Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients
Abstract
1. Introduction
- To patients with stage III melanoma and gross nodal disease who were deemed unresectable, in that they cannot be resected due to an expected OR delay of ≥8 weeks;
- With discussion and approval in multidisciplinary rounds;
- With an audit of outcomes.
2. Materials and Methods
3. Results
4. Discussion
- When the response to therapy is as anticipated;
- When the response to therapy is limited;
- When the response to therapy supersedes expectations.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Canadian Institute for Health Information. COVID-19′s Impact on Hospital Services. Available online: https://www.cihi.ca/en/covid-19-resources/impact-of-covid-19-on-canadas-health-care-systems/hospital-services (accessed on 11 March 2023).
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- A Ascierto, P.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef]
- Hauschild, A.; Dummer, R.; Schadendorf, D.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Longer Follow-Up Confirms Relapse-Free Survival Benefit With Adjuvant Dabrafenib Plus Trametinib in Patients With Resected BRAF V600–Mutant Stage III Melanoma. J. Clin. Oncol. 2018, 36, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage IIIBRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: An international, prospective, cohort study. Lancet Oncol. 2021, 22, 1507–1517. [Google Scholar] [CrossRef]
- Conic, R.; Cabrera, C.I.; Khorana, A.; Gastman, B.R. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J. Am. Acad. Dermatol. 2017, 78, 40–46.e7. [Google Scholar] [CrossRef]
- Degeling, K.; Baxter, N.N.; Emery, J.; Jenkins, M.A.; Franchini, F.; Gibbs, P.; Mann, G.B.; McArthur, G.; Solomon, B.J.; Ijzerman, M.J. An inverse stage-shift model to estimate the excess mortality and health economic impact of delayed access to cancer services due to the COVID-19 pandemic. Asia Pacific J. Clin. Oncol. 2021, 17, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Basnet, A.; Wang, D.; Sinha, S.; Sivapiragasam, A. Effect of a delay in definitive surgery in melanoma on overall survival: A NCDB analysis. J. Clin. Oncol. 2018, 36, e21586. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; Van De Wiel, B.; Kvistborg, P.; Krijgsman, O.; Braber, M.V.D.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Hoefsmit, E.P.; Reijers, I.L.M.; Saw, R.P.M.; Versluis, J.M.; Krijgsman, O.; Dimitriadis, P.; Sikorska, K.; van de Wiel, B.A.; Eriksson, H.; et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat. Med. 2021, 27, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; Heuvel, N.M.J.v.D.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: The PRADO trial. Nat. Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef]
- Troiani, T.; De Falco, V.; Napolitano, S.; Trojaniello, C.; Ascierto, P. How we treat locoregional melanoma. ESMO Open 2021, 6, 100136. [Google Scholar] [CrossRef]
- Tetzlaff, M.; Messina, J.; Stein, J.; Xu, X.; Amaria, R.; Blank, C.; van de Wiel, B.; Ferguson, P.; Rawson, R.; Ross, M.; et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 2018, 29, 1861–1868. [Google Scholar] [CrossRef]
- Cancer Institute, N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Published Online 2017. Available online: https://www.meddra.org/ (accessed on 25 June 2022).
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 2022, 105, 102375. [Google Scholar] [CrossRef] [PubMed]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Selli, C.; Sims, A.H. Neoadjuvant Therapy for Breast Cancer as a Model for Translational Research. Breast Cancer Basic Clin. Res. 2019, 13, 1178223419829072. [Google Scholar] [CrossRef] [PubMed]

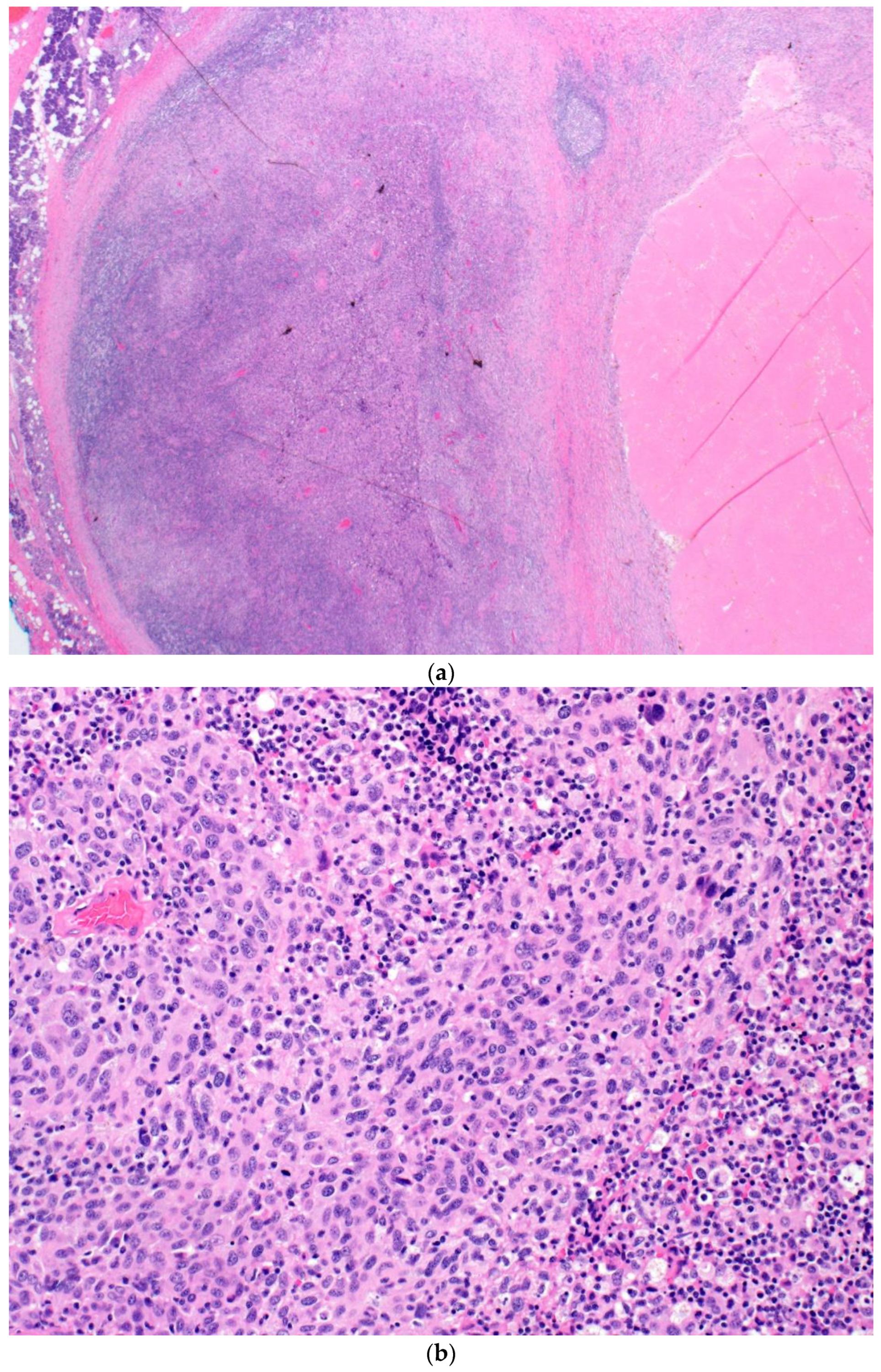
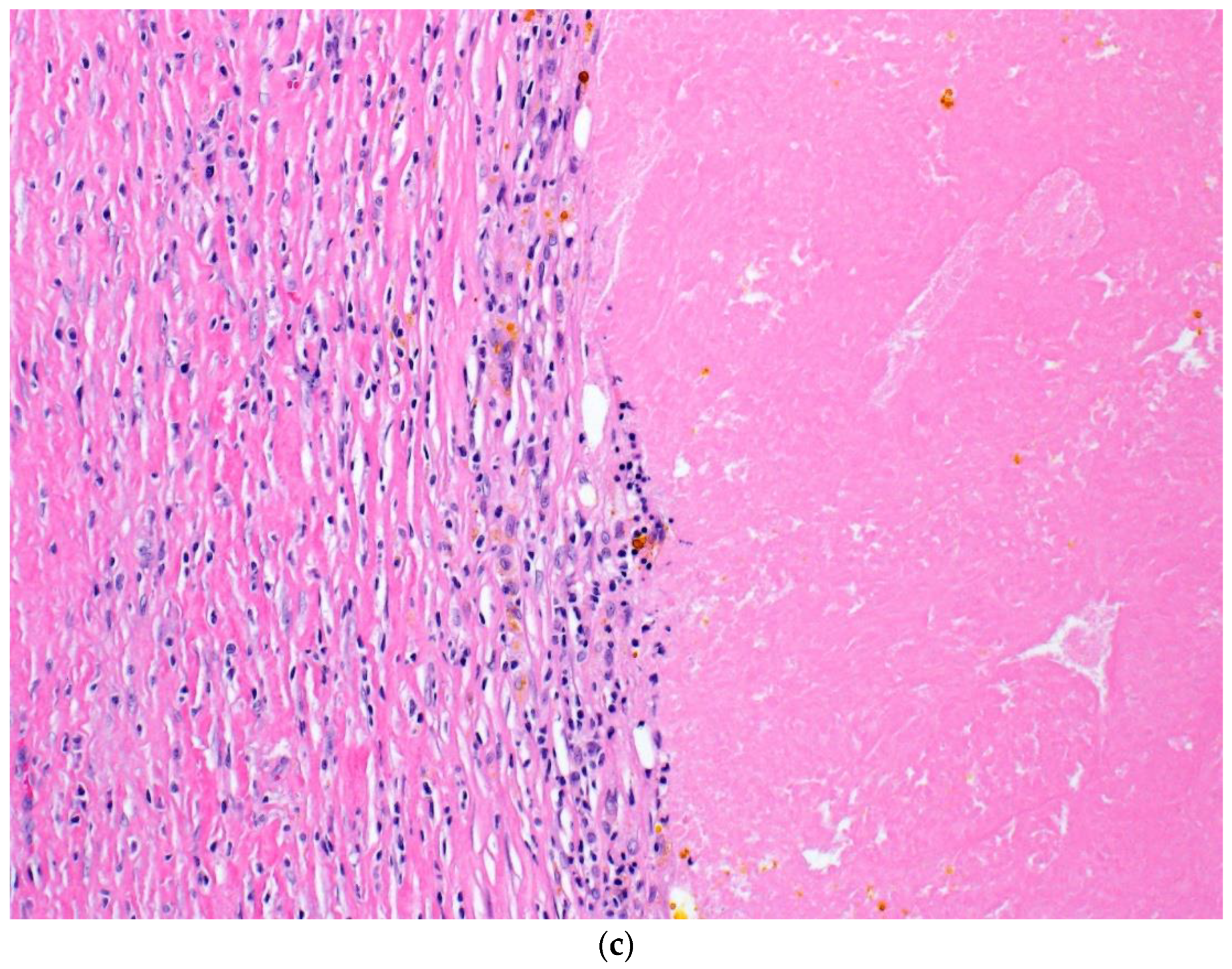
| Patient | Age | Sex | Primary Site | BRAF Status; Variant | Evidence of Systemic Disease | Locally Advanced Presentation | NAT Agent(s) | NAT Cycles Completed | Time from Last NAT to Sx (Days) | Post-Sx NT (# of Cycles) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | L temple | (+) V600E | None | L parotid adenopathy | ipilimumab + nivolumab | 3 | 20 | 10 |
| 2 | 62 | M | L tragus | (+) V600R | None | L parotid adenopathy | nivolumab | 2 | 20 | 10 |
| 3 | 27 | F | R ear | (−) | None | R parotid and neck adenopathy | nivolumab | 2 | 41 | 10 |
| 4 | 65 | F | midline back | (−) | None | R axillary adenopathy | nivolumab | 2 | 48 | 10 |
| 5 | 65 | F | L posterior neck | (−) | None | L neck adenopathy | nivolumab | 3 | 28 | 9 |
| 6 | 55 | F | L distal. medial thigh | (+) V600E | Lung nodules and L supraclavicular LN * | L thigh mass > 10 cm (20 cm of surrounding skin discolouration) | dabrafenib + trametinib | 5 | 0 † | 7 |
| Treatment-Related Adverse Event | Incidence | Grade 3–4 (%) | Associated NAT (Y/N) | ||
|---|---|---|---|---|---|
| Nivolumab Alone (n = 4) | Ipilimumab and Nivolumab (n = 1) | Dabrafenib and Trametinib (n = 1) | |||
| Fatigue | 3 * | 0 | Y | Y | N |
| Insomnia | 1 | 0 | Y | N | N |
| Rash | 4 | 0 | Y | Y | Y |
| Pruritus | 1 | 0 | Y | N | N |
| Nausea | 2 | 0 | Y | Y | N |
| Abdominal cramping | 1 | 0 | N | Y | N |
| Diarrhea | 1 | 0 | Y | N | N |
| Arthritis | 1 | 0 | Y | N | N |
| Increased TSH | 1 | 0 | Y | N | N |
| Headache ⁺ | 1 | 0 | Y | N | N |
| Dyspnea ⁺ | 1 | 0 | Y | N | N |
| Multisystem inflammatory syndromes | 1 | 0 | N | Y | N |
| Alopecia | 1 | 0 | N | Y | N |
| Hypophysitis and related symptoms † | 2 | 100% | N | Y | N |
| Pancreatitis | 1 | 0 | N | Y | N |
| Patient | Procedure | # of (+) LNs/Total LNs | Size of Largest Metastatic Deposit in LN | Location of (+) LN | Extracapsular Extension (Y/N) | Response |
|---|---|---|---|---|---|---|
| 1 | L parotidectomy, neck dissection | 1/58 | 6 mm | parenchymal | N | PR |
| 2 | Wide excision melanoma L cheek, L parotidectomy, L functional neck dissection | 6/20 | 5 mm | parenchymal | N | PR |
| 3 | R parotidectomy, R neck dissection | 1/32 | 10 × 7 mm | parenchymal | Y | CR |
| 4 | R axillary LN dissection | 1/23 | 8 × 7 mm | parenchymal | N | CR |
| 5 | L modified neck dissection | 0/29 | NA | NA | N | CR |
| 6 | L thigh | 0 | NA | Dermis and subcutaneous tissues | N | CR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinaschuk, K.; Cheng, T.; Brenn, T.; McKinnon, J.G.; Temple-Oberle, C. Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients. Curr. Oncol. 2023, 30, 4402-4411. https://doi.org/10.3390/curroncol30050335
Kinaschuk K, Cheng T, Brenn T, McKinnon JG, Temple-Oberle C. Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients. Current Oncology. 2023; 30(5):4402-4411. https://doi.org/10.3390/curroncol30050335
Chicago/Turabian StyleKinaschuk, Katie, Tina Cheng, Thomas Brenn, J. Gregory McKinnon, and Claire Temple-Oberle. 2023. "Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients" Current Oncology 30, no. 5: 4402-4411. https://doi.org/10.3390/curroncol30050335
APA StyleKinaschuk, K., Cheng, T., Brenn, T., McKinnon, J. G., & Temple-Oberle, C. (2023). Not Waiting to Progress; How the COVID-19 Pandemic Nudged Neoadjuvant Therapy for Stage III Locally Advanced Melanoma Patients. Current Oncology, 30(5), 4402-4411. https://doi.org/10.3390/curroncol30050335






