 (±0.98) | |
| Histology | ||
| NSCLC | 12 (33.3) | |
| Melanoma | 8 (22.2) | |
| Breast | 4 (11.1) | |
| SCLC | 3 (8.3) | |
| CRC | 2 (5.6) | |
| Esophageal | 1 (2.8) | |
| Pancreatic | 1 (2.8) | |
| Thyroid | 1 (2.8) | |
| Ovarian | 1 (2.8) | |
| SCC | 1 (2.8) | |
| RCC | 1 (2.8) | |
| Sarcoma | 1 (2.8) | |
| Immunotherapy | ||
| Yes | 9 (25) | |
| No | 27 (75) | |
| Previous RT | ||
| Yes | 7 (19.4) | |
| No | 29 (80.6) | |
| Sequential RT to distant lesions | ||
| SRS | 6 (16.7) | |
| FSRT | 3 (8.3) | |
| WBRT | 5 (13.9 |
| Lesion Characteristic | n (%) | Median (Range)/Mean (±SD) |
|---|---|---|
| Total number | 49 (100) | |
| Location | ||
| Frontal | 14 (28.6) | |
| Occipital | 9 (18.4) | |
| Cerebellum | 9 (18.4) | |
| Temporal | 7 (14.3) | |
| Parietal | 7 (14.3) | |
| Central | 3 (6.1) | |
| Treatment setting | ||
| Definitive | 34 (69.4) | |
| Adjuvant | 15 (30.6) | |
| Immunotherapy | ||
| Yes | 12 (24.5) | |
| No | 37 (75.5) | |
| Previous RT | ||
| Yes | 12 (24.5) | |
| No | 37 (75.5) | |
| Radiation necrosis | ||
| Yes | 7 (14.3) | |
| No | 42 (85.3) | |
| PTV (cc) | 13 (0.7–74.4)/15.8 (±14.4) | |
| Conformity index | 1.06 (0.21–3.5)/1.14 (±0.43) |
| Grade | Acute Toxicity | Late Toxicity | Total |
|---|---|---|---|
| Grade 1 | 33 (61.1%) | 7 (13%) | 40 (74.1%) |
| Grade 2 | 11 (20.3%) | 3 (5.6%) | 14 (25.9%) |
| Grade 3+ | 0 | 0 | 0 |
| Total | 44 (81.4%) | 10 (18.6%) | 54 (100%) |
| Adverse Event | Grade 1 | Grade 2 | Grade 3+ | Total |
|---|---|---|---|---|
| Fatigue | 5 | 6 | 0 | 11 (30.6%) |
| Cephalgia | 7 | 2 | 0 | 5 (13.9%) |
| Vertigo | 4 | 1 | 0 | 5 (13.9%) |
| Nausea | 5 | 0 | 0 | 5 (13.9%) |
| Alopecia | 3 | 1 | 0 | 4 (11.1%) |
| Neuropathies | 1 | 1 | 0 | 2 (5.6%) |
| Cognitive deterioration | 2 | 0 | 0 | 2 (5.6%) |
| Fever | 2 | 0 | 0 | 2 (5.6%) |
| Gait deterioration | 2 | 0 | 0 | 2 (5.6%) |
| Skin reactions | 2 | 0 | 0 | 2 (5.6%) |
| Mucositis | 0 | 1 | 0 | 1 (2.8%) |
| Dysphagia | 0 | 1 | 0 | 1 (2.8%) |
| Seizures | 0 | 1 | 0 | 1 (2.8%) |
| Anemia | 1 | 0 | 0 | 1 (2.8%) |
| Ataxia | 1 | 0 | 0 | 1 (2.8%) |
| Cramps | 1 | 0 | 0 | 1 (2.8%) |
| Gastrointestinal | 1 | 0 | 0 | 1 (2.8%) |
| Tremor | 1 | 0 | 0 | 1 (2.8%) |
| Viscerocranial pain | 1 | 0 | 0 | 1 (2.8%) |
| Visional impairment | 1 | 0 | 0 | 1 (2.8%) |
| 40 | 14 | 0 | 54 (100%) |
| Authors | Year | Dose (Gy) | RT Setting | BM Size (Median) | RT Technique | Lesions | Histology | 1y-LCR (%) | 2y-LCR (%) | RN Rate (%) | Median Time to RN (Months) | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current series | 2022 | 7/35 | both | 13 cc | Conventional FSRT | 49 | mixed | 83.1 | 50 | 14.3 | 12.7 | 0% G3+ |
| Di Perri et. al. [43] | 2020 | 7/35 | both | 11 cc | Cyberknife FSRT | 89 | mixed | 62.5 | n. a. | >40 | n. a. | n. a. |
| Ernst-Stecken et al. [24] | 2006 | 7/35 | definitive | 13 cc | Conventional FSRT | 72 | mixed | 76 | n. a. | n. a. | n. a. | 2% G3+ |
| Jeong et al. [44] | 2015 | 7/35 | definitive | 17.6 cc | Cyberknife FSRT | 38 | mixed | 87 | 65.2 | 15.8 | 10.5 | n. a. |
| Koide et al. [45] | 2019 | 7/35 | definitive | 7.2 cc | Conventional FSRT | 58 | mixed | 64.7 | n. a. | 3.5 | n. a. | 0% G3+ |
| Mengue et al. [46] | 2020 | 7/35 | both | 2.3 cm | Cyberknife FSRT | 158 | mixed | <80 | <60 | NA | n. a. | n. a. |
| Authors | Year | Dose (Gy) | RT Setting | BM Size (Median) | RT Technique | Lesions | Histology | 1y-LCR (%) | 2y-LCR (%) | RN Rate (%) | Median Time to RN (Months) | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown et al. [47] | 2017 | 12–20 | adjuvant | <3 cm | Conventional SRS | 93 | mixed | 61.8 | n. a. | n. a. | n. a. | 39% G3+ |
| Choi et al. [48] | 2021 | Median 20 | definitive | 0.5 cc | Conventional SRS | 311 | melanoma | n. a. | n. a. | 6.1 | 10.2 | n. a. |
| Doré et al. [49] | 2017 | 7.7/23.3 | adjuvant | 11.4 cc | Conventional FSRT | 95 | mixed | 84 | n. a. | 20.6 | 15 | n. a. |
| Eitz et al. [50] | 2020 | Median 6/30 | adjuvant | 23.9 cc | Conventional FSRT | 581 | mixed | 84 | 75 | 8.6 | 13.1 | 6.9% G3 |
| Fokas et al. [18] | 2012 | 5/35 | both | 2 cc | Conventional FSRT | 61 | mixed | 75 | n. a. | 1.6 | n. a. | 2% G3+ |
| Jhaveri et al. [51] | 2019 | 5–7/21–35 | adjuvant | 15/20 cc | Conventional FSRT | 139 | mixed | 84.8 | n. a. | 21.1 | n. a. | n. a. |
| Kohutek et al. [16] | 2015 | 15–22 | definitive | 1.1 cm | Conventional SRS | 271 | mixed | n. a. | n. a. | 25.8 | 10.7 | n. a. |
| Lehrer et al. [52] | 2022 | Median 20 | definitive | 1.6 cc | Conventional SRS | 4,536 | mixed | 90.5 | n. a. | 9.8 | n. a. | n. a. |
| Lischalk et al. [53] | 2015 | 6–8/30–40 | definitive | 5.6 cc | Cyberknife FSRT | 13 | NSCLC | 90 | 90 | 15.4 | 11 | 15% G3+ |
| Minniti et al. [12] | 2011 | 15–20 | definitive | 1.9 cc | Conventional SRS | 310 | mixed | 92 | 84 | 24 | 11 | 5.8% G3+ |
| Minniti et al. [54] | 2014 | 9–12/27–36 | definitive | 16.4 cc | Conventional FSRT | 171 | mixed | 88 | 72 | 18 | 12 | 4% G3+ |
| Minniti et al. [17] | 2016 | 9/27 | definitive | 17.9 cc | Conventional FSRT | 138 | mixed | 91 | n. a. | 8 | 12 | n. a. |
| 2016 | 15–18 | definitive | 12.2 cc | Conventional SRS | 151 | mixed | 77 | n. a. | 20 | 10 | n. a. | |
| Piras et al. [55] | 2022 | 6–8/30–40 | definitive | 1.8 cc | Conventional FSRT | 57 | mixed | n. a. | n. a. | 2.4 | 9 | 7% G2 0% G3 |
| Wegner et al. [56] | 2015 | 8/24 | definitive | 15.6 cc | Conventional FSRT | 36 | mixed | 63 | n. a. | 0 | n. d. | 0% G3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layer, J.P.; Layer, K.; Sarria, G.R.; Röhner, F.; Dejonckheere, C.S.; Friker, L.L.; Zeyen, T.; Koch, D.; Scafa, D.; Leitzen, C.; et al. Five-Fraction Stereotactic Radiotherapy for Brain Metastases—A Retrospective Analysis. Curr. Oncol. 2023, 30, 1300-1313. https://doi.org/10.3390/curroncol30020101
Layer JP, Layer K, Sarria GR, Röhner F, Dejonckheere CS, Friker LL, Zeyen T, Koch D, Scafa D, Leitzen C, et al. Five-Fraction Stereotactic Radiotherapy for Brain Metastases—A Retrospective Analysis. Current Oncology. 2023; 30(2):1300-1313. https://doi.org/10.3390/curroncol30020101
Chicago/Turabian StyleLayer, Julian P., Katharina Layer, Gustavo R. Sarria, Fred Röhner, Cas S. Dejonckheere, Lea L. Friker, Thomas Zeyen, David Koch, Davide Scafa, Christina Leitzen, and et al. 2023. "Five-Fraction Stereotactic Radiotherapy for Brain Metastases—A Retrospective Analysis" Current Oncology 30, no. 2: 1300-1313. https://doi.org/10.3390/curroncol30020101
APA StyleLayer, J. P., Layer, K., Sarria, G. R., Röhner, F., Dejonckheere, C. S., Friker, L. L., Zeyen, T., Koch, D., Scafa, D., Leitzen, C., Köksal, M., Schmeel, F. C., Schäfer, N., Landsberg, J., Hölzel, M., Herrlinger, U., Schneider, M., Giordano, F. A., & Schmeel, L. C. (2023). Five-Fraction Stereotactic Radiotherapy for Brain Metastases—A Retrospective Analysis. Current Oncology, 30(2), 1300-1313. https://doi.org/10.3390/curroncol30020101






