Osteopenia Is Associated with Shorter Survival in Patients with Intrahepatic Cholangiocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Definition of Osteopenia
2.3. Definition of Sarcopenia and Myosteatosis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
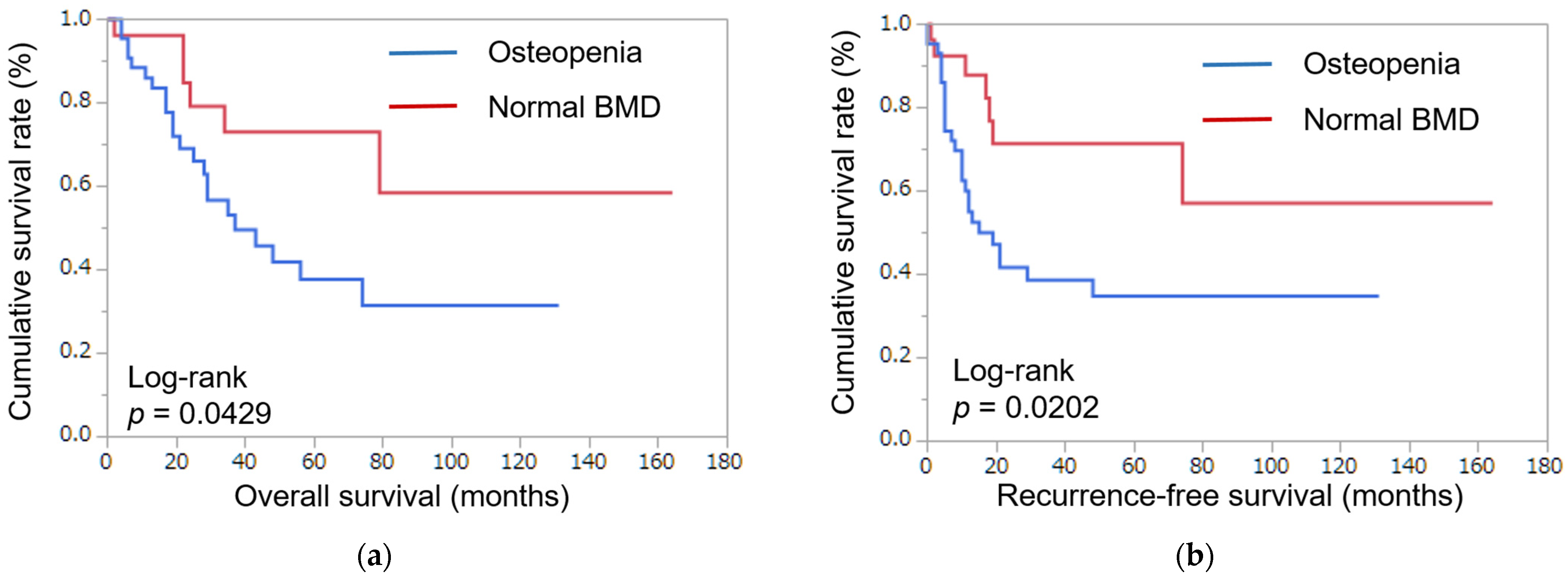
3.2. Survival
3.3. Univariable and Multivariable Analysis for Overall Survival
3.4. Univariable and Multivariable Analysis for Recurrence-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spolverato, G.; Yakoob, M.Y.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Pulitano, C.; et al. Impact of complications on long-term survival after resection of intrahepatic cholangiocarcinoma. Cancer 2015, 121, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Kim, B.H. Cholangiolocellular carcinoma with satellite nodules showing intermediate differentiation. Clin. Mol. Hepatol. 2015, 21, 183–186. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.C.; Nathan, H.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J. Clin. Oncol. 2011, 29, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Takashima, A.; Ueno, M.; Ikeda, M.; Hamamoto, Y.; Ishii, H.; Boku, N.; Furuse, J. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: A Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 2013, 104, 1211–1216. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Hendifar, A.E.; Chang, J.I.; Huang, B.Z.; Tuli, R.; Wu, B.U. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J. Gastrointest. Oncol. 2018, 9, 17–23. [Google Scholar] [CrossRef]
- Kahl, C.; Krahl, R.; Becker, C.; Al-Ali, H.K.; Sayer, H.G.; Schulze, A.; Herold, M.; Hänel, M.; Scholl, S.; Hochhaus, A.; et al. Long-term follow-up of the AML97 study for patients aged 60 years and above with acute myeloid leukaemia: A study of the East German Haematology and Oncology Study Group (OSHO). J. Cancer Res. Clin. Oncol. 2016, 142, 305–315. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Antunes, J.M.M.; Ferreira, R.M.P.; Moreira-Gonçalves, D. Exercise Training as Therapy for Cancer-Induced Cardiac Cachexia. Trends Mol. Med. 2018, 24, 709–727. [Google Scholar] [CrossRef]
- Yakabe, M.; Hosoi, T.; Akishita, M.; Ogawa, S. Updated concept of sarcopenia based on muscle-bone relationship. J. Bone Miner. Metab. 2020, 38, 7–13. [Google Scholar] [CrossRef]
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E. Determinants of Muscle and Bone Aging. J. Cell Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.E.; Underwood, P.W.; Williams, I.E.; George, T.J.; Judge, S.M.; Yarrow, J.F.; Trevino, J.G.; Judge, A.R. Osteopenia is associated with wasting in pancreatic adenocarcinoma and predicts survival after surgery. Cancer Med. 2022, 11, 50–60. [Google Scholar] [CrossRef]
- Sharma, P.; Parikh, N.D.; Yu, J.; Barman, P.; Derstine, B.A.; Sonnenday, C.J.; Wang, S.C.; Su, G.L. Bone mineral density predicts posttransplant survival among hepatocellular carcinoma liver transplant recipients. Liver Transplant. 2016, 22, 1092–1098. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Taniai, T.; Haruki, K.; Yanagaki, M.; Igarashi, Y.; Furukawa, K.; Onda, S.; Yasuda, J.; Matsumoto, M.; Tsunematsu, M.; Ikegami, T. Osteosarcopenia predicts poor prognosis for patients with intrahepatic cholangiocarcinoma after hepatic resection. Surg. Today 2022, 53, 82–89. [Google Scholar] [CrossRef]
- Pang, Y.Y. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB 2002, 4, 99, author reply 99–100. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Sakuma, Y.; Ohzawa, H.; Saito, A.; Meguro, Y.; Watanabe, J.; Kazue, M.; Endo, K.; Sasanuma, H.; Shimizu, A.; et al. Clearance of the liver remnant predicts short-term outcome in patients undergoing resection of hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 5614–5625. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Watanabe, J.; Miki, A.; Sakuma, Y.; Shimodaira, K.; Aoki, Y.; Meguro, Y.; Morishima, K.; Endo, K.; Sasanuma, H.; Lefor, A.K.; et al. Preoperative Osteopenia Is Associated with Significantly Shorter Survival in Patients with Perihilar Cholangiocarcinoma. Cancers 2022, 14, 2213. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishikawa, K.; Furukawa, K.; Tanishima, Y.; Ishikawa, Y.; Kurogochi, T.; Yuda, M.; Tanaka, Y.; Matsumoto, A.; Mitsumori, N.; et al. Prognostic Significance of Preoperative Osteopenia in Patients Undergoing Esophagectomy for Esophageal Cancer. World J. Surg. 2021, 45, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Guida, J.L.; Ahles, T.A.; Belsky, D.; Campisi, J.; Cohen, H.J.; DeGregori, J.; Fuldner, R.; Ferrucci, L.; Gallicchio, L.; Gavrilov, L.; et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J. Natl. Cancer Inst. 2019, 111, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Osaki, T.; Ueyama, T.; Koyama, M.; Iki, M.; Endo, K.; Tatebe, S.; Hirooka, Y. The Combination of Preoperative Skeletal Muscle Quantity and Quality is an Important Indicator of Survival in Elderly Patients Undergoing Curative Gastrectomy for Gastric Cancer. World J. Surg. 2021, 45, 2868–2877. [Google Scholar] [CrossRef] [PubMed]
- Çınar, H.U.; Çelik, B.; Taşkın, G.; İnce, Ö. Impact of preoperative computed tomography-determined quantity and quality of skeletal muscle on survival after resected non-small cell lung carcinoma. Eur. J. Surg. Oncol. 2022, 48, 1937–1946. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Masui, T.; Mizumoto, M.; Hammad, A.; Mori, A.; Takaori, K.; Uemoto, S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015, 157, 1088–1098. [Google Scholar] [CrossRef]
- Miyachi, Y.; Kaido, T.; Yao, S.; Shirai, H.; Kobayashi, A.; Hamaguchi, Y.; Kamo, N.; Yagi, S.; Uemoto, S. Bone Mineral Density as a Risk Factor for Patients Undergoing Surgery for Hepatocellular Carcinoma. World J. Surg. 2019, 43, 920–928. [Google Scholar] [CrossRef]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef]
- Langen, R.C.; Schols, A.M.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001, 15, 1169–1180. [Google Scholar] [CrossRef]
- Lee, D.; Goldberg, A.L. Muscle Wasting in Fasting Requires Activation of NF-κB and Inhibition of AKT/Mechanistic Target of Rapamycin (mTOR) by the Protein Acetylase, GCN5. J. Biol. Chem. 2015, 290, 30269–30279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhaos, J.; Liu, X.; Yan, B.; Chen, D.; Gao, Y.; Hu, X.; Liu, S.; Zhang, D.; Zhou, C. Activation of NF-B upregulates Snail and consequent repression of E-cadherin in cholangiocarcinoma cell invasion. Hepatogastroenterology 2011, 58, 1–7. [Google Scholar]
- Krenn-Pilko, S.; Langsenlehner, U.; Thurner, E.M.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br. J. Cancer 2014, 110, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Castro Santa, E.; Rico Juri, J.M.; Pinheiro, R.S.; Lerut, J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transplant. Int. 2014, 27, 32–41. [Google Scholar] [CrossRef]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C.; Luyten, E.J.; Mahadev, S.; Witkowski, P.; Siegel, A.B.; Brown, R.S.; Emond, J.C. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Dai, Z.; Yin, D.; Yang, L.X.; Wang, Z.; Xiao, Y.S.; Fan, J.; Zhou, J. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine 2015, 94, e574. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144. [Google Scholar] [CrossRef]
- Disis, M.L. Immune regulation of cancer. J. Clin. Oncol. 2010, 28, 4531–4538. [Google Scholar] [CrossRef]
- Chen, M.F.; Hsieh, C.C.; Chen, P.T.; Lu, M.S. Role of Nutritional Status in the Treatment Outcome for Esophageal Squamous Cell Carcinoma. Nutrients 2021, 13, 2997. [Google Scholar] [CrossRef]
- Kaido, T.; Ogawa, K.; Fujimoto, Y.; Ogura, Y.; Hata, K.; Ito, T.; Tomiyama, K.; Yagi, S.; Mori, A.; Uemoto, S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am. J. Transplant. 2013, 13, 1549–1556. [Google Scholar] [CrossRef]
- Benzo, R.; Wigle, D.; Novotny, P.; Wetzstein, M.; Nichols, F.; Shen, R.K.; Cassivi, S.; Deschamps, C. Preoperative pulmonary rehabilitation before lung cancer resection: Results from two randomized studies. Lung Cancer 2011, 74, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Mayo, N.E.; Feldman, L.; Scott, S.; Zavorsky, G.; Kim, D.J.; Charlebois, P.; Stein, B.; Carli, F. Impact of preoperative change in physical function on postoperative recovery: Argument supporting prehabilitation for colorectal surgery. Surgery 2011, 150, 505–514. [Google Scholar] [CrossRef]
- Inoue, J.; Ono, R.; Makiura, D.; Kashiwa-Motoyama, M.; Miura, Y.; Usami, M.; Nakamura, T.; Imanishi, T.; Kuroda, D. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis. Esophagus 2013, 26, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Saitsu, A.; Miki, A.; Kotani, K.; Sata, N. Prognostic value of preoperative low bone mineral density in patients with digestive cancers: A systematic review and meta-analysis. Arch. Osteoporos. 2022, 17, 33. [Google Scholar] [CrossRef] [PubMed]
| Variables | Normal BMD (n = 27) | Osteopenia (n = 44) | p-Value |
|---|---|---|---|
| Age (y), mean ± SD | 64.9 ± 9.7 | 70.3 ± 7.2 | 0.0095 |
| Gender, Female vs. Male | 5 vs. 22 | 20 vs. 24 | 0.0211 |
| Myosteatosis, yes vs. no | 21 vs. 6 | 30 vs. 14 | 0.3829 |
| Sarcopenia, yes vs. no | 23 vs. 4 | 38 vs. 5 | 0.6982 |
| ASA Score, 1, 2 vs. 3 | 23 vs. 3 | 38 vs. 6 | 0.8000 |
| Neutrophil–lymphocyte ratio, mean ± SD | 4.3 ± 5.7 | 3.9 ± 3.8 | 0.7498 |
| Platelet–lymphocyte ratio, mean ± SD | 0.18 ± 0.12 | 0.17 ± 0.13 | 0.9355 |
| Prognostic Nutrition Index, mean ± SD | 37.7 ± 6.6 | 38.9 ± 4.1 | 0.4096 |
| Intraoperative blood loss, mean ± SD | 948 ± 940 | 1437 ± 1454 | 0.1601 |
| CEA (mg/dl), mean ± SD | 3.8 ± 3.4 | 5.3 ± 6.2 | 0.2899 |
| CA19-9 (IU/mL), mean ± SD | 5380 ± 23,780 | 570 ± 1130 | 0.1880 |
| R0 resection, yes vs. no | 22 vs. 5 | 40 vs. 4 | 0.2464 |
| Stage, I, II vs. III, IV | 19 vs. 8 | 29 vs. 15 | 0.6956 |
| Differentiation, well, moderate vs. poorly | 18 vs. 4 | 38 vs. 1 | 0.0327 |
| T stage, I, II vs. III, IV | 20 vs. 2 | 37 vs. 3 | 0.8285 |
| N stage, positive vs. negative | 3 vs. 19 | 9 vs. 31 | 0.3980 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (y), ≥70 | 1.85 | 0.87–4.07 | 0.1083 | |||
| Gender, male | 0.69 | 0.32–1.52 | 0.3431 | |||
| Osteopenia, yes | 2.46 | 1.06–6.69 | 0.0357 | 3.66 | 1.16–14.1 | 0.0258 |
| Myosteatosis, yes | 1.73 | 0.71–5.14 | 0.2423 | |||
| Sarcopenia, yes | 3.08 | 0.13–0.99 | 0.0492 | 2.89 | 0.62–10.4 | 0.1593 |
| ASA Score, 1 or 2 | 0.82 | 0.28–3.48 | 0.7535 | |||
| Neutrophil–lymphocyte ratio, ≥1.9 | 6.26 | 2.27–15.9 | 0.0008 | 1.40 | 0.07–7.93 | 0.7650 |
| Platelet–lymphocyte ratio, ≥0.16 | 12.78 | 3.76–39.4 | 0.0002 | 17.23 | 1.80–401 | 0.0126 |
| Prognostic Nutrition Index, ≥40 | 0.51 | 0.19–1.25 | 0.1420 | |||
| Intraoperative blood loss, ≥760 mL | 2.05 | 0.91–5.01 | 0.0816 | |||
| CEA (mg/dl), ≥4.5 | 2.02 | 0.92–4.39 | 0.0803 | |||
| CA19-9 (IU/mL), ≥37 | 2.16 | 0.95–5.52 | 0.0658 | |||
| R0 resection, no | 1.15 | 0.27–3.30 | 0.8179 | |||
| Stage, I or II | 2.01 | 0.92–4.25 | 0.0773 | |||
| Differentiation, well or moderate | 0.84 | 0.05–4.01 | 0.8597 | |||
| T stage, 1 or 2 | 0.37 | 0.02–1.79 | 0.2635 | |||
| N stage, positive | 3.00 | 1.23–6.95 | 0.0169 | 1.82 | 0.60–5.24 | 0.2799 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (y) ≥70 | 1.61 | 0.82–3.28 | 0.1735 | |||
| Gender, male | 0.49 | 0.25–0.89 | 0.0464 | 0.50 | 0.20–1.21 | 0.1230 |
| Osteopenia, yes | 2.57 | 1.28–6.44 | 0.0168 | 1.67 | 0.62–5.03 | 0.3158 |
| Myosteatosis, yes | 1.50 | 0.69–3.75 | 0.3255 | |||
| Sarcopenia, yes | 2.05 | 0.76–4.71 | 0.1447 | |||
| ASA Score, 1 or 2 | 1.12 | 0.44–3.78 | 0.8323 | |||
| Neutrophil–lymphocyte ratio, ≥1.9 | 5.34 | 2.12–12.4 | 0.0008 | 3.17 | 0.67–11.4 | 0.1311 |
| Platelet–lymphocyte ratio, ≥0.16 | 12.77 | 3.58–43.7 | 0.0003 | 4.11 | 0.85–21.0 | 0.0795 |
| Prognostic Nutrition Index, ≥40 | 0.71 | 0.31–1.55 | 0.3918 | |||
| Intraoperative blood loss, ≥760 mL | 2.05 | 0.98–4.53 | 0.0566 | |||
| CEA (mg/dl), ≥4.5 | 1.57 | 0.75–3.20 | 0.2214 | |||
| CA19-9 (IU/mL), ≥37 | 2.10 | 0.98–5.02 | 0.0561 | |||
| R0 resection, no | 2.14 | 0.95–4.30 | 0.0637 | |||
| Stage, I or II | 1.71 | 0.84–3.41 | 0.1366 | |||
| Differentiation, well or moderate | 0.46 | 0.03–2.17 | 0.3923 | |||
| T stage, 1 or 2 | 1.41 | 0.42–8.78 | 0.6206 | |||
| N stage, positive | 2.14 | 0.95–4.53 | 0.0637 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miki, A.; Sakuma, Y.; Watanabe, J.; Endo, K.; Sasanuma, H.; Teratani, T.; Lefor, A.K.; Kitayama, J.; Sata, N. Osteopenia Is Associated with Shorter Survival in Patients with Intrahepatic Cholangiocarcinoma. Curr. Oncol. 2023, 30, 1860-1868. https://doi.org/10.3390/curroncol30020144
Miki A, Sakuma Y, Watanabe J, Endo K, Sasanuma H, Teratani T, Lefor AK, Kitayama J, Sata N. Osteopenia Is Associated with Shorter Survival in Patients with Intrahepatic Cholangiocarcinoma. Current Oncology. 2023; 30(2):1860-1868. https://doi.org/10.3390/curroncol30020144
Chicago/Turabian StyleMiki, Atsushi, Yasunaru Sakuma, Jun Watanabe, Kazuhiro Endo, Hideki Sasanuma, Takumi Teratani, Alan Kawarai Lefor, Joji Kitayama, and Naohiro Sata. 2023. "Osteopenia Is Associated with Shorter Survival in Patients with Intrahepatic Cholangiocarcinoma" Current Oncology 30, no. 2: 1860-1868. https://doi.org/10.3390/curroncol30020144
APA StyleMiki, A., Sakuma, Y., Watanabe, J., Endo, K., Sasanuma, H., Teratani, T., Lefor, A. K., Kitayama, J., & Sata, N. (2023). Osteopenia Is Associated with Shorter Survival in Patients with Intrahepatic Cholangiocarcinoma. Current Oncology, 30(2), 1860-1868. https://doi.org/10.3390/curroncol30020144






