A Retrospective Study of Renal Growth Changes after Proton Beam Therapy for Pediatric Malignant Tumor
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Proton Beam Therapy
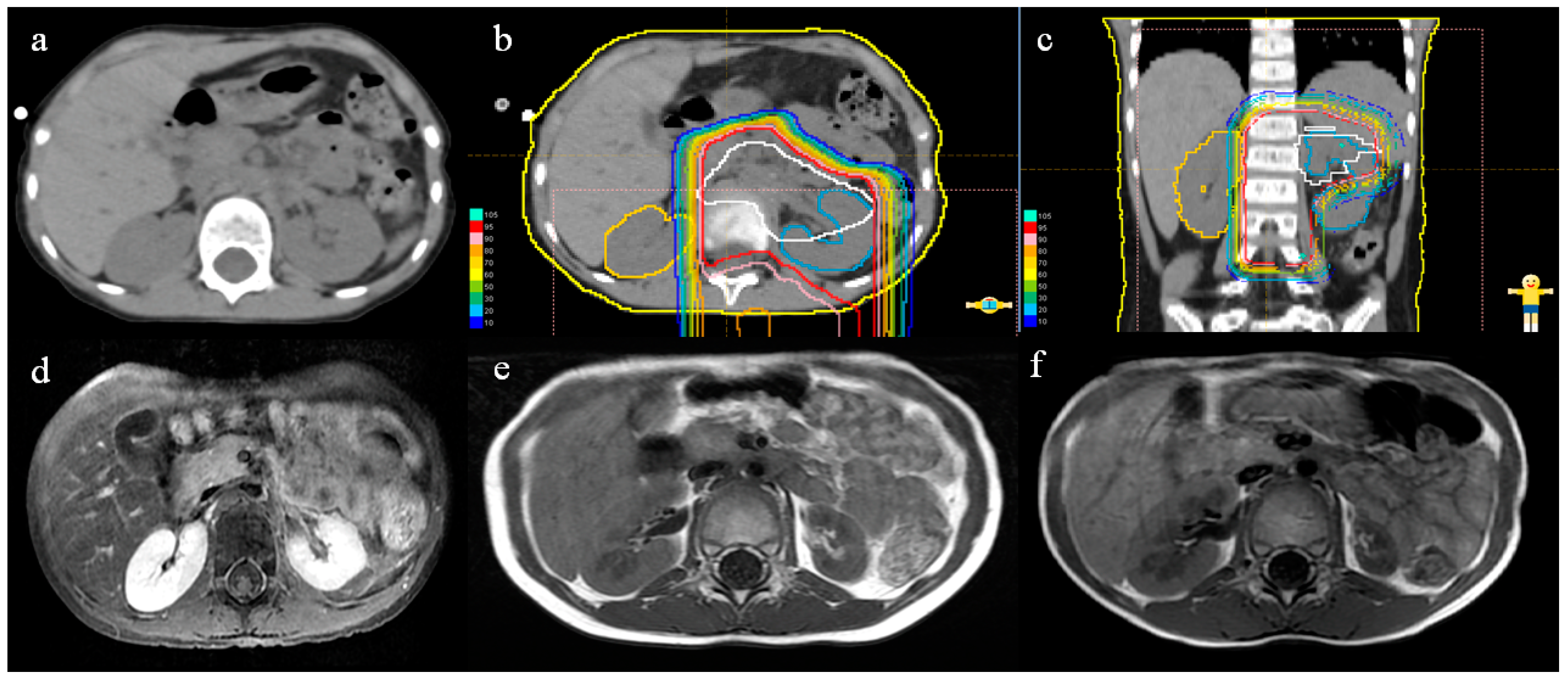
2.3. Renal Morphology and DVH Analysis
2.4. Statistical Analysis
3. Results
3.1. Treatment Characteristics
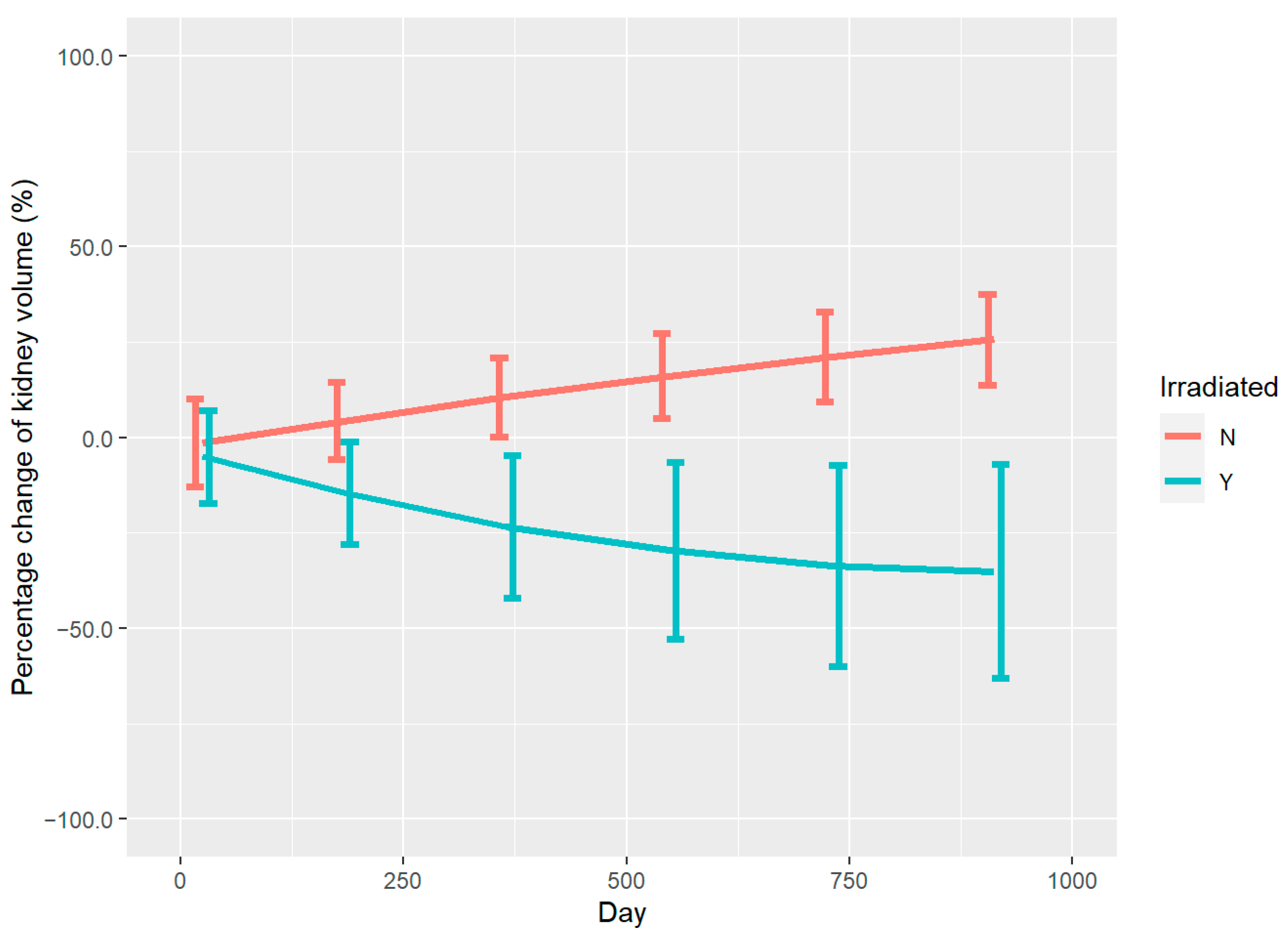
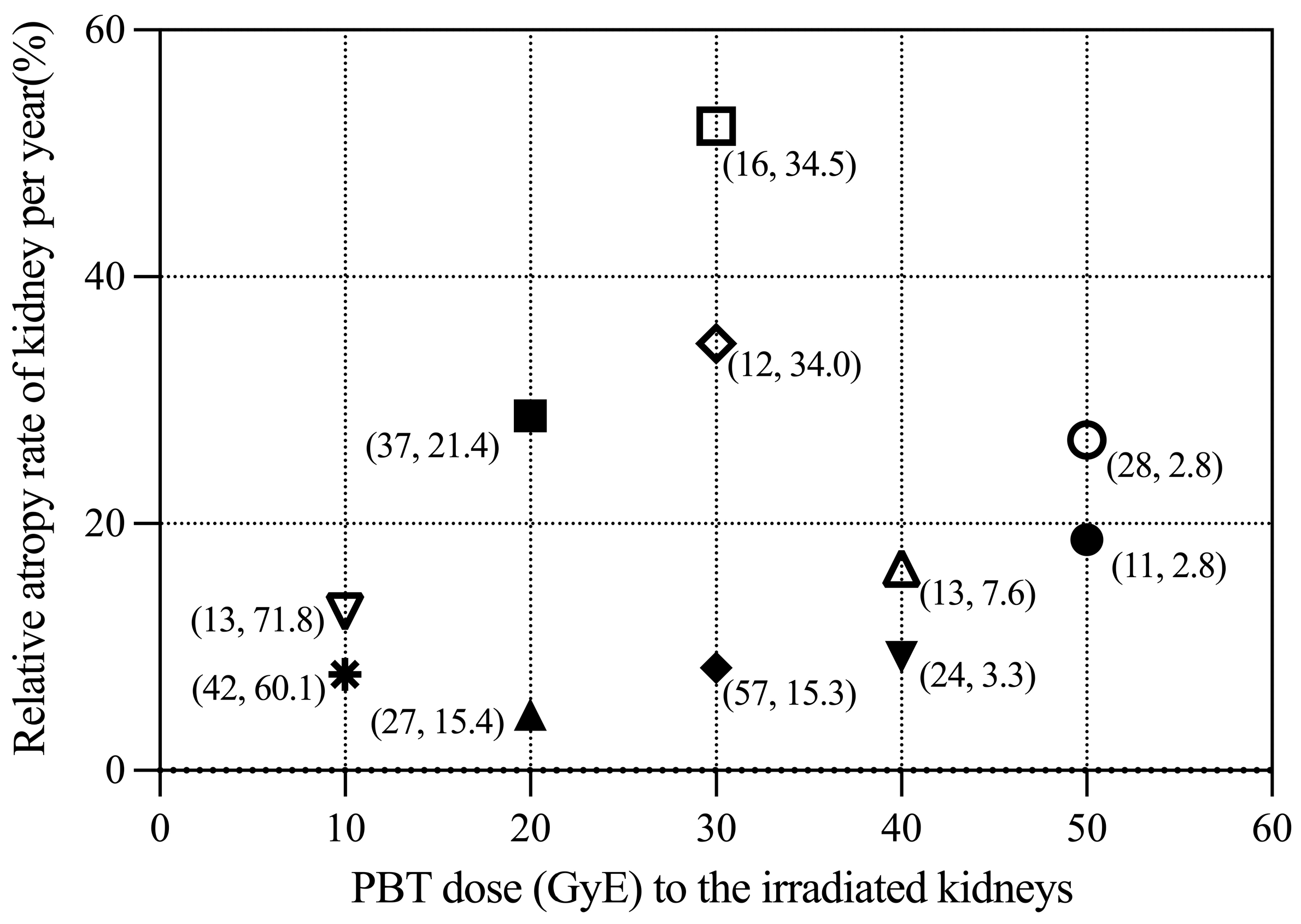
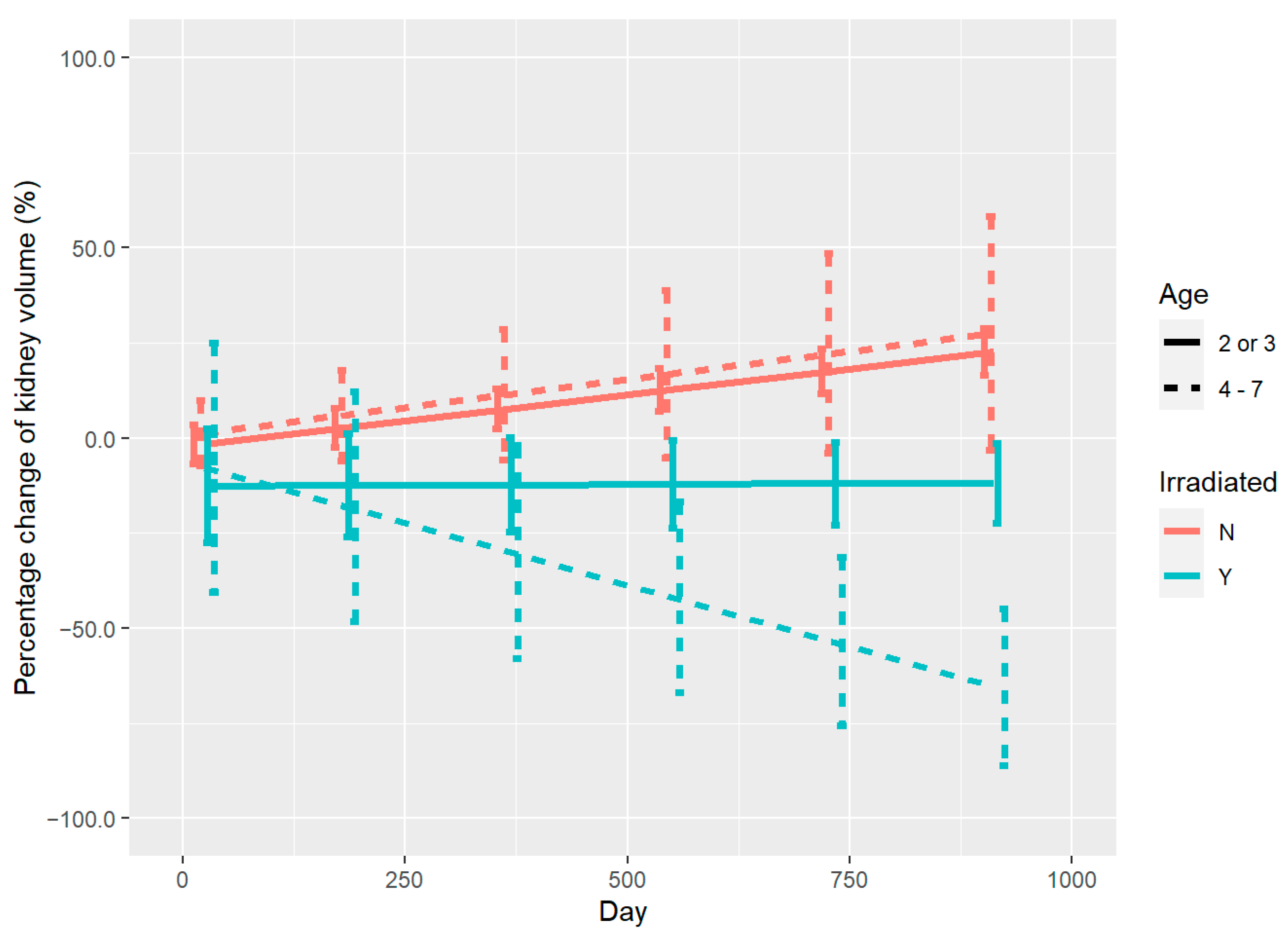
3.2. Renal Volume Changes and DVH Analysis
3.3. Analysis of Renal Function Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esiashvili, N.; Prabhu, R.; Kahn, S.; Paulino, A.C. Current Strategies and Challenges in Treatment of Childhood Rhabdomyosarcoma. J. Radiat. Oncol. 2012, 2, 159–168. [Google Scholar] [CrossRef]
- Palmer, J.D.; Tsang, D.S.; Tinkle, C.L.; Olch, A.J.; Kremer, L.C.M.; Ronckers, C.M.; Gibbs, I.C.; Constine, L.S. Late Effects of Radiation Therapy in Pediatric Patients and Survivorship. Pediatr. Blood Cancer 2021, 68, e28349. [Google Scholar] [CrossRef]
- Huang, T.T.; Hudson, M.M.; Stokes, D.C.; Krasin, M.J.; Spunt, S.L.; Ness, K.K. Pulmonary Outcomes in Survivors of Childhood Cancer: A Systematic Review. Chest 2011, 140, 881–901. [Google Scholar] [CrossRef] [PubMed]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.P.; Wasilewski-Masker, K.; Constine, L.S.; Robison, L.L.; Ness, K.K. A Systematic Review of Selected Musculoskeletal Late Effects in Survivors of Childhood Cancer. Curr. Pediatr. Rev. 2014, 10, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y.; Zhang, Y.; Meng, L.; Wei, J.; Wang, B.; Wang, H.; Xin, Y.; Dong, L.; Jiang, X. Role and Toxicity of Radiation Therapy in Neuroblastoma Patients: A Literature Review. Crit. Rev. Oncol. Hematol. 2020, 149, 102924. [Google Scholar] [CrossRef]
- Yeung, C.K.; Ward, H.C.; Ransley, P.G.; Duffy, P.G.; Pritchard, J. Bladder and Kidney Function after Cure of Pelvic Rhabdomyosarcoma in Childhood. Br. J. Cancer 1994, 70, 1000–1003. [Google Scholar] [CrossRef]
- Lenarczyk, M.; Laiakis, E.C.; Mattson, D.L.; Johnson, B.D.; Kronenberg, A.; North, P.E.; Komorowski, R.; Mäder, M.; Baker, J.E. Irradiation of the Kidneys Causes Pathologic Remodeling in the Nontargeted Heart: A Role for the Immune System. FASEB Bioadv. 2020, 2, 705–719. [Google Scholar] [CrossRef]
- Bölling, T.; Ernst, I.; Pape, H.; Martini, C.; Rübe, C.; Timmermann, B.; Fischedick, K.; Kortmann, R.D.; Willich, N. Dose-Volume Analysis of Radiation Nephropathy in Children: Preliminary Report of the Risk Consortium. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 840–844. [Google Scholar] [CrossRef]
- Tian, X.; Liu, K.; Hou, Y.; Cheng, J.; Zhang, J. The Evolution of Proton Beam Therapy: Current and Future Status. Mol Clin Oncol 2018, 8, 15–21. [Google Scholar] [CrossRef]
- Baliga, S.; Yock, T.I. Proton Beam Therapy in Pediatric Oncology. Curr. Opin. Pediatr. 2019, 31, 28–34. [Google Scholar] [CrossRef]
- Sakurai, H.; Ishikawa, H.; Okumura, T. Proton Beam Therapy in Japan: Current and Future Status. Jpn J. Clin. Oncol. 2016, 46, 885–892. [Google Scholar] [CrossRef]
- Bölling, T.; Könemann, S.; Ernst, I.; Willich, N. Late Effects of Thoracic Irradiation in Children. Strahlenther. Onkol. 2008, 184, 289–295. [Google Scholar] [CrossRef]
- Kal, H.B.; van Kempen-Harteveld, M.L. Renal Dysfunction after Total Body Irradiation: Dose-Effect Relationship. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1228–1232. [Google Scholar] [CrossRef]
- Mizumoto, M.; Murayama, S.; Akimoto, T.; Demizu, Y.; Fukushima, T.; Ishida, Y.; Oshiro, Y.; Numajiri, H.; Fuji, H.; Okumura, T.; et al. Long-term Follow-up after Proton Beam Therapy for Pediatric Tumors: A Japanese National Survey. Cancer Sci. 2017, 108, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, M.; Fuji, H.; Miyachi, M.; Soejima, T.; Yamamoto, T.; Aibe, N.; Demizu, Y.; Iwata, H.; Hashimoto, T.; Motegi, A.; et al. Proton Beam Therapy for Children and Adolescents and Young Adults (AYAs): JASTRO and JSPHO Guidelines. Cancer Treat Rev. 2021, 98, 102209. [Google Scholar] [CrossRef] [PubMed]
- Stokkevåg, C.H.; Engeseth, G.M.; Ytre-Hauge, K.S.; Röhrich, D.; Odland, O.H.; Muren, L.P.; Brydoy, M.; Hysing, L.B.; Szostak, A.; Palmer, M.B.; et al. Estimated Risk of Radiation-Induced Cancer Following Paediatric Cranio-Spinal Irradiation with Electron, Photon and Proton Therapy. Acta Oncol. 2014, 53, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Title Cluster-Robust (Sandwich) Variance Estimators with Small-Sample Corrections. Available online: https://cran.r-project.org/web/packages/clubSandwich/clubSandwich.pdf (accessed on 12 October 2022).
- Package “emmeans” Type Package Title Estimated Marginal Means, Aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 17 January 2023).
- Marc, M.; Mazerolle, J. Type Package Title Model Selection and Multimodel Inference Based on (Q)AIC(c) Depends R (>= 3.2.0). Available online: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 12 October 2022).
- Ward, E.; Desantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and Adolescent Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Seth, R.; Singh, A.; Seth, S.; Sapra, S. Late Effects of Treatment in Survivors of Childhood Cancers: A Single-Centre Experience. Indian J. Med. Res. 2017, 146, 216–223. [Google Scholar] [CrossRef]
- Schwartz, C.L. Long-Term Survivors of Childhood Cancer: The Late Effects of Therapy. Oncologist 1999, 4, 45–54. [Google Scholar] [CrossRef]
- Chang, W.H.; Katsoulis, M.; Tan, Y.Y.; Mueller, S.H.; Green, K.; Lai, A.G. Late Effects of Cancer in Children, Teenagers and Young Adults: Population-Based Study on the Burden of 183 Conditions, in-Patient and Critical Care Admissions and Years of Life Lost. Lancet Reg. Health—Eur. 2022, 12, 100248. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological Response of Cancer Cells to Radiation Treatment. Front. Mol. Biosci 2014, 1, 24. [Google Scholar] [CrossRef]
- Larson, D.L.; Kroll, S.; Jaffe, N.; Serure, A.; Geopfert, H. Long-Term Effects of Radiotherapy in Childhood and Adolescence. Am. J. Surg. 1990, 160, 348–351. [Google Scholar] [CrossRef]
- Paulino, A.C.; Wen, B.C.; Brown, C.K.; Tannous, R.; Mayr, N.A.; Zhen, W.K.; Weidner, G.J.; Hussey, D.H. Late Effects in Children Treated with Radiation Therapy for Wilms’ Tumor. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Jiang, L.; Cui, X.; Zhang, J.; Yu, J. Proton Beam Therapy for Cancer in the Era of Precision Medicine. J. Hematol. Oncol. 2018, 11, 136. [Google Scholar] [CrossRef]
- Mohamed, N.; Lee, A.; Lee, N.Y. Proton Beam Radiation Therapy Treatment for Head and Neck Cancer. Precis. Radiat. Oncol. 2022, 6, 59–68. [Google Scholar] [CrossRef]
- Baba, K.; Mizumoto, M.; Oshiro, Y.; Shimizu, S.; Nakamura, M.; Hiroshima, Y.; Iizumi, T.; Saito, T.; Numajiri, H.; Nakai, K.; et al. An Analysis of Vertebral Body Growth after Proton Beam Therapy for Pediatric Cancer. Cancers 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, Y.; Mizumoto, M.; Pan, H.; Kaste, S.C.; Gajjar, A.; Merchant, T.E. Spinal Changes after Craniospinal Irradiation in Pediatric Patients. Pediatr. Blood Cancer 2020, 67, e28728. [Google Scholar] [CrossRef]
- Dörr, W.; Kallfels, S.; Herrmann, T. Late Bone and Soft Tissue Sequelae of Childhood Radiotherapy. Relevance of Treatment Age and Radiation Dose in 146 Children Treated between 1970 and 1997. Strahlenther. Onkol. 2013, 189, 529–534. [Google Scholar] [CrossRef]
- Phipps, L.M.; Chen, T.; Harris, D.C.H. Radiation Nephropathy. In Case Studies in Clinical Psychological Science: Bridging the Gap from Science to Practice; Oxford University Press: Oxford, UK, 2015; pp. 1–7. [Google Scholar]
- Ritchey, M.L.; Green, D.M.; Thomas, P.R.; Smith, G.R.; Haase, G.; Shochat, S.; Moksness, J.; Breslow, N.E. Renal failure in Wilms’ tumor patients: A report from the National Wilms’ Tumor Study Group. Med. Pediatr. Oncol. 1996, 26, 75–80. [Google Scholar] [CrossRef]
- Maarten Egeler, R.; Wolff, J.E.A.; Anderson, R.A.; Coppes, M.J. Long-Term Complications and Post-Treatment Follow-up of Patients with Wilms’ Tumor. Semin. Urol. Oncol. 1999, 17, 55–61. [Google Scholar]
- Smith, G.R.; Thomas, P.R.M.; Ritchey, M.; Norkool, P. Long-Term Renal Function in Patients with Irradiated Bilateral Wilms Tumor. National Wilms’ Tumor Study Group. Am. J. Clin. Oncol. 1998, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-Induced Kidney Toxicity: Molecular and Cellular Pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, N.; Ishikawa, H.; Arimura, T.; Wada, H.; Okimoto, T.; Sato, Y.; Iwata, H.; Shimizu, S.; Sakurai, H. Proton Therapy for Primary Renal Cell Carcinoma: The First Nationwide Retrospective Study in Japan. In Vivo 2020, 34, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Gong, I.H.; Hwang, J.; Choi, D.K.; Lee, S.R.; Hong, Y.K.; Hong, J.Y.; Park, D.S.; Jeon, H.G. Relationship Among Total Kidney Volume, Renal Function and Age. J. Urol. 2012, 187, 344–349. [Google Scholar] [CrossRef]
- Shin, H.S.; Chung, B.H.; Lee, S.E.; Kim, W.J.; Ha, H.I.; Yang, C.W. Measurement of Kidney Volume with Multi-Detector Computed Tomography Scanning in Young Korean. Yonsei Med. J. 2009, 50, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Rongviriyapanich, C.; Sakunchit, T.; Sudla, C.; Mungkung, S.; Pongnapang, N.; Yeong, C.H. Sonographic renal length and volume of normal Thai children versus their Chinese and Western counterparts. Clin. Exp. Pediatr. 2020, 63, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R. Late renal toxicity of treatment for childhood malignancy: Risk factors, long-term outcomes, and surveillance. Pediatr. Nephrol. 2017, 33, 215–225. [Google Scholar] [CrossRef]
- Stewart, F.A. Radiation Nephropathy after Abdominal Irradiation or Total-Body Irradiation. Radiat. Res. 1995, 143, 235–237. [Google Scholar] [CrossRef]
| Items | n | % |
|---|---|---|
| Age at start of PBT | ||
| 2 or 3 years | 6 | 54.5% |
| 4–7 years | 5 | 45.5% |
| Gender | ||
| Male | 5 | 45.5% |
| Female | 6 | 54.5% |
| Primary disease | ||
| Rhabdomyosarcoma | 2 | 18.2% |
| Neuroblastoma | 8 | 72.7% |
| Osteosarcoma | 1 | 9.1% |
| Total dose and fractions of PBT | ||
| 19.8 GyE/11 fr | 4 | 36.4% |
| 30.6 GyE/17 fr | 4 | 36.4% |
| 41.4 GyE/23 fr | 1 | 9.09% |
| 55.8 GyE/31 fr | 1 | 9.09% |
| 70.4 GyE/32 fr | 1 | 9.09% |
| Chemotherapy | ||
| Pre-radiation chemotherapy | 8 | 72.7% |
| Concurrent chemoradiotherapy | 2 | 18.2% |
| Post-radiation chemotherapy | 1 | 9.09% |
| Median period of follow-up (range) (months) | 24.5 (11–57) |
| Primary Disease | Total Dose (GyE) | Fractions | Chemotherapy | V10 (%) | V20 (%) | V30 (%) | Follow-Up (Month) | Volume Change of Irradiated Kidney/Year (%) | Volume Change of Control Kidney/Year (%) | Relative Volume Change of Irradiated Kidney/Year (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Rhabdomyosarcoma | 55.8 | 31 | Post-rad | 18.25 | 13.2 | 9.61 | 11 | −8.17 | +12.66 | −18.70 |
| #2 | Neuroblastoma | 19.8 | 11 | Pre-rad | 74.31 | 21.37 | 0 | 37 | −26.41 | +17.87 | −28.73 |
| #3 | Neuroblastoma | 19.8 | 11 | Pre-rad | 54.47 | 15.42 | 0 | 27 | +10.17 | +16.38 | −4.53 |
| #4 | Rhabdomyosarcoma | 41.4 | 23 | Pre-rad | 42.42 | 28.91 | 15.03 | 24 | −10.89 | −2.13 | −9.15 |
| #5 | Neuroblastoma | 30.6 | 17 | Pre-rad | 78.4 | 52.74 | 15.3 | 57 | −2.52 | +9.53 | −8.32 |
| #6 | Osteosarcoma | 70.4 | 32 | CCRT | 32.3 | 25.12 | 15.33 | 28 | −26.38 | +1.04 | −26.78 |
| #7 | Neuroblastoma | 30.6 | 17 | Pre-rad | 99.92 | 98.11 | 34.46 | 16 | −47.52 | +15.08 | −52.21 |
| #8 | Neuroblastoma | 30.6 | 17 | CCRT | 72.12 | 58.52 | 45.49 | 13 | +15.51 | +38.78 | −16.42 |
| #9 | Neuroblastoma | 19.8 | 11 | Pre-rad | 71.84 | 0.47 | 0 | 13 | −8.55 | +5.00 | −12.86 |
| #10 | Neuroblastoma | 30.6 | 17 | Pre-rad | 73.29 | 55.13 | 33.96 | 12 | −34.52 | +0.09 | −34.58 |
| #11 | Neuroblastoma | 19.8 | 11 | Pre-rad | 60.14 | 0.66 | 0 | 42 | −2.68 | +6.98 | −7.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Mizumoto, M.; Oshiro, Y.; Nitta, H.; Saito, T.; Iizumi, T.; Kawano, C.; Yamaki, Y.; Fukushima, H.; Hosaka, S.; et al. A Retrospective Study of Renal Growth Changes after Proton Beam Therapy for Pediatric Malignant Tumor. Curr. Oncol. 2023, 30, 1560-1570. https://doi.org/10.3390/curroncol30020120
Li Y, Mizumoto M, Oshiro Y, Nitta H, Saito T, Iizumi T, Kawano C, Yamaki Y, Fukushima H, Hosaka S, et al. A Retrospective Study of Renal Growth Changes after Proton Beam Therapy for Pediatric Malignant Tumor. Current Oncology. 2023; 30(2):1560-1570. https://doi.org/10.3390/curroncol30020120
Chicago/Turabian StyleLi, Yinuo, Masashi Mizumoto, Yoshiko Oshiro, Hazuki Nitta, Takashi Saito, Takashi Iizumi, Chie Kawano, Yuni Yamaki, Hiroko Fukushima, Sho Hosaka, and et al. 2023. "A Retrospective Study of Renal Growth Changes after Proton Beam Therapy for Pediatric Malignant Tumor" Current Oncology 30, no. 2: 1560-1570. https://doi.org/10.3390/curroncol30020120
APA StyleLi, Y., Mizumoto, M., Oshiro, Y., Nitta, H., Saito, T., Iizumi, T., Kawano, C., Yamaki, Y., Fukushima, H., Hosaka, S., Maruo, K., Kamizawa, S., & Sakurai, H. (2023). A Retrospective Study of Renal Growth Changes after Proton Beam Therapy for Pediatric Malignant Tumor. Current Oncology, 30(2), 1560-1570. https://doi.org/10.3390/curroncol30020120





