The Potential of Adding Mammography to Handheld Ultrasound or Automated Breast Ultrasound to Reduce Unnecessary Biopsies in BI-RADS Ultrasound Category 4a: A Multicenter Hospital-Based Study in China
Abstract
1. Introduction
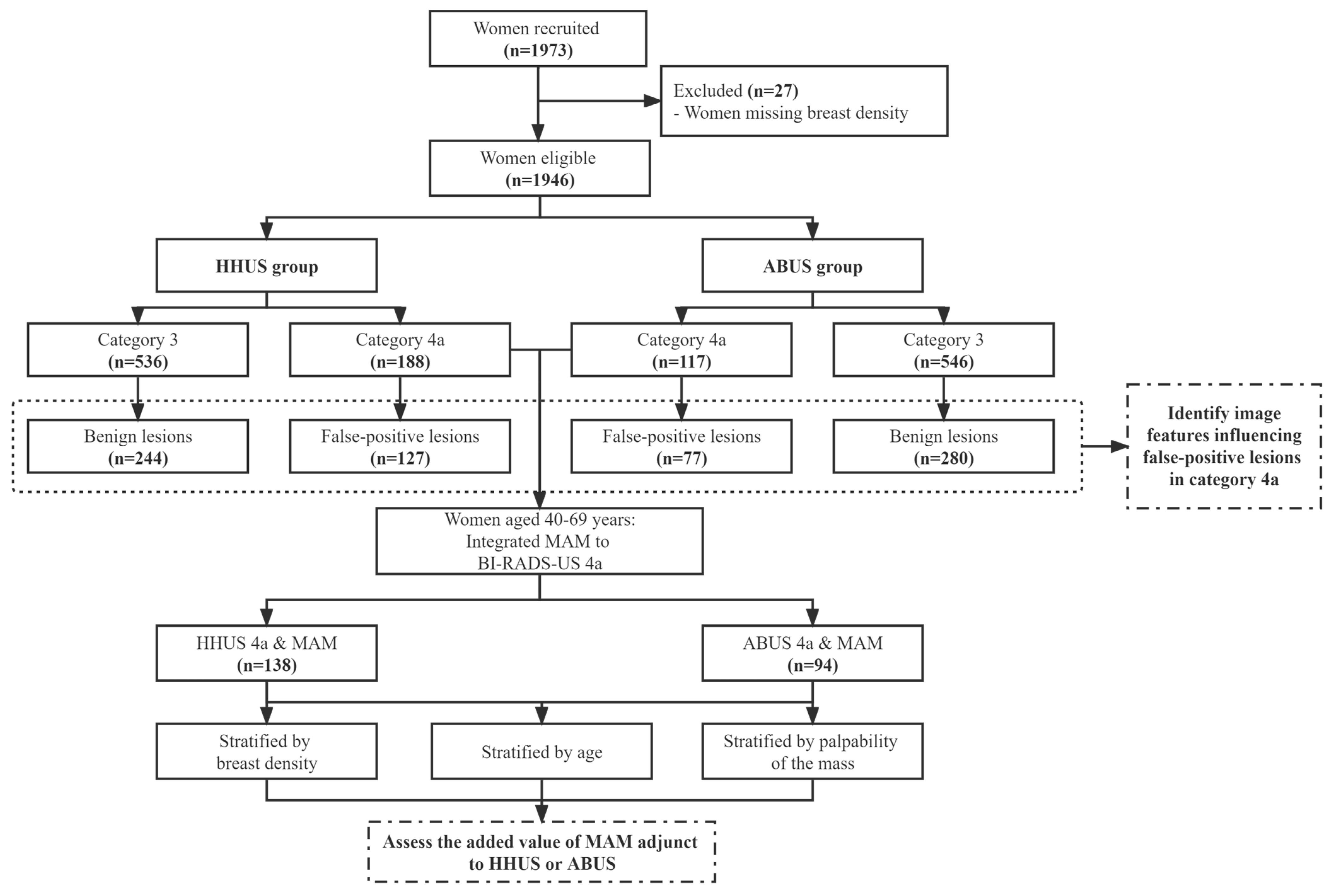
2. Materials and Methods
2.1. Study Population and Design
2.2. Image Acquisition and Interpretation
2.3. Statistical Analysis
3. Results
3.1. Distribution of Benign and Malignant Lesions According to BI-RADS-US Category
3.2. Clinical and Imaging Factors Associated with False-Positive Lesions in Category 4a
3.3. Diagnostic Performance of Adding MAM to HHUS or ABUS
3.4. Value of Adding MAM to HHUS or ABUS in Reducing Unnecessary Biopsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Tohno, E.; Umemoto, T.; Sasaki, K.; Morishima, I.; Ueno, E. Effect of adding screening ultrasonography to screening mammography on patient recall and cancer detection rates: A retrospective study in Japan. Eur. J. Radiol. 2013, 82, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sarma, E.A. Barriers to screening mammography. Health Psychol. Rev. 2015, 9, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Ding, L.; Liang, X.; Wang, Y.; Greuter, M.J.W.; de Bock, G.H.; Lu, W. Is Ultrasound an Accurate Alternative for Mammography in Breast Cancer Screening in an Asian Population? A Meta-Analysis. Diagnostics 2020, 10, 985. [Google Scholar] [CrossRef]
- Zanotel, M.; Bednarova, I.; Londero, V.; Linda, A.; Lorenzon, M.; Girometti, R.; Zuiani, C. Automated breast ultrasound: Basic principles and emerging clinical applications. Radiol. Med. 2018, 123, 1–12. [Google Scholar] [CrossRef]
- Breast Imaging Reporting and Data System, the 5th Version. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed on 26 June 2022).
- Gao, L.; Li, J.; Gu, Y.; Ma, L.; Xu, W.; Tao, X.; Wang, R.; Zhang, R.; Zhang, Y.; Wang, H.; et al. Breast ultrasound in Chinese hospitals: A cross-sectional study of the current status and influencing factors of BI-RADS utilization and diagnostic accuracy. Lancet Reg. Health West. Pac. 2022, 29, 100576. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, Y.; Chai, W.; Zong, S.; Xu, S.; Zhan, W.; Zhang, X. Downgrade BI-RADS 4A Patients Using Nomogram Based on Breast Magnetic Resonance Imaging, Ultrasound, and Mammography. Front. Oncol. 2022, 12, 807402. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, B.K.; Ko, E.Y.; Ko, E.S.; Shin, J.H.; Kim, G.R. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur. Radiol. 2016, 26, 3542–3549. [Google Scholar] [CrossRef]
- Brewer, N.T.; Salz, T.; Lillie, S.E. Systematic review: The long-term effects of false-positive mammograms. Ann. Intern. Med. 2007, 146, 502–510. [Google Scholar] [CrossRef]
- Zagouri, F.; Sergentanis, T.N.; Gounaris, A.; Koulocheri, D.; Nonni, A.; Domeyer, P.; Fotiadis, C.; Bramis, J.; Zografos, G.C. Pain in different methods of breast biopsy: Emphasis on vacuum-assisted breast biopsy. Breast 2008, 17, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Yazici, B.; Sever, A.R.; Mills, P.; Fish, D.; Jones, S.E.; Jones, P.A. Scar formation after stereotactic vacuum-assisted core biopsy of benign breast lesions. Clin. Imaging 2006, 61, 619–624. [Google Scholar]
- Covington, M.F. Ultrasound Elastography May Better Characterize BI-RADS 3 and BI-RADS 4A Lesions to Decrease False-Positive Breast Biopsy Rates and Enable Earlier Detection of Breast Cancer. J. Am. Coll. Radiol. 2022, 19, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Golatta, M.; Pfob, A.; Busch, C.; Bruckner, T.; Alwafai, Z.; Balleyguier, C.; Clevert, D.A.; Duda, V.; Goncalo, M.; Gruber, I.; et al. The potential of combined shear wave and strain elastography to reduce unnecessary biopsies in breast cancer diagnostics—An international, multicentre trial. Eur. J. Cancer 2022, 161, 1–9. [Google Scholar] [CrossRef]
- Choi, E.J.; Choi, H.; Park, E.H.; Song, J.S.; Youk, J.H. Evaluation of an automated breast volume scanner according to the fifth edition of BI-RADS for breast ultrasound compared with hand-held ultrasound. Eur. J. Radiol. 2018, 99, 138–145. [Google Scholar] [CrossRef]
- Nyante, S.J.; Lee, S.S.; Benefield, T.S.; Hoots, T.N.; Henderson, L.M. The association between mammographic calcifications and breast cancer prognostic factors in a population-based registry cohort. Cancer 2017, 123, 219–227. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; Tan, Y.; Zhu, Y.; Wang, H.; Feng, R.; Tang, G.; Zhou, X.; Li, A.; Qiao, Y. A multicenter hospital-based diagnosis study of automated breast ultrasound system in detecting breast cancer among Chinese women. Chin. J. Cancer Res. 2018, 30, 231–239. [Google Scholar] [CrossRef]
- Berg, W.A.; Bandos, A.I.; Mendelson, E.B.; Lehrer, D.; Jong, R.A.; Pisano, E.D. Ultrasound as the Primary Screening Test for Breast Cancer: Analysis From ACRIN 6666. J. Natl. Cancer Inst. 2016, 108, djv367. [Google Scholar] [CrossRef]
- Elverici, E.; Barca, A.N.; Aktas, H.; Ozsoy, A.; Zengin, B.; Cavusoglu, M.; Araz, L. Nonpalpable BI-RADS 4 breast lesions: Sonographic findings and pathology correlation. Diagn. Interv. Radiol. 2015, 21, 189–194. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Diao, X.H.; Fang, L.; Pang, Y.; Cheng, A.Q.; Li, W.P.; Wang, Y. Comparative study of automated breast 3-D ultrasound and handheld B-mode ultrasound for differentiation of benign and malignant breast masses. Ultrasound Med. Biol. 2013, 39, 1735–1742. [Google Scholar] [CrossRef]
- Lin, X.; Wang, J.; Han, F.; Fu, J.; Li, A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur. J. Radiol. 2012, 81, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Gristina, L.; Tosto, S.; Massone, E.; De Giorgis, S.; Garlaschi, A.; Tagliafico, A.; Calabrese, M. The value of coronal view as a stand-alone assessment in women undergoing automated breast ultrasound. Radiol. Med. 2021, 126, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jia, M.; Zhou, X.; Bao, L.; Chen, Y.; Liu, P.; Feng, R.; Zhang, X.; Zhu, L.; Wang, H.; et al. The diagnostic performance of automated versus handheld breast ultrasound and mammography in symptomatic outpatient women: A multicenter, cross-sectional study in China. Eur. Radiol. 2021, 31, 947–957. [Google Scholar] [CrossRef]
- Jia, M.; Lin, X.; Zhou, X.; Yan, H.; Chen, Y.; Liu, P.; Bao, L.; Li, A.; Basu, P.; Qiao, Y.; et al. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res. Treat. 2020, 181, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Lee, E.H.; Kim, Y.M.; Chang, Y.W.; Lee, J.H.; Park, Y.M.; Kim, K.W.; Kim, Y.J.; Jun, J.K.; Hong, S.; et al. Interobserver agreement in breast ultrasound categorization in the Mammography and Ultrasonography Study for Breast Cancer Screening Effectiveness (MUST-BE) trial: Results of a preliminary study. Ultrasonography 2019, 38, 172–180. [Google Scholar] [CrossRef]
- Song, S.E.; Yie, A.; Seo, B.K.; Lee, S.H.; Cho, K.R.; Woo, O.H.; Lee, K.Y.; Kim, Y.S. A prospective study about abnormal ductal dilatations without associated masses on breast US: What is the significance for us? Acad. Radiol. 2012, 19, 296–302. [Google Scholar] [CrossRef]
- Raza, S.; Goldkamp, A.L.; Chikarmane, S.A.; Birdwell, R.L. US of breast masses categorized as BI-RADS 3, 4, and 5: Pictorial review of factors influencing clinical management. Radiographics 2010, 30, 1199–1213. [Google Scholar] [CrossRef]
- Hooley, R.J.; Scoutt, L.M.; Philpotts, L.E. Breast ultrasonography: State of the art. Radiology 2013, 268, 642–659. [Google Scholar] [CrossRef]
- Ouyang, Y.L.; Zhou, Z.H.; Wu, W.W.; Tian, J.; Xu, F.; Wu, S.C.; Tsui, P.H. A review of ultrasound detection methods for breast microcalcification. Math. Biosci. Eng. 2019, 16, 1761–1785. [Google Scholar] [CrossRef]
- Ma, L.; Lian, Z.; Zhao, Y.; Di, J.; Song, B.; Ren, W.; Miao, Z.; Wu, J.; Wang, Q. Breast ultrasound optimization process analysis based on breast cancer screening for 1 501 753 rural women in China. Zhonghua Zhong Liu Za Zhi 2021, 43, 497–503. [Google Scholar]
- Dickerson, L.K.; Rositch, A.F.; Lucas, S.; Harvey, S.C. Pilot Educational Intervention and Feasibility Assessment of Breast Ultrasound in Rural South Africa. J. Glob. Oncol. 2017, 3, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Rositch, A.F.; Shakoor, D.; Ambinder, E.; Pool, K.-L.; Pollack, E.; Mollura, D.J.; Mullen, L.A.; Harvey, S.C. Ultrasound for Breast Cancer Detection Globally: A Systematic Review and Meta-Analysis. J. Glob. Oncol. 2019, 5, 1–17. [Google Scholar]
- Stewart, K.A.; Navarro, S.M.; Kambala, S.; Tan, G.; Poondla, R.; Lederman, S.; Barbour, K.; Lavy, C. Trends in Ultrasound Use in Low and Middle Income Countries: A Systematic Review. Int. J. MCH AIDS 2020, 9, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.K.; Neal, C.H.; Jeffries, D.O.; Joe, A.; Klein, K.; Bailey, J.; Pinsky, R.; Paramagul, C.; Watcharotone, K. Outcomes of solid palpable masses assessed as BI-RADS 3 or 4A: A retrospective review. Breast Cancer Res. Treat. 2014, 147, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; Moonka, R. Normal mammography and ultrasonography in the setting of palpable breast cancer. Am. J. Surg. 2003, 185, 416–419. [Google Scholar] [CrossRef]
- Posso, M.; Alcantara, R.; Vazquez, I.; Comerma, L.; Bare, M.; Louro, J.; Quintana, M.J.; Roman, M.; Marcos-Gragera, R.; Vernet-Tomas, M.; et al. Mammographic features of benign breast lesions and risk of subsequent breast cancer in women attending breast cancer screening. Eur. Radiol. 2022, 32, 621–629. [Google Scholar] [CrossRef]
- Vourtsis, A.; Kachulis, A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1,886 women. Eur. Radiol. 2018, 28, 592–601. [Google Scholar] [CrossRef]
- Roman, M.; Hofvind, S.; von Euler-Chelpin, M.; Castells, X. Long-term risk of screen-detected and interval breast cancer after false-positive results at mammography screening: Joint analysis of three national cohorts. Br. J. Cancer 2019, 120, 269–275. [Google Scholar] [CrossRef]
- Hofvind, S.; Sagstad, S.; Sebuodegard, S.; Chen, Y.; Roman, M.; Lee, C.I. Interval Breast Cancer Rates and Histopathologic Tumor Characteristics after False-Positive Findings at Mammography in a Population-based Screening Program. Radiology 2018, 287, 58–67. [Google Scholar] [CrossRef]
- Castells, X.; Torá-Rocamora., I.; Posso., M.; Román., M.; Vernet-Tomas., M.; Rodríguez-Arana., A.; Domingo., L.; Vidal., C.; Baré., M.; Ferrer., J.; et al. Rrisk of Breast cancer in Women with False-Positive results according to Mammographic Features. Radiology 2016, 280, 379–386. [Google Scholar] [CrossRef]
| BI-RADS US Category | Total (N, %) * | Normal/Benign (n, %) | DCIS (n, %) | IC (n, %) |
|---|---|---|---|---|
| HHUS | ||||
| 3 | 536 (27.54) | 518 (96.64) | 9 (1.68) | 9 (1.68) |
| 4a | 188 (9.66) | 127 (67.55) | 10 (5.32) | 51 (27.13) |
| 4b | 72 (3.70) | 19 (26.39) | 11 (15.28) | 42 (58.33) |
| 4c | 79 (4.05) | 15 (18.99) | 6 (7.59) | 58 (73.42) |
| p for trend | - | <0.001 | - | <0.001 |
| ABUS | ||||
| 3 | 546 (28.06) | 520 (95.24) | 5 (0.91) | 21 (3.85) |
| 4a | 117 (6.01) | 77 (65.81) | 12 (10.26) | 28 (23.93) |
| 4b | 71 (3.65) | 17 (23.94) | 10 (14.09) | 44 (61.97) |
| 4c | 105 (5.40) | 9 (8.57) | 9 (8.57) | 87 (82.86) |
| p for trend | - | <0.001 | - | <0.001 |
| HHUS & ABUS | ||||
| 3 | 436 (22.40) | 424 (97.25) | 4 (0.92) | 8 (1.83) |
| 4a | 81 (4.16) | 59 (72.84) | 6 (7.41) | 16 (19.75) |
| 4b | 21 (1.08) | 5 (23.81) | 3 (14.29) | 13 (61.90) |
| 4c | 32 (1.64) | 2 (6.25) | 3 (9.37) | 27 (84.38) |
| p for trend | - | <0.001 | - | <0.001 |
| Variables | BI-RADS 4a (Benign, n = 127) | BI-RADS 3 (Benign, n = 244) | OR (95% CI) | aOR (95% CI) ** |
|---|---|---|---|---|
| Age (y) | ||||
| 30–39 | 48 | 102 | 1.00 | |
| 40–69 | 79 | 142 | 1.18 (0.76, 1.84) | - |
| Menopausal status | ||||
| Premenopausal | 25 | 44 | 1.00 | |
| Postmenopausal | 102 | 200 | 0.90 (0.52, 1.55) | - |
| Breast density * | ||||
| Less dense | 11 | 21 | 1.00 | |
| More dense | 68 | 121 | 1.17 (0.76, 1.80) | - |
| Palpability of the mass | ||||
| Palpable | 62 | 77 | 1.00 | 1.00 |
| Non palpable | 65 | 147 | 0.69 (0.45, 1.07) | 0.84 (0.48, 1.49) |
| Size (cm) * | ||||
| ≤2 | 79 | 184 | 1.00 | 1.00 |
| >2 | 43 | 60 | 1.57 (0.98, 2.51) | 1.61 (0.87, 2.97) |
| Shape * | ||||
| Oval and Round | 56 | 174 | 1.00 | 1.00 |
| Irregular | 66 | 70 | 2.69 (1.72, 4.20) | 1.69 (0.95, 3.03) |
| Orientation * | ||||
| Parallel | 102 | 236 | 1.00 | 1.00 |
| Nonparallel | 20 | 8 | 5.51 (2.35, 12.92) | 5.30 (1.98, 14.16) |
| Margin * | ||||
| Regular | 74 | 204 | 1.00 | 1.00 |
| Irregular | 48 | 40 | 3.10 (1.89, 5.07) | 1.68 (0.88, 3.20) |
| Posterior feature * | ||||
| None | 87 | 182 | 1.00 | |
| Enhancement and/or Shadowing | 35 | 62 | 1.12 (0.69, 1.81) | - |
| Calcification * | ||||
| None | 97 | 213 | 1.00 | 1.00 |
| Present | 25 | 31 | 1.68 (0.95, 3.00) | 1.82 (0.91, 3.61) |
| Distorted structure | ||||
| None | 102 | 227 | 1.00 | 1.00 |
| Architectural distortion | 25 | 17 | 3.27 (1.69, 6.33) | 2.86 (1.33, 6.15) |
| Duct change | ||||
| None | 102 | 236 | 1.00 | 1.00 |
| Dilation or with filling | 25 | 8 | 7.23 (3.16, 16.57) | 8.92 (3.49, 22.77) |
| Vascularity | ||||
| Absent | 70 | 171 | 1.00 | 1.00 |
| Internal and/or vessels vascularity | 57 | 73 | 1.91 (1.22, 2.97) | 1.24 (0.71, 2.16) |
| Variables | BI-RADS 4a (Benign, n = 77) | BI-RADS 3 (Benign, n = 280) | OR (95% CI) | aOR (95% CI) ** |
|---|---|---|---|---|
| Age (y) | ||||
| 30–39 | 26 | 119 | 1.00 | |
| 40–69 | 51 | 161 | 1.45 (0.86, 2.46) | - |
| Menopausal status | ||||
| Premenopausal | 24 | 48 | 1.00 | 1.00 |
| Postmenopausal | 53 | 232 | 0.46 (0.26, 0.81) | 0.37 (0.19, 0.74) |
| Breast density * | ||||
| Less dense | 10 | 25 | 1.00 | |
| More dense | 45 | 136 | 1.21 (0.73, 2.00) | - |
| Palpability of the mass | ||||
| Palpable | 38 | 122 | 1.00 | 1.00 |
| Non palpable | 39 | 158 | 0.79 (0.48, 1.31) | 0.78 (0.41, 1.48) |
| Size (cm) * | ||||
| ≤2 | 48 | 215 | 1.00 | 1.00 |
| >2 | 23 | 56 | 1.70 (0.96, 3.01) | 1.90 (0.91, 3.97) |
| Shape * | ||||
| Oval and Round | 35 | 201 | 1.00 | 1.00 |
| Irregular | 36 | 70 | 2.63 (1.56, 4.44) | 2.23 (0.99, 4.99) |
| Orientation * | ||||
| Parallel | 57 | 242 | 1.00 | 1.00 |
| Nonparallel | 14 | 29 | 1.92 (0.96, 3.85) | 1.42 (0.56, 3.56) |
| Margin * | ||||
| Regular | 30 | 177 | 1.00 | 1.00 |
| Irregular | 41 | 94 | 2.25 (1.35, 3.76) | 0.96 (0.44, 2.11) |
| Posterior feature | ||||
| None | 44 | 183 | 1.00 | |
| Enhancement and/or Shadowing | 33 | 97 | 1.42 (0.85, 2.37) | - |
| Calcification | ||||
| None | 52 | 243 | 1.00 | 1.00 |
| Present | 25 | 37 | 3.16 (1.75, 5.69) | 2.27 (1.11, 4.62) |
| Distorted structure | ||||
| None | 62 | 270 | 1.00 | 1.00 |
| Architectural distortion | 15 | 10 | 6.53 (2.80, 15.22) | 4.05 (1.44, 11.44) |
| Duct change | ||||
| None | 62 | 257 | 1.00 | 1.00 |
| Dilation or with filling | 15 | 23 | 2.70 (1.33, 5.48) | 2.20 (0.90, 5.39) |
| Retraction phenomenon | ||||
| None | 71 | 280 | ||
| Present | 6 | 0 | - | - |
| Biopsy Thresholds | HHUS + MAM (N = 138) | ABUS + MAM (N = 94) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | PPV (%, 95% CI) | NPV (%, 95% CI) | AUC Value (95% CI) | Sensitivity (%, 95% CI) | Specificity (%, 95% CI) | PPV (%, 95% CI) | NPV (%, 95% CI) | AUC Value (95% CI) | |
| Current scenario | 77.22 (66.14, 85.60) | 80.31 (76.98, 83.27) | 32.45 (25.92, 39.71) | 96.64 (94.64, 97.64) | 0.80 (0.75, 0.85) | 60.61 (47.80, 72.18) | 87.10 (84.08, 89.63) | 34.19 (25.83, 43.60) | 95.24 (93.01, 96.81) | 0.77 (0.70, 0.84) |
| Scenario #1 | 59.49 (47.84, 70.21) | 91.01 (88.47, 93.05) | 44.76 (35.15, 54.76) | 94.83 (92.70, 96.38) | 0.78 (0.72, 0.84) | 50.00 (37.56, 62.44) | 94.30 (92.05, 95.96) | 49.25 (36.95, 61.64) | 94.46 (92.23, 96.10) | 0.74 (0.68, 0.81) |
| Scenario #2 | 51.90 (40.44, 63.17) | 93.95 (91.75, 95.61) | 51.25 (39.89, 62.48) | 94.10 (91.92, 95.74) | 0.76 (0.70, 0.82) | 40.91 (29.18, 53.70) | 95.64 (93.59, 97.08) | 50.94 (37.00, 64.75) | 93.61 (91.29, 95.36) | 0.70 (0.63, 0.76) |
| * p value1 | <0.001 | <0.001 | 0.036 | 0.131 | 0.238 | 0.016 | <0.001 | 0.044 | 0.555 | 0.277 |
| ** p value2 | <0.001 | <0.001 | 0.004 | 0.041 | 0.095 | <0.001 | <0.001 | 0.038 | 0.229 | 0.018 |
| Biopsy Thresholds | HHUS + MAM (N = 138) | ABUS + MAM (N = 94) | ||||
|---|---|---|---|---|---|---|
| Unnecessary Biopsy Rate (n, %) | IC Detection Rate (n, %) | Malignancy Rate of Biopsy (n, %) | Unnecessary Biopsy Rate(n, %) | IC Detection Rate (n, %) | Malignancy Rate of Biopsy (n, %) | |
| Total | ||||||
| Current scenario | 84 (60.87) | 46 (33.33) | 54 (39.13) | 55 (58.51) | 28 (29.78) | 39 (41.49) |
| Scenario #1 | 55 (39.86) * | 38 (27.54) † | 46 (45.54) | 33 (35.11) * | 24 (25.53) | 33 (50.00) |
| Scenario #2 | 39 (28.26) * | 34 (24.64) † | 41 (51.25) | 26 (27.66) * | 20 (21.28) † | 27 (50.94) |
| Stratified by breast density | ||||||
| Less dense | ||||||
| Current scenario | 11 (52.38) | 8 (38.10) | 10 (47.62) | 10 (55.56) | 4 (22.22) | 8 (44.44) |
| Scenario #1 | 8 (38.10) | 8 (38.10) | 10 (55.56) | 4 (22.22) * | 4 (22.22) | 6 (60.00) |
| Scenario #2 | 5 (23.81) * | 7 (33.33) | 9 (64.29) | 3 (16.67) * | 3 (16.67) | 5 (62.50) |
| More dense | ||||||
| Current scenario | 73 (72.39) | 38 (32.48) | 44 (37.61) | 45 (59.21) | 24 (31.58) | 31 (40.79) |
| Scenario #1 | 47 (40.17) * | 30 (25.64) † | 36 (43.37) | 29 (38.16) * | 20 (26.32) | 27 (48.21) |
| Scenario #2 | 34 (29.06) * | 27 (23.08) † | 32 (48.48) | 23 (30.26) * | 17 (22.37) † | 22 (48.89) |
| Stratified by age | ||||||
| 40–49 years | ||||||
| Current scenario | 52 (73.24) | 17 (23.94) | 19 (26.76) | 34 (68.00) | 14 (28.00) | 16 (32.00) |
| Scenario #1 | 34 (47.89) * | 13 (18.31) | 15 (30.61) | 22 (44.00) * | 12 (24.00) | 14 (38.89) |
| Scenario #2 | 29 (40.85) * | 11 (15.49) † | 13 (30.95) | 19 (38.00) * | 10 (20.00) | 12 (38.71) |
| 50–69 years | ||||||
| Current scenario | 32 (47.76) | 29 (43.28) | 35 (52.24) | 21 (47.72) | 14 (31.81) | 23 (52.27) |
| Scenario #1 | 21 (31.34) * | 25 (37.31) | 31 (59.62) | 11 (25.00) * | 12 (27.27) | 19 (63.33) |
| Scenario #2 | 10 (14.93) * | 23 (34.33) † | 28 (73.68) § | 7 (15.91) * | 10 (22.73) | 15 (68.18) |
| Stratified by palpability of the mass | ||||||
| Palpable | ||||||
| Current scenario | 36 (50.70) | 32 (45.07) | 35 (49.30) | 25 (50.00) | 20 (40.00) | 25 (50.00) |
| Scenario #1 | 27 (38.03) * | 30 (42.25) | 33 (55.00) | 17 (34.00) * | 18 (36.00) | 23 (57.50) |
| Scenario #2 | 20 (28.17) * | 27 (38.03) | 30 (60.00) | 14 (28.00) * | 16 (32.00) | 20 (58.82) |
| Non-Palpable | ||||||
| Current scenario | 48 (71.64) | 14 (20.90) | 19 (28.36) | 30 (68.18) | 8 (18.18) | 14 (31.82) |
| Scenario #1 | 28 (41.79) * | 8 (11.94) † | 13 (31.71) | 16 (36.36) * | 6 (13.64) | 10 (38.46) |
| Scenario #2 | 19 (28.36) * | 7 (10.45) † | 11 (36.67) | 12 (27.27) * | 4 (9.09) | 7 (36.84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Zhao, X.; Zhao, X.; Yan, H.; Hu, S.; Qiao, Y.; Xu, Z.; Zhao, F. The Potential of Adding Mammography to Handheld Ultrasound or Automated Breast Ultrasound to Reduce Unnecessary Biopsies in BI-RADS Ultrasound Category 4a: A Multicenter Hospital-Based Study in China. Curr. Oncol. 2023, 30, 3301-3314. https://doi.org/10.3390/curroncol30030251
Ren W, Zhao X, Zhao X, Yan H, Hu S, Qiao Y, Xu Z, Zhao F. The Potential of Adding Mammography to Handheld Ultrasound or Automated Breast Ultrasound to Reduce Unnecessary Biopsies in BI-RADS Ultrasound Category 4a: A Multicenter Hospital-Based Study in China. Current Oncology. 2023; 30(3):3301-3314. https://doi.org/10.3390/curroncol30030251
Chicago/Turabian StyleRen, Wenhui, Xuelian Zhao, Xiaowei Zhao, Huijiao Yan, Shangying Hu, Youlin Qiao, Zhijian Xu, and Fanghui Zhao. 2023. "The Potential of Adding Mammography to Handheld Ultrasound or Automated Breast Ultrasound to Reduce Unnecessary Biopsies in BI-RADS Ultrasound Category 4a: A Multicenter Hospital-Based Study in China" Current Oncology 30, no. 3: 3301-3314. https://doi.org/10.3390/curroncol30030251
APA StyleRen, W., Zhao, X., Zhao, X., Yan, H., Hu, S., Qiao, Y., Xu, Z., & Zhao, F. (2023). The Potential of Adding Mammography to Handheld Ultrasound or Automated Breast Ultrasound to Reduce Unnecessary Biopsies in BI-RADS Ultrasound Category 4a: A Multicenter Hospital-Based Study in China. Current Oncology, 30(3), 3301-3314. https://doi.org/10.3390/curroncol30030251





