Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis
Abstract
:1. Introduction
2. Incidence and Mortality Rates Worldwide
3. Prostate Cancer Risk Factors
3.1. Unmodifiable Risk Factors: Ethnicity, Family History, and Genetic Factors
3.2. Modifiable Risk Factors: Lifestyle, Diet, and Environment
4. Prostate Cancer Screening
4.1. Prostate-Specific Membrane Antigen: A Theranostic Approach
4.1.1. Molecular Imaging
4.1.2. Radioligand Targeted Therapy
4.2. Tumor Biomarkers
4.2.1. Molecular Biomarkers
4.2.2. Long Non-Coding RNAs
4.2.3. Liquid Biopsy Biomarkers
4.3. Active Surveillance and Risk-Stratification Algorithms
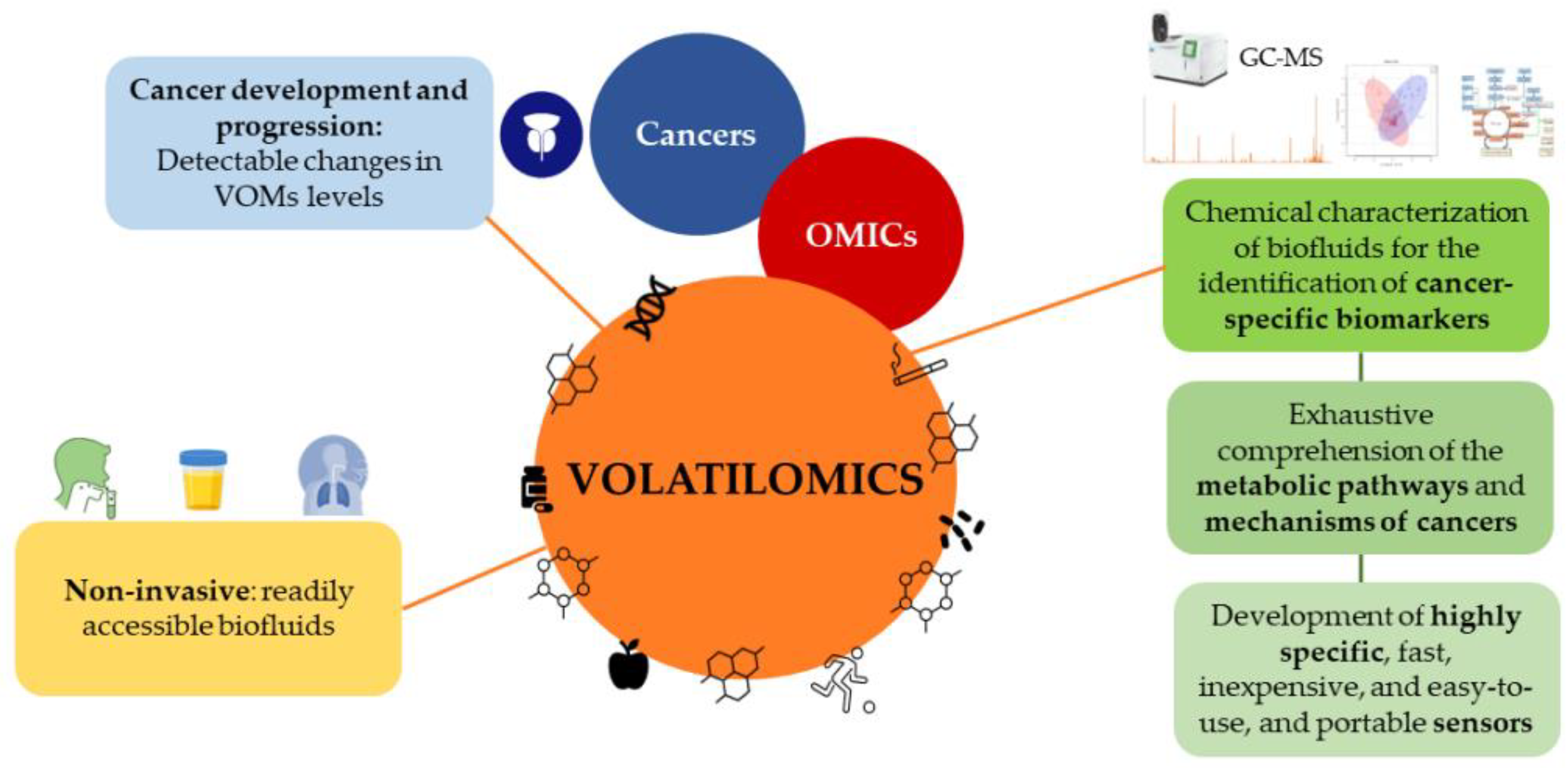
4.4. Volatilomics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanji, Y.; Isaacs, W.B.; Xu, J.; Cooney, K.A. Prostate Cancer Predisposition. Urol. Clin. N. Am. 2021, 48, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Tikkinen, K.A.O.; Dahm, P.; Lytvyn, L.; Heen, A.F.; Vernooij, R.W.M.; Siemieniuk, R.A.C.; Wheeler, R.; Vaughan, B.; Fobuzi, A.C.; Blanker, M.H.; et al. Prostate cancer screening with prostate-specific antigen (PSA) test: A clinical practice guideline. BMJ 2018, 362, k3581. [Google Scholar] [CrossRef] [Green Version]
- Braga, R.; Costa, A.R.; Pina, F.; Moura-Ferreira, P.; Lunet, N. Prostate cancer screening in Portugal: Prevalence and perception of potential benefits and adverse effects. Eur. J. Cancer Prev. 2020, 29, 248–251. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Liver Int. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Marhold, M.; Kramer, G.; Krainer, M.; Le Magnen, C. The prostate cancer landscape in Europe: Current challenges, future opportunities. Cancer Lett. 2022, 526, 304–310. [Google Scholar] [CrossRef]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef]
- Coughlin, S.S. A review of social determinants of prostate cancer risk, stage, and survival. Prostate Int. 2020, 8, 49–54. [Google Scholar] [CrossRef]
- Loeb, S.; Katz, M.S.; Langford, A.; Byrne, N.; Ciprut, S. Prostate cancer and social media. Nat. Rev. Urol. 2018, 15, 422–429. [Google Scholar] [CrossRef]
- Nossiter, J.; Morris, M.; Parry, M.G.; Sujenthiran, A.; Cathcart, P.; van der Meulen, J.; Aggarwal, A.; Payne, H.; Clarke, N.W. Impact of the COVID-19 pandemic on the diagnosis and treatment of men with prostate cancer. BJU Int. 2022, 130, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Markozannes, G.; Tzoulaki, I.; Karli, D.; Evangelou, E.; Ntzani, E.; Gunter, M.J.; Norat, T.; Ioannidis, J.P.; Tsilidis, K.K. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur. J. Cancer 2016, 69, 61–69. [Google Scholar] [CrossRef]
- Tonon, L.; Fromont, G.; Boyault, S.; Thomas, E.; Ferrari, A.; Sertier, A.S.; Kielbassa, J.; Le Texier, V.; Kamoun, A.; Elarouci, N.; et al. Mutational Profile of Aggressive, Localised Prostate Cancer from African Caribbean Men Versus European Ancestry Men. Eur. Urol. 2019, 75, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebbeck, T.R. Prostate Cancer Disparities by Race and Ethnicity: From Nucleotide to Neighborhood. Cold Spring Harb. Perspect. Med. 2018, 8, a030387. [Google Scholar] [CrossRef] [PubMed]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Men’s Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAllister, B.J. The association between ethnic background and prostate cancer. Br. J. Nurs. 2019, 28, S4–S10. [Google Scholar] [CrossRef]
- Brown, C.R.; Hambleton, I.; Hercules, S.M.; Unwin, N.; Murphy, M.M.; Nigel Harris, E.; Wilks, R.; MacLeish, M.; Sullivan, L.; Sobers-Grannum, N. Social determinants of prostate cancer in the Caribbean: A systematic review and meta-analysis. BMC Public Health 2018, 18, 900. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; Kemper, A.R.; et al. Screening for prostate cancer USPreventive servicestaskforcerecommendation statement. JAMA J. Am. Med. Assoc. 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.; Paulo, P.; Teixeira, M.R. Hereditary Predisposition to Prostate Cancer: From Genetics to Clinical Implications. Int. J. Mol. Sci. 2020, 21, 5036. [Google Scholar] [CrossRef] [PubMed]
- Bree, K.K.; Hensley, P.J.; Pettaway, C.A. Germline Predisposition to Prostate Cancer in Diverse Populations. Urol. Clin. N. Am. 2021, 48, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S.; Vernon, M.; Klaassen, Z.; Tingen, M.S.; Cortes, J.E. Knowledge of prostate cancer among African American men: A systematic review. Prostate 2021, 81, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, E.; Salonia, A.; Briganti, A.; Montorsi, F. Re: Family History and Probability of Prostate Cancer, Differentiated by Risk Category—A Nationwide Population-based Study. Eur. Urol. 2017, 71, 143–144. [Google Scholar] [CrossRef]
- Takata, R.; Takahashi, A.; Fujita, M.; Momozawa, Y.; Saunders, E.J.; Yamada, H.; Maejima, K.; Nakano, K.; Nishida, Y.; Hishida, A.; et al. 12 new susceptibility loci for prostate cancer identified by genome-wide association study in Japanese population. Nat. Commun. 2019, 10, 4422. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.C.; Freedland, S.J. Obesity and Prostate Cancer: A Focused Update on Active Surveillance, Race, and Molecular Subtyping. Eur. Urol. 2017, 72, 78–83. [Google Scholar] [CrossRef]
- Wilson, R.L.; Taaffe, D.R.; Newton, R.U.; Hart, N.H.; Lyons-Wall, P.; Galvao, D.A. Obesity and prostate cancer: A narrative review. Crit. Rev. Oncol. Hematol. 2022, 169, 103543. [Google Scholar] [CrossRef]
- Adesunloye, B.A. Mechanistic Insights into the Link between Obesity and Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 3935. [Google Scholar] [CrossRef]
- Bandini, M.; Gandaglia, G.; Briganti, A. Obesity and prostate cancer. Curr. Opin. Urol. 2017, 27, 415–421. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.M.; Mucci, L.A. Diet and Lifestyle in Prostate Cancer. Prostate Cancer Cell. Genet. Mech. Dis. Dev. Progress. 2019, 1210, 1–27. [Google Scholar] [CrossRef]
- Kaiser, A.; Haskins, C.; Siddiqui, M.M.; Hussain, A.; D’Adamo, C. The evolving role of diet in prostate cancer risk and progression. Curr. Opin. Oncol. 2019, 31, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Darcey, E.; Boyle, T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 70, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Shiota, M.; Shiga, K.I.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; Yokomizo, A.; Naito, S.; Eto, M. Effect of Smoking on Oncological Outcome among Prostate Cancer Patients after Radical Prostatectomy with Neoadjuvant Hormonal Therapy. Cancer Investig. 2020, 38, 559–564. [Google Scholar] [CrossRef]
- Khan, S.; Thakkar, S.; Drake, B. Smoking history, intensity, and duration and risk of prostate cancer recurrence among men with prostate cancer who received definitive treatment. Ann. Epidemiol. 2019, 38, 4–10. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; Fritz, J.; Häggström, C.; Järvholm, B.; Stattin, P.; Stocks, T. Smoking and Risk of Prostate Cancer and Prostate Cancer Death: A Pooled Study. Eur. Urol. 2022, 82, 571–579. [Google Scholar] [CrossRef]
- Foerster, B.; Pozo, C.; Abufaraj, M.; Mari, A.; Kimura, S.; D’Andrea, D.; John, H.; Shariat, S.F. Association of Smoking Status With Recurrence, Metastasis, and Mortality Among Patients With Localized Prostate Cancer Undergoing Prostatectomy or Radiotherapy: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Fraser, G.E.; Jacobsen, B.K.; Knutsen, S.F.; Mashchak, A.; Lloren, J.I. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: The Adventist Health Study-2. Cancer Causes Control 2020, 31, 341–351. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Soares, N.; Elias, M.B.; Lima Machado, C.; Trindade, B.B.; Borojevic, R.; Teodoro, A.J. Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines. Foods 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Oczkowski, M.; Dziendzikowska, K.; Pasternak-Winiarska, A.; Włodarek, D.; Gromadzka-Ostrowska, J. Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.A.; Richmond, R.C.; Santos Ferreira, D.L.; Ness, A.R.; May, M.; Smith, G.D.; Vincent, E.E.; Adams, C.; Ala-Korpela, M.; Würtz, P.; et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: The ProDiet randomised controlled trial. Int. J. Cancer 2019, 144, 1918–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksymchuk, O.V.; Kashuba, V.I. Altered expression of cytochrome P450 enzymes involved in metabolism of androgens and vitamin D in the prostate as a risk factor for prostate cancer. Pharmacol. Rep. 2020, 72, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Capiod, T.; Barry Delongchamps, N.; Pigat, N.; Souberbielle, J.C.; Goffin, V. Do dietary calcium and vitamin D matter in men with prostate cancer? Nat. Rev. Urol. 2018, 15, 453–461. [Google Scholar] [CrossRef]
- Grant, W.B. Review of Recent Advances in Understanding the Role of Vitamin D in Reducing Cancer Risk: Breast, Colorectal, Prostate, and Overall Cancer. Anticancer. Res. 2020, 40, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Ardura, J.A.; Álvarez-Carrión, L.; Gutiérrez-Rojas, I.; Alonso, V. Role of Calcium Signaling in Prostate Cancer Progression: Effects on Cancer Hallmarks and Bone Metastatic Mechanisms. Cancers (Basel) 2020, 12, 1071. [Google Scholar] [CrossRef]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Tsugane, S. Why has Japan become the world’s most long-lived country: Insights from a food and nutrition perspective. Eur. J. Clin. Nutr. 2021, 75, 921–928. [Google Scholar] [CrossRef]
- Rogovskii, V.S.; Popov, S.V.; Sturov, N.V.; Shimanovskii, N.L. The Possibility of Preventive and Therapeutic Use of Green Tea Catechins in Prostate Cancer. Anticancer. Agents Med. Chem. 2019, 19, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-Cancer Effects of Green Tea Polyphenols Against Prostate Cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, P.M.P.; Rodrigues, L.; de Alencar Carnib, L.P.; de Lima Sousa, P.V.; Nolasco Lugo, L.M.; Nunes, N.M.F.; do Nascimento Silva, J.; da Silva Araûjo, L.; de Macêdo Gonçalves Frota, K. Cruciferous Vegetables as Antioxidative, Chemopreventive and Antineoplasic Functional Foods: Preclinical and Clinical Evidences of Sulforaphane Against Prostate Cancers. Curr. Pharm. Des. 2018, 24, 4779–4793. [Google Scholar] [CrossRef]
- Gunderson, K.; Wang, C.Y.; Wang, R. Global prostate cancer incidence and the migration, settlement, and admixture history of the Northern Europeans. Cancer Epidemiol. 2011, 35, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheirandish, P.; Chinegwundoh, F. Ethnic differences in prostate cancer. Br. J. Cancer 2011, 105, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011, 7, e1001387. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Devesa, S.S.; Chang, B.-L.; Bunker, C.H.; Cheng, I.; Cooney, K.; Eeles, R.; Fernandez, P.; Giri, V.N.; Gueye, S.M.; et al. Global Patterns of Prostate Cancer Incidence, Aggressiveness, and Mortality in Men of African Descent. Prostate Cancer 2013, 2013, 560857. [Google Scholar] [CrossRef] [Green Version]
- Suuriniemi, M.; Agalliu, I.; Schaid, D.J.; Johanneson, B.; McDonnell, S.K.; Iwasaki, L.; Stanford, J.L.; Ostrander, E.A. Confirmation of a Positive Association between Prostate Cancer Risk and a Locus at Chromosome 8q24. Cancer Epidemiol. Biomark. Prev. 2007, 16, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Okobia, M.N.; Zmuda, J.M.; Ferrell, R.E.; Patrick, A.L.; Bunker, C.H. Chromosome 8q24 variants are associated with prostate cancer risk in a high risk population of African ancestry. Prostate 2011, 71, 1054–1063. [Google Scholar] [CrossRef]
- Freedman, M.L.; Haiman, C.A.; Patterson, N.; McDonald, G.J.; Tandon, A.; Waliszewska, A.; Penney, K.; Steen, R.G.; Ardlie, K.; John, E.M.; et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl. Acad. Sci. USA 2006, 103, 14068–14073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grammatikopoulou, M.G.; Gkiouras, K.; Papageorgiou, S.; Myrogiannis, I.; Mykoniatis, I.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. Dietary Factors and Supplements Influencing Prostate Specific-Antigen (PSA) Concentrations in Men with Prostate Cancer and Increased Cancer Risk: An Evidence Analysis Review Based on Randomized Controlled Trials. Nutrients 2020, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Fujita, K.; Nonomura, N. Influence of Diet and Nutrition on Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 1447. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Gathirua-Mwangi, W.G.; Zhang, J. Dietary factors and risk for advanced prostate cancer. Eur. J. Cancer Prev. 2014, 23, 96–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Allen, J.D.; Arnold, J.T.; Blackman, M.R. Lycopene inhibits IGF-I signal transduction and growth in normal prostate epithelial cells by decreasing DHT-modulated IGF-I production in co-cultured reactive stromal cells. Carcinogenesis 2008, 29, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniyal, M.; Siddiqui, Z.A.; Akram, M.; Asif, H.M.; Sultana, S.; Khan, A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9575–9578. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; McCullough, M.L.; Mondul, A.M.; Jacobs, E.J.; Fakhrabadi-Shokoohi, D.; Giovannucci, E.L.; Thun, M.J.; Calle, E.E. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol. Biomark. Prev. 2003, 12, 597–603. [Google Scholar]
- Sinha, R.; Park, Y.; Graubard, B.I.; Leitzmann, M.F.; Hollenbeck, A.; Schatzkin, A.; Cross, A.J. Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am. J. Epidemiol. 2009, 170, 1165–1177. [Google Scholar] [CrossRef] [Green Version]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Investig. 2007, 117, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Banez, L.L.; Hamilton, R.J.; Partin, A.W.; Vollmer, R.T.; Sun, L.; Rodriguez, C.; Wang, Y.; Terris, M.K.; Aronson, W.J.; Presti, J.C., Jr.; et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 2007, 298, 2275–2280. [Google Scholar] [CrossRef]
- Kaaks, R.; Stattin, P. Obesity, Endogenous Hormone Metabolism, and Prostate Cancer Risk: A Conundrum of “Highs” and “Lows”. Cancer Prev. Res. 2010, 3, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huncharek, M.; Sue Haddock, K.; Reid, R.; Kupelnick, B. Smoking as a risk factor for prostate cancer: A meta-analysis of 24 prospective cohort studies. Am. J. Public Health 2010, 100, 693–701. [Google Scholar] [CrossRef]
- Rohrmann, S.; Genkinger, J.M.; Burke, A.; Helzlsouer, K.J.; Comstock, G.W.; Alberg, A.J.; Platz, E.A. Smoking and Risk of Fatal Prostate Cancer in a Prospective U.S. Study. Urology 2007, 69, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J.; Dong, L.; Amend, S.R.; Cho, Y.-K.; Pienta, K.J. The role of liquid biopsies in prostate cancer management. Lab A Chip 2021, 21, 3263–3288. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Pinto, J.; Amaro, F.; Bastos, M.d.L.; Carvalho, M.; de Pinho, P.G. Advances and Perspectives in Prostate Cancer Biomarker Discovery in the Last 5 Years through Tissue and Urine Metabolomics. Metabolites 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Louie, K.S.; Seigneurin, A.; Cathcart, P.; Sasieni, P. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann. Oncol. 2015, 26, 848–864. [Google Scholar] [CrossRef]
- Das, C.J.; Razik, A.; Sharma, S.; Verma, S. Prostate biopsy: When and how to perform. Clin. Radiol. 2019, 74, 853–864. [Google Scholar] [CrossRef]
- McDunn, J.E.; Li, Z.; Adam, K.P.; Neri, B.P.; Wolfert, R.L.; Milburn, M.V.; Lotan, Y.; Wheeler, T.M. Metabolomic signatures of aggressive prostate cancer. Prostate 2013, 73, 1547–1560. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.L.; Lagana, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.M.; Di Pierro, G.B.; Ricciuti, G.P.; Del Giudice, F.; et al. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Dimakakos, A.; Armakolas, A.; Koutsilieris, M. Novel Tools for Prostate Cancer Prognosis, Diagnosis, and Follow-Up. BioMed. Res. Int. 2014, 2014, 890697. [Google Scholar] [CrossRef]
- Barry, M.J. Prevention of Prostate Cancer Morbidity and Mortality Primary Prevention and Early Detection. Med. Clin. NA 2017, 101, 787–806. [Google Scholar] [CrossRef]
- Pal, R.P.; Maitra, N.U.; Mellon, J.K.; Khan, M.A. Defining prostate cancer risk before prostate biopsy. Urol. Oncol. 2013, 31, 1408–1418. [Google Scholar] [CrossRef]
- Rigau, M.; Olivan, M.; Garcia, M.; Sequeiros, T.; Montes, M.; Colás, E.; Llauradó, M.; Planas, J.; Torres, I.D.; Morote, J.; et al. The present and future of prostate cancer urine biomarkers. Int. J. Mol. Sci. 2013, 14, 12620–12649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.R.; Pinto, J.; Azevedo, A.I.; Barros-Silva, D.; Jerónimo, C.; Henrique, R.; de Lourdes Bastos, M.; de Pinho, P.G.; Carvalho, M. Identification of a biomarker panel for improvement of prostate cancer diagnosis by volatile metabolic profiling of urine. Br. J. Cancer 2019, 121, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Gaglani, S.; Gonzalez-Kozlova, E.; Lundon, D.J.; Tewari, A.K.; Dogra, N.; Kyprianou, N. Exosomes as A Next-Generation Diagnostic and Therapeutic Tool in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 10131. [Google Scholar] [CrossRef]
- Lima, A.R.; Bastos, M.d.L.; Carvalho, M.; Guedes de Pinho, P. Biomarker Discovery in Human Prostate Cancer: An Update in Metabolomics Studies. Transl. Oncol. 2016, 9, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Campos-Fernández, E.; Barcelos, L.S.; de Souza, A.G.; Goulart, L.R.; Alonso-Goulart, V. Research landscape of liquid biopsies in prostate cancer. Am. J. Cancer Res. 2019, 9, 1309–1328. [Google Scholar]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L., III; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Isaacs, C.; Kvale, P.A.; Reding, D.J.; et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J. Natl. Cancer Inst. 2012, 104, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Spur, E.M.; Decelle, E.A.; Cheng, L.L. Metabolomic imaging of prostate cancer with magnetic resonance spectroscopy and mass spectrometry. Eur. J. Nucl. Med. Mol. Imaging 2013, 40 (Suppl. S1), S60–S71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsi, M.; Desai, M.H.; Desai, D.; Singhal, S.; Khandwala, P.M.; Potdar, R.R. PSMA: A game changer in the diagnosis and treatment of advanced prostate cancer. Med. Oncol. 2021, 38, 89. [Google Scholar] [CrossRef]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.A.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA radioligand therapy for solid tumors other than prostate cancer: Background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4350–4368. [Google Scholar] [CrossRef]
- Seifert, R.; Alberts, I.L.; Afshar-Oromieh, A.; Rahbar, K. Prostate Cancer Theranostics: PSMA Targeted Therapy. PET Clin. 2021, 16, 391–396. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Hofman, M.; Violet, J.A.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Akhurst, T.J.; Mooi, J.; et al. Results of a 50 patient single-center phase II prospective trial of Lutetium-177 PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 228. [Google Scholar] [CrossRef]
- Calais, J.; Fendler, W.P.; Eiber, M.; Lassmann, M.; Dahlbom, M.; Esfandiari, R.; Gartmann, J.; Nguyen, K.; Thin, P.; Lok, V.; et al. RESIST-PC phase 2 trial: 177Lu-PSMA-617 radionuclide therapy for metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2019, 37, 5028. [Google Scholar] [CrossRef]
- Moradi, F.; Farolfi, A.; Fanti, S.; Iagaru, A. Prostate cancer: Molecular imaging and MRI. Eur. J. Radiol. 2021, 143, 109893. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Black, P.C.; Rais-Bahrami, S.; Gorin, M.; Allaf, M.; Choyke, P. Novel PET imaging methods for prostate cancer. World J. Urol. 2021, 39, 687–699. [Google Scholar] [CrossRef]
- Thomas, L.; Balmus, C.; Ahmadzadehfar, H.; Essler, M.; Strunk, H.; Bundschuh, R.A. Assessment of Bone Metastases in Patients with Prostate Cancer-A Comparison between (99m)Tc-Bone-Scintigraphy and [(68)Ga]Ga-PSMA PET/CT. Pharmaceuticals (Basel) 2017, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Oey, O.; Ghaffari, M.; Li, J.J.; Hosseini-Beheshti, E. Application of extracellular vesicles in the diagnosis and treatment of prostate cancer: Implications for clinical practice. Crit. Rev. Oncol. Hematol. 2021, 167, 103495. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Rajvansh, R.; Drake, J.M. Emerging Role of Extracellular Vesicles in Prostate Cancer. Endocrinology 2021, 162, bqab139. [Google Scholar] [CrossRef]
- Lorenc, T.; Klimczyk, K.; Michalczewska, I.; Słomka, M.; Kubiak-Tomaszewska, G.; Olejarz, W. Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2118. [Google Scholar] [CrossRef] [Green Version]
- McKiernan, J.; Donovan, M.J.; O’Neill, V.; Bentink, S.; Noerholm, M.; Belzer, S.; Skog, J.; Kattan, M.W.; Partin, A.; Andriole, G.; et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016, 2, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Chen, R.; Zhao, L.; Xu, Y.; Cao, Z.; Xu, H.; Chen, X.; Shi, X.; Zhu, Y.; Lyu, J.; et al. Circulating exosomal mRNA profiling identifies novel signatures for the detection of prostate cancer. Mol. Cancer 2021, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Deng, J.L.; Wang, G.; Zhu, Y.S. Long non-coding RNAs in prostate cancer: Functional roles and clinical implications. Cancer Lett. 2019, 464, 37–55. [Google Scholar] [CrossRef]
- Li, W.; Dou, Z.; We, S.; Zhu, Z.; Pan, D.; Jia, Z.; Liu, H.; Wang, X.; Yu, G. Long noncoding RNA BDNF-AS is associated with clinical outcomes and has functional role in human prostate cancer. Biomed. Pharmacother. 2018, 102, 1105–1110. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, F.; Bei, X.; Wang, X.; Zhu, Y.; Jiang, C.; Zhao, F.; Han, B.; Xia, S. Upregulation of the long non-coding RNA FALEC promotes proliferation and migration of prostate cancer cell lines and predicts prognosis of PCa patients. Prostate 2017, 77, 1107–1117. [Google Scholar] [CrossRef]
- Hu, J.C.; Wang, S.S.; Chou, Y.E.; Chiu, K.Y.; Li, J.R.; Chen, C.S.; Hung, S.C.; Yang, C.K.; Ou, Y.C.; Cheng, C.L.; et al. Associations between LncRNA MALAT1 Polymorphisms and Lymph Node Metastasis in Prostate Cancer. Diagnostics (Basel) 2021, 11, 1692. [Google Scholar] [CrossRef]
- Li, Y.; Ji, J.; Lyu, J.; Jin, X.; He, X.; Mo, S.; Xu, H.; He, J.; Cao, Z.; Chen, X.; et al. A Novel Urine Exosomal lncRNA Assay to Improve the Detection of Prostate Cancer at Initial Biopsy: A Retrospective Multicenter Diagnostic Feasibility Study. Cancers (Basel) 2021, 13, 4075. [Google Scholar] [CrossRef]
- Kidd, S.G.; Carm, K.T.; Bogaard, M.; Olsen, L.G.; Bakken, A.C.; Løvf, M.; Lothe, R.A.; Axcrona, K.; Axcrona, U.; Skotheim, R.I. High expression of SCHLAP1 in primary prostate cancer is an independent predictor of biochemical recurrence, despite substantial heterogeneity. Neoplasia 2021, 23, 634–641. [Google Scholar] [CrossRef]
- Huang, W.; Su, X.; Yan, W.; Kong, Z.; Wang, D.; Huang, Y.; Zhai, Q.; Zhang, X.; Wu, H.; Li, Y.; et al. Overexpression of AR-regulated lncRNA TMPO-AS1 correlates with tumor progression and poor prognosis in prostate cancer. Prostate 2018, 78, 1248–1261. [Google Scholar] [CrossRef]
- Beltran, H.; Demichelis, F. Intrapatient heterogeneity in prostate cancer. Nat. Rev. Urol. 2015, 12, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Dudka, I.; Thysell, E.; Lundquist, K.; Antti, H.; Iglesias-Gato, D.; Flores-Morales, A.; Bergh, A.; Wikström, P.; Gröbner, G. Comprehensive metabolomics analysis of prostate cancer tissue in relation to tumor aggressiveness and TMPRSS2-ERG fusion status. BMC Cancer 2020, 20, 437. [Google Scholar] [CrossRef]
- Coleman, R.E.; Lipton, A.; Roodman, G.D.; Guise, T.A.; Boyce, B.F.; Brufsky, A.M.; Clézardin, P.; Croucher, P.I.; Gralow, J.R.; Hadji, P.; et al. Metastasis and bone loss: Advancing treatment and prevention. Cancer Treat. Rev. 2010, 36, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffioli, L.; Florimonte, L.; Costa, D.; Castanheira Correira, J.; Grana, C.; Luster, M.; Bodei, L.; Chinol, M. New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 420–438. [Google Scholar] [PubMed]
- Du, Y.; Dizdarevic, S. Molecular radiotheragnostics in prostate cancer. Clin. Med. J. R. Coll. Physicians Lond. 2017, 17, 458–461. [Google Scholar] [CrossRef] [Green Version]
- Retter, A.; Gong, F.; Syer, T.; Singh, S.; Adeleke, S.; Punwani, S. Emerging methods for prostate cancer imaging: Evaluating cancer structure and metabolic alterations more clearly. Mol. Oncol. 2021, 15, 2565–2579. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kübler, H.; Beer, A.J.; et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Cursano, M.C.; Iuliani, M.; Casadei, C.; Stellato, M.; Tonini, G.; Paganelli, G.; Santini, D.; De Giorgi, U. Combination radium-223 therapies in patients with bone metastases from castration-resistant prostate cancer: A review. Crit. Rev. Oncol. Hematol. 2020, 146, 102864. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The Rise of PSMA Ligands for Diagnosis and Therapy of Prostate Cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpa, L.; Buxbaum, S.; Kendler, D.; Fink, K.; Bektic, J.; Gruber, L.; Decristoforo, C.; Uprimny, C.; Lukas, P.; Horninger, W.; et al. The (68)Ga/(177)Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: Correlation of SUV(max) values and absorbed dose estimates. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 788–800. [Google Scholar] [CrossRef] [Green Version]
- Morrison, G.J.; Goldkorn, A. Development and Application of Liquid Biopsies in Metastatic Prostate Cancer. Curr. Oncol. Rep. 2018, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, M.; Schalken, J.A.; Salagierski, M. Prostate Cancer Liquid Biopsy Biomarkers’ Clinical Utility in Diagnosis and Prognosis. Cancers 2021, 13, 3373. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Yang, S.H.; Kim, A.; Kim, H.G. RNA-based biomarkers for the diagnosis, prognosis, and therapeutic response monitoring of prostate cancer. Urol. Oncol. 2022, 40, 105.e1–105.e10. [Google Scholar] [CrossRef]
- Kan, Y.; Li, B.; Yang, D.; Liu, Y.; Liu, J.; Yang, C.; Mao, L. Emerging Roles of Long Non-coding RNAs as Novel Biomarkers in the Diagnosis and Prognosis of Prostate Cancer. Discov. Med. 2021, 32, 29–37. [Google Scholar]
- Kretschmer, A.; Tilki, D. Biomarkers in prostate cancer—Current clinical utility and future perspectives. Crit. Rev. Oncol. Hematol. 2017, 120, 180–193. [Google Scholar] [CrossRef]
- Hanjani, N.A.; Esmaelizad, N.; Zanganeh, S.; Gharavi, A.T.; Heidarizadeh, P.; Radfar, M.; Omidi, F.; MacLoughlin, R.; Doroudian, M. Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit. Rev. Oncol. Hematol. 2022, 169, 103565. [Google Scholar] [CrossRef]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020, 145, 102860. [Google Scholar] [CrossRef]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.F.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA J. Am. Med. Assoc. 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Ossoliński, K.; Nizioł, J.; Arendowski, A.; Ossolińska, A.; Ossoliński, T.; Kucharz, J.; Wiechno, P.; Ruman, T. Mass spectrometry-based metabolomic profiling of prostate cancer—A pilot study. J. Cancer Metastasis Treat. 2019, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- de Cobelli, O.; Terracciano, D.; Tagliabue, E.; Raimondi, S.; Galasso, G.; Cioffi, A.; Cordima, G.; Musi, G.; Damiano, R.; Cantiello, F.; et al. Body mass index was associated with upstaging and upgrading in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Urol. Oncol. 2015, 33, 201.e1–208.e8. [Google Scholar] [CrossRef] [Green Version]
- Ferro, M.; Lucarelli, G.; Bruzzese, D.; Di Lorenzo, G.; Perdonà, S.; Autorino, R.; Cantiello, F.; La Rocca, R.; Busetto, G.M.; Cimmino, A.; et al. Low serum total testosterone level as a predictor of upstaging and upgrading in low-risk prostate cancer patients meeting the inclusion criteria for active surveillance. Oncotarget 2017, 8, 18424–18434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. A European Model for an Organised Risk-stratified Early Detection Programme for Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Yusim, I.; Krenawi, M.; Mazor, E.; Novack, V.; Mabjeesh, N.J. The use of prostate specific antigen density to predict clinically significant prostate cancer. Sci. Rep. 2020, 10, 20015. [Google Scholar] [CrossRef]
- Omri, N.; Kamil, M.; Alexander, K.; Alexander, K.; Edmond, S.; Ariel, Z.; David, K.; Gilad, A.E.; Azik, H. Association between PSA density and pathologically significant prostate cancer: The impact of prostate volume. Prostate 2020, 80, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Drost, F.J.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 6, CD012663. [Google Scholar] [CrossRef]
- Gravina, M.; Spirito, L.; Celentano, G.; Capece, M.; Creta, M.; Califano, G.; Collà Ruvolo, C.; Morra, S.; Imbriaco, M.; Di Bello, F.; et al. Machine Learning and Clinical-Radiological Characteristics for the Classification of Prostate Cancer in PI-RADS 3 Lesions. Diagnostics (Basel) 2022, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Belue, M.J.; Turkbey, B. Tasks for artificial intelligence in prostate MRI. Eur. Radiol. Exp. 2022, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, M.R.S.; Saha, A.; Hosseinzadeh, M.; Elschot, M.; Huisman, H. Artificial intelligence for prostate MRI: Open datasets, available applications, and grand challenges. Eur. Radiol. Exp. 2022, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, C.V.; Pereira, F.; Pereira, J.A.M.; Câmara, J.S. Volatilomics: An Emerging and Promising Avenue for the Detection of Potential Prostate Cancer Biomarkers. Cancers 2022, 14, 3982. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Breast Cancer Metabolomics: From Analytical Platforms to Multivariate Data Analysis. A Review. Metabolites 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Lee, W.Y. Urinary metabolites for urological cancer detection: A review on the application of volatile organic compounds for cancers. Am. J. Clin. Exp. Urol. 2019, 7, 232–248. [Google Scholar] [PubMed]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Al-Kateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef] [Green Version]
- Struck-Lewicka, W.; Kordalewska, M.; Bujak, R.; Yumba Mpanga, A.; Markuszewski, M.; Jacyna, J.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Urine metabolic fingerprinting using LC-MS and GC-MS reveals metabolite changes in prostate cancer: A pilot study. J. Pharm. Biomed. Anal. 2015, 111, 351–361. [Google Scholar] [CrossRef]
- Gao, Q.; Su, X.; Annabi, M.H.; Schreiter, B.R.; Prince, T.; Ackerman, A.; Morgas, S.; Mata, V.; Williams, H.; Lee, W.-Y. Application of Urinary Volatile Organic Compounds (VOCs) for the Diagnosis of Prostate Cancer. Clin. Genitourin. Cancer 2019, 17, 183–190. [Google Scholar] [CrossRef]
- Bax, C.; Taverna, G.; Eusebio, L.; Sironi, S.; Grizzi, F.; Guazzoni, G.; Capelli, L. Innovative Diagnostic Methods for Early Prostate Cancer Detection through Urine Analysis: A Review. Cancers (Basel) 2018, 10, 123. [Google Scholar] [CrossRef] [Green Version]
- da Costa, B.R.B.; De Martinis, B.S. Analysis of urinary VOCs using mass spectrometric methods to diagnose cancer: A review. Clin. Mass Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, H.; Daulton, E.; Bannaga, A.S.; Arasaradnam, R.P.; Covington, J.A. Urinary Volatiles and Chemical Characterisation for the Non-Invasive Detection of Prostate and Bladder Cancers. Biosensors (Basel) 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Pinto, J.; Carvalho-Maia, C.; Jerónimo, C.; Henrique, R.; Bastos, M.d.L.; Carvalho, M.; Guedes de Pinho, P. A Panel of Urinary Volatile Biomarkers for Differential Diagnosis of Prostate Cancer from Other Urological Cancers. Cancers 2020, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Taverna, G.; Bellini, A.; Eusebio, L.; Buffi, N.; Lazzeri, M.; Guazzoni, G.; Bozzini, G.; Seveso, M.; Mandressi, A.; et al. Application and Uses of Electronic Noses for Clinical Diagnosis on Urine Samples: A Review. Sensors (Basel) 2016, 16, 1708. [Google Scholar] [CrossRef] [PubMed]




| Risk Factor | Role in PCa | Reference |
|---|---|---|
| Ethnicity | PCa incidence, morbidity, and mortality rates vary significantly by race and ethnicity. African-American, Black, and Caribbean men show the highest PCa rates worldwide. These disparities are mostly related to differences in access to screening and treatment, exposure to PCa risk factors, and variations in genomic susceptibility (e.g., risk loci found at chromosome 8q24), among other biological factors. | [15,16,17,18,19,20] |
| Family history and genetic factors | According to estimates, around 5 to 15% of PCa cases have been related to hereditary factors. In genome-wide association studies, almost 170 loci of susceptibility for hereditary PCa (about 33% of familial PCa risks) have been identified. Many genes show a strong association with hereditary PCa risk, including BRCA1, BRCA2, ATM, CHEK2, and PALB2, and Lynch syndrome MLH1, MSH2, MSH6, and PMS2 genes. Other genes, however, have an unclear cancer risk and unknown clinical importance. | [4,20,21,22,23,24,25,26] |
| Obesity, overweight and physical inactivity | Obesity is implicated in the dysregulation of various hormonal pathways, leading to higher levels of insulin and IGF, oxidative stress, and inflammatory cytokines, and lower levels of adiponectin, testosterone, and sex hormone-binding globulin. Obesity is associated with an increased risk of PCa mortality and recurrence, worsened treatment-related adverse effects, development of obesity-related comorbidities, and the earlier progression and development of metastatic disease. Nevertheless, the physiological mechanisms associated between obesity and poor PCa outcomes remain unknown. | [3,27,28,29,30,31,32,33] |
| Tobacco use | Smoking increases the risk of death from PCa, which increases with obesity, specifically for advanced PCa. Moreover, tobacco smoking increases the risk of biochemical recurrence and metastasis. Nevertheless, the association between tobacco smoking and PCa prognosis needs to be explored. | [3,32,34,35,36,37,38] |
| Lycopene and tomato-based products | Epidemiologic studies have focused on tomatoes as a specific source of lycopene, with more consistent findings supporting the protective effect of a higher intake of tomatoes on PCa risk. Furthermore, studies have shown a reduced risk of advanced PCa with the consumption of cooked tomatoes, since these products have more available lycopene. Current epidemiologic evidence is not definitive but suggests that a higher intake of tomato-based products is associated with a reduced risk of PCa and a potentially lower risk of progression. Further studies are required to determine whether the effect is because of lycopene or other components of tomatoes. | [3,32,39,40,41,42,43,44] |
| Calcium, dairy products, and vitamin D | An intake of dairy products above the daily recommended dose has been positively associated with PCa risk. A potential mechanism underlying the association with calcium is through suppressing circulating levels of dihydroxyvitamin D, which seems to have a protective effect against PCa. The mechanisms behind this association are not yet fully understood, but researchers suggest reducing dairy intake while increasing the consumption of fish and tomato products for PCa prevention. | [3,32,45,46,47,48] |
| Cruciferous, soy, and green tea | Cruciferous, soy, and green tea seem to have a role in decreasing the risk of PCa due to compounds with anticarcinogenic properties in their composition. Asian populations consume soy foods as a part of their regular diet, which might contribute to the lower PCa incidence found in these countries. However, the preventive action of these compounds needs to be further explored. | [32,43,49,50,51,52,53,54] |
| Method | Evidence/Aim | Reference |
|---|---|---|
| PSMA radioligand targeted therapy and molecular imaging | Evidence: Molecular imaging techniques detect PCa lesions that are occult on anatomic imaging. PSMA radioligand therapy shows promising response rates with low toxicity in extensively pre-treated patients with PCa. Aim: Theragnostic applications—diagnosis, management, and treatment of metastatic PCa. | [92,93,94,95,96,97,98,99,100] |
| EVs | Evidence: EVs can mediate PCa progression and metastasis. EVs have great potential to be used as liquid biopsy biomarkers in the diagnosis of PCa. EVs can be used in risk stratification and to predict the response to hormonal, chemo-, immune- and targeted therapy. Aim: Diagnosis and treatment. Can be used to personalize and guide treatment decisions. | [76,87,89,101,102,103,104,105] |
| lncRNAs (PCA3, MALAT1, SChLAP1, BDNF-AS, FALEC) | Evidence: lncRNAs provide new insights into cancer signaling networks, along with novel strategies and methods for PCa diagnosis and treatment. lncRNAs analysis has the potential to improve the specificity and sensitivity of existing biomarkers. Aim: Novel biomarkers (predictive, diagnostic, prognostic) and therapeutic targets. | [106,107,108,109,110,111,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300-2321. https://doi.org/10.3390/curroncol30020178
Berenguer CV, Pereira F, Câmara JS, Pereira JAM. Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Current Oncology. 2023; 30(2):2300-2321. https://doi.org/10.3390/curroncol30020178
Chicago/Turabian StyleBerenguer, Cristina V., Ferdinando Pereira, José S. Câmara, and Jorge A. M. Pereira. 2023. "Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis" Current Oncology 30, no. 2: 2300-2321. https://doi.org/10.3390/curroncol30020178
APA StyleBerenguer, C. V., Pereira, F., Câmara, J. S., & Pereira, J. A. M. (2023). Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Current Oncology, 30(2), 2300-2321. https://doi.org/10.3390/curroncol30020178






