Direct Comparison of Two Different Definitions with Biochemical Recurrence after Low-Dose-Rate Brachytherapy for Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment
2.3. Post-Dosimetric Evaluation
2.4. Follow-Up Schedule
2.5. Statistical Analysis
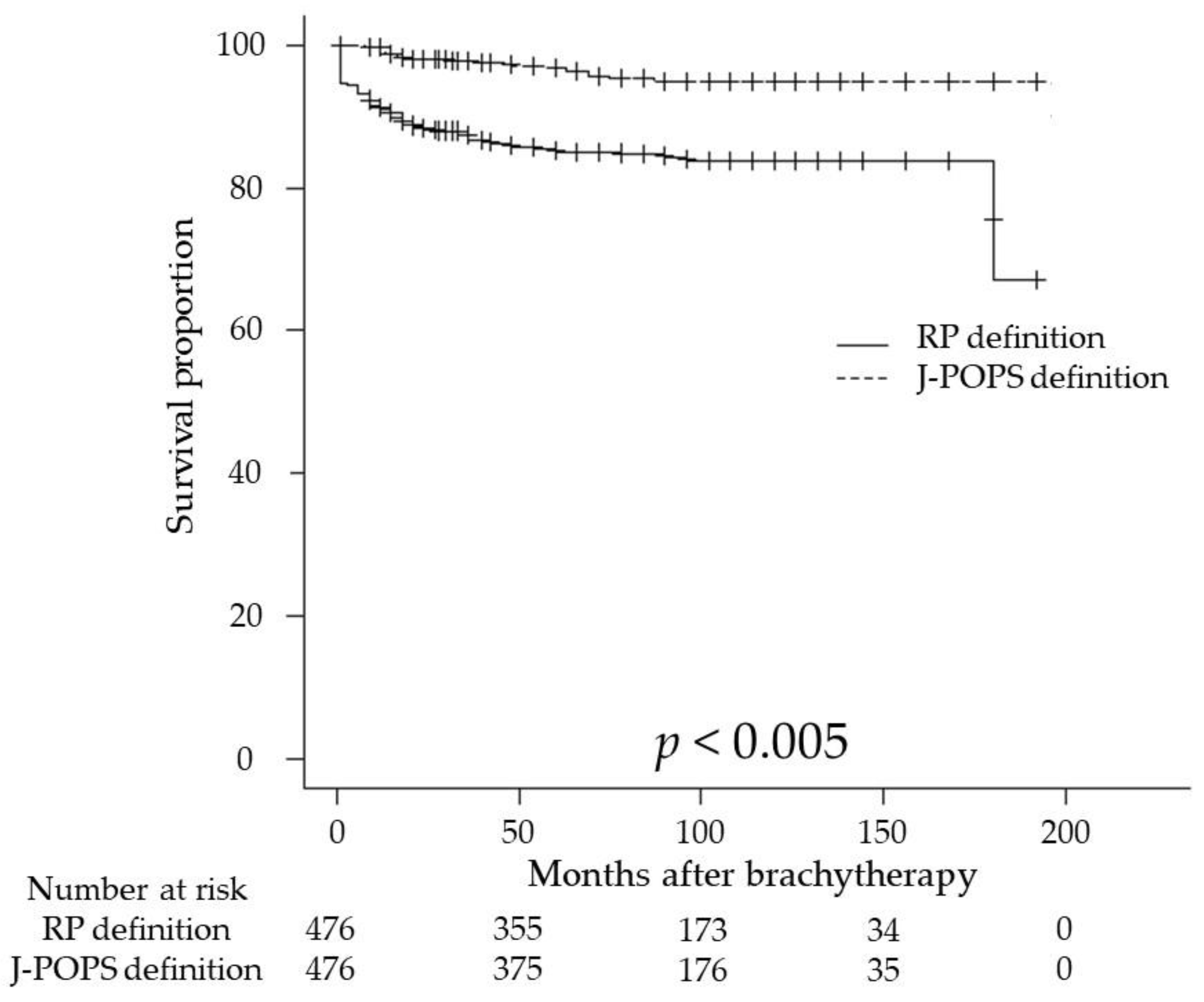
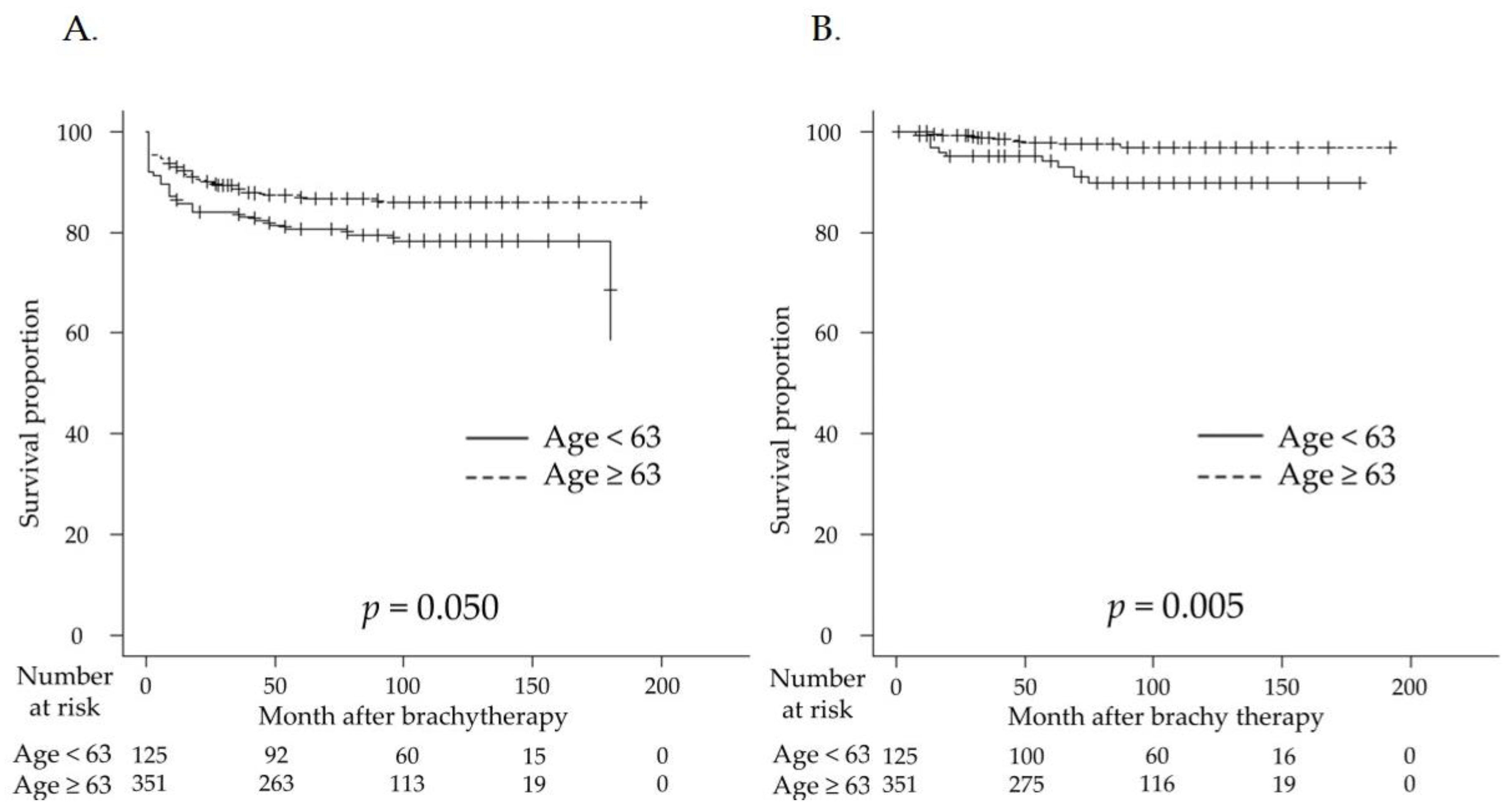
3. Results
3.1. Patient Characteristics
3.2. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer (2022) NCCN guidelines®. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. (accessed on 27 December 2022).
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.; Humphrey, P.A.; Committee, G. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ash, D.; Flynn, A.; Battermann, J.; de Reijke, T.; Lavagnini, P.; Blank, L. ESTRO/EAU/EORTC recommendations on permanent seed implantation for localized prostate cancer. Radiother. Oncol. 2000, 57, 315–321. [Google Scholar] [CrossRef]
- Oh, J.; Morris, W.J.; Spadinger, I.; Tyldesley, S.; Keyes, M.; Halperin, R.; Crook, J.; Lapointe, V.; Pickles, T. After ASCENDE-RT: Biochemical and survival outcomes following combined external beam radiotherapy and low-dose-rate brachytherapy for high-risk and unfavourable intermediate-risk prostate cancer, a population-based analysis. Brachytherapy 2022, 21, 605–616. [Google Scholar] [CrossRef]
- Iinuma, K.; Nakano, M.; Kato, T.; Kato, D.; Takai, M.; Maekawa, Y.M.; Nakane, K.; Mizutani, K.; Tsuchiya, T.; Ishihara, T.; et al. Assessment of Long-term Changes in Lower Urinary Tract Symptoms in Patients with Prostate Cancer Who Underwent Low-dose-rate Prostate Brachytherapy. Urology 2020, 142, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Iinuma, K.; Nakano, M.; Kawase, M.; Kato, D.; Kawase, K.; Takai, M.; Nakane, K.; Ito, M.; Kumano, T.; et al. Patient age as a predictive factor in biochemical recurrence following brachytherapy: Oncological outcomes at a single center. Prostate Int. 2022, 10, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Tanaka, N.; Oguchi, T.; Owari, T.; Nakai, Y.; Asakawa, I.; Iijima, K.; Kato, H.; Hashida, I.; Tabata, K.I.; et al. Direct comparison of low-dose-rate brachytherapy versus radical prostatectomy using the surgical definition of biochemical recurrence for patients with intermediate-risk prostate cancer. Radiat. Oncol. 2022, 17, 71. [Google Scholar] [CrossRef]
- Eccles, B.K.; Cross, W.; Rosario, D.J.; Doble, A.; Parker, C.; Logue, J.; Little, L.; Stanton, L.; Bottomley, D. SABRE 1 (Surgery Against Brachytherapy—A Randomised Evaluation): Feasibility randomised controlled trial (RCT) of brachytherapy vs radical prostatectomy in low-intermediate risk clinically localised prostate cancer. BJU Int. 2013, 112, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [PubMed]
- Thompson, I.M.; Valicenti, R.K.; Albertsen, P.; Davis, B.J.; Goldenberg, S.L.; Hahn, C.; Klein, E.; Michalski, J.; Roach, M.; Sartor, O.; et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J. Urol. 2013, 190, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.J.; Pickles, T.; Keyes, M.; McKenzie, M.; Spadinger, I. Pride or prejudice: Does Phoenix flatter radiation therapy? Brachytherapy 2014, 13, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.J.; Pickles, T.; Keyes, M. Using a surgical prostate-specific antigen threshold of >0.2 ng/mL to define biochemical failure for intermediate- and high-risk prostate cancer patients treated with definitive radiation therapy in the ASCENDE-RT randomized control trial. Brachytherapy 2018, 17, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Saito, S.; Yorozu, A.; Kojima, S.; Kikuchi, T.; Higashide, S.; Aoki, M.; Koga, H.; Satoh, T.; Ohashi, T.; et al. Nationwide Japanese Prostate Cancer Outcome Study of Permanent Iodine-125 Seed Implantation (J-POPS): First analysis on survival. Int. J. Clin. Oncol. 2018, 23, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Buyyounouski, M.K.; Choyke, P.L.; McKenney, J.K.; Sartor, O.; Howard, M.; Sandler, H.W.; Amin, M.B.; Kattan, M.W.; Lin, D.W. Prostate cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lester-Coll, N.H.; Goldhaber, S.Z.; Sher, D.J.; D’Amico, A.V. Death from high-risk prostate cancer versus cardiovascular mortality with hormone therapy. Cancer 2013, 119, 1808–1815. [Google Scholar] [CrossRef]
- Taniguchi, T.; Iinuma, K.; Kato, D.; Takai, M.; Maekawa, Y.M.; Nakane, K.; Mizutani, K.; Tsuchiya, T.; Nakano, M.; Kato, T.; et al. Predictive factors of rectal hemorrhage in patients with localized prostate cancer who underwent low-dose-rate brachytherapy. Int. J. Clin. Oncol. 2020, 25, 1711–1717. [Google Scholar] [CrossRef]
- Stone, N.N.; Gerber, N.K.; Blacksburg, S.; Stone, J.; Stock, R.G. Factors influencing urinary symptoms 10 years after permanent prostate seed implantation. J. Urol. 2012, 187, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Yorozu, A.; Toya, K.; Saito, S.; Momma, T.; Nagata, H.; Nagata, H.; Kosugi, M.; Shigematsu, N.; Kubo, A. Comparison of intraoperative ultrasound with postimplant computed tomography--dosimetric values at Day 1 and Day 30 after prostate brachytherapy. Brachytherapy 2007, 6, 246–253. [Google Scholar] [CrossRef]
- Tanaka, O.; Hayashi, S.; Matsuo, M.; Nakano, M.; Uno, H.; Ohtakara, K.; Miyoshi, T.; Deguchi, T.; Hoshi, H. Effect of edema on postimplant dosimetry in prostate brachytherapy using CT/MRI fusion. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 614–618. [Google Scholar] [CrossRef]
- Lo, A.C.; Morris, W.J.; Lapointe, V.; Hamm, J.; Keyes, M.; Pickles, T.; McKenzie, M.; Spadinger, I. Prostate-specific antigen at 4 to 5 years after low-dose-rate prostate brachytherapy is a strong predictor of disease-free survival. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [PubMed]
- Zaorsky, N.G.; Palmer, J.D.; Hurwitz, M.D.; Keith, S.W.; Dicker, A.P.; Den, R.B. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother. Oncol. 2015, 115, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gul, Z.G.; Say, R.; Skouteris, V.M.; Stock, R.G.; Stone, N.N. Comparison of AUA and phoenix definitions of biochemical failure following permanent brachytherapy for prostate cancer. Brachytherapy 2022, 21, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Kindts, I.; Stellamans, K.; Billiet, I.; Pottel, H.; Lambrecht, A. 125I brachytherapy in younger prostate cancer patients: Outcomes in low- and intermediate-risk disease. Strahlenther. Onkol. 2017, 193, 707–713. [Google Scholar] [CrossRef] [PubMed]
| Age (year, median, IQR) | 66 (50–81) |
| PSA (ng/mL, median, IQR) | 6.44 (1.7–60.8) |
| Clinical T stage (number, %) | |
| T1c | 252 (52.9) |
| T2a | 136 (28.6) |
| T2b | 29 (6.1) |
| T2c | 47 (9.9) |
| T3a | 11 (2.3) |
| T3b | 1 (0.2) |
| Gleason Group Grade (number, %) | 2 (1–5) |
| 1 | 199 (41.8) |
| 2 | 166 (34.9) |
| 3 | 67 (14.1) |
| 4 | 28 (5.9) |
| 5 | 16 (3.3) |
| NCCN risk classification (number, %) | |
| Low | 169 (35.5) |
| Intermediate | 248 (52.1) |
| High | 59 (12.4) |
| Prostate volume at LDR-BT (mL, median, IQR) | 23.4 (13.8–53.0) |
| Neoadjuvant ADT (number, %) | 369 (77.5) |
| Follow-up period (month, median, IQR) | 84 (1–216) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, S.; Iinuma, K.; Nakane, K.; Nakano, M.; Kawase, M.; Kawase, K.; Takai, M.; Kato, D.; Mori, T.; Takano, H.; et al. Direct Comparison of Two Different Definitions with Biochemical Recurrence after Low-Dose-Rate Brachytherapy for Prostate Cancer. Curr. Oncol. 2023, 30, 2792-2800. https://doi.org/10.3390/curroncol30030212
Takeuchi S, Iinuma K, Nakane K, Nakano M, Kawase M, Kawase K, Takai M, Kato D, Mori T, Takano H, et al. Direct Comparison of Two Different Definitions with Biochemical Recurrence after Low-Dose-Rate Brachytherapy for Prostate Cancer. Current Oncology. 2023; 30(3):2792-2800. https://doi.org/10.3390/curroncol30030212
Chicago/Turabian StyleTakeuchi, Shinichi, Koji Iinuma, Keita Nakane, Masahiro Nakano, Makoto Kawase, Kota Kawase, Manabu Takai, Daiki Kato, Takayuki Mori, Hirota Takano, and et al. 2023. "Direct Comparison of Two Different Definitions with Biochemical Recurrence after Low-Dose-Rate Brachytherapy for Prostate Cancer" Current Oncology 30, no. 3: 2792-2800. https://doi.org/10.3390/curroncol30030212
APA StyleTakeuchi, S., Iinuma, K., Nakane, K., Nakano, M., Kawase, M., Kawase, K., Takai, M., Kato, D., Mori, T., Takano, H., Kumano, T., Matsuo, M., & Koie, T. (2023). Direct Comparison of Two Different Definitions with Biochemical Recurrence after Low-Dose-Rate Brachytherapy for Prostate Cancer. Current Oncology, 30(3), 2792-2800. https://doi.org/10.3390/curroncol30030212






