Surgical Management of Brain Tumors with Focused Ultrasound
Abstract
:1. Introduction
2. Indications for Use of FUS
3. FUS—From Past to Present—Preclinical Investigations
4. FUS: Past to Present
5. Optimizing Treatment Efficacy
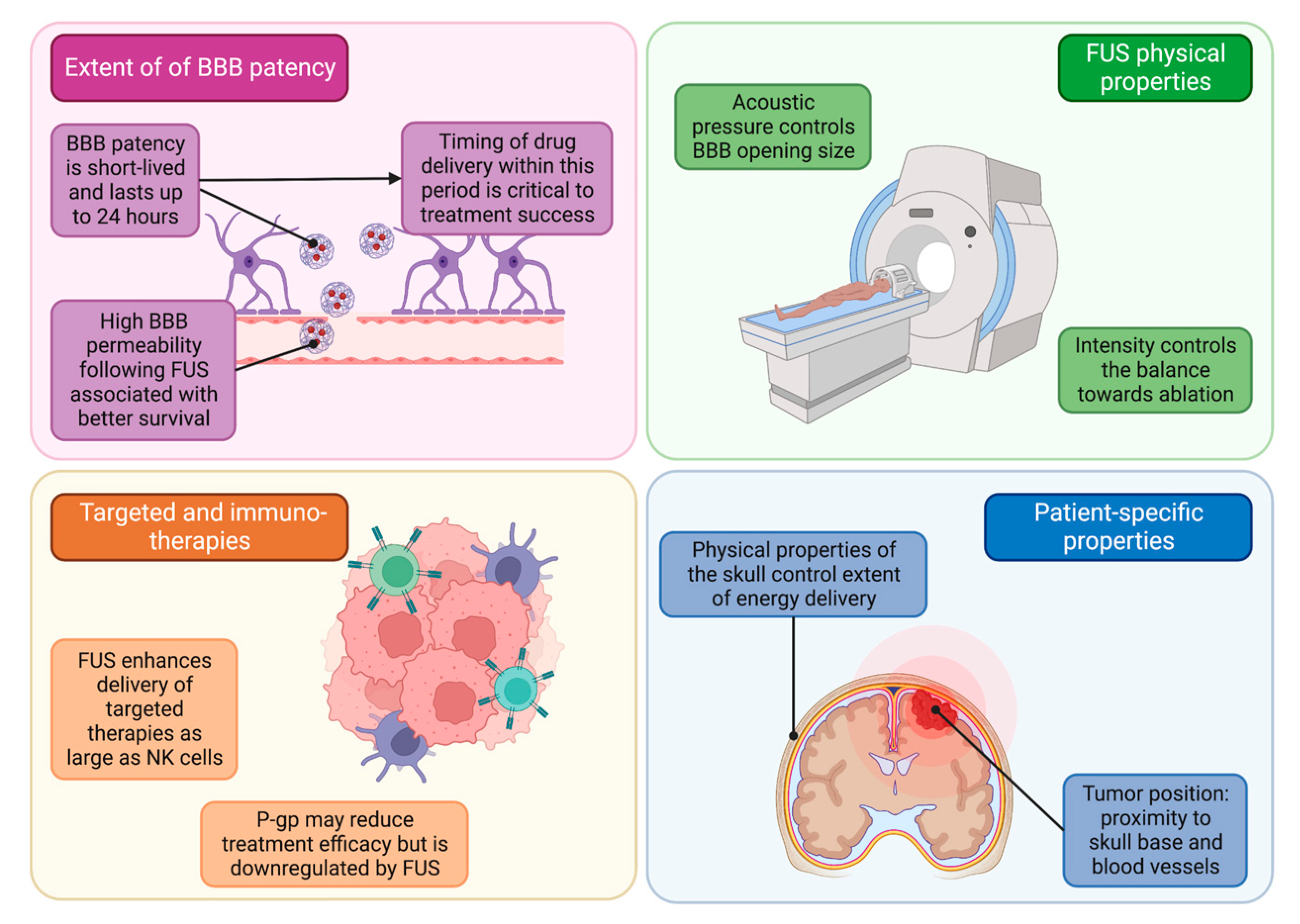
5.1. Blood–Brain Barrier Patency for Pharmaceutical Delivery
5.2. FUS Properties and Patient-Specific Considerations
5.3. FUS Delivery of Immuno-Therapeutics
5.4. Sonodynamic Therapy
5.5. Histotripsy
5.6. Liquid Biopsy
5.7. Tumor Types
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quadri, S.A.; Waqas, M.; Khan, I.; Khan, M.A.; Suriya, S.S.; Farooqui, M.; Fiani, B. High-intensity focused ultrasound: Past, present, and future in neurosurgery. Neurosurg. Focus 2018, 44, E16. [Google Scholar] [CrossRef]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology: Advances in Diagnosis and Treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef]
- Harary, M.; Segar, D.J.; Huang, K.T.; Tafel, I.J.; Valdes, P.A.; Cosgrove, G.R. Focused ultrasound in neurosurgery: A historical perspective. Neurosurg. Focus 2018, 44, E2. [Google Scholar] [CrossRef]
- Jung, N.Y.; Chang, J.W. Magnetic Resonance-Guided Focused Ultrasound in Neurosurgery: Taking Lessons from the Past to Inform the Future. J. Korean Med. Sci. 2018, 33, e279. [Google Scholar] [CrossRef] [PubMed]
- Phenix, C.P.; Togtema, M.; Pichardo, S.; Zehbe, I.; Curiel, L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J. Pharm. Pharm. Sci. 2014, 17, 136–153. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, M.O.; Haqshenas, S.R.; Pahk, K.J.; Saffari, N. The effects of ultrasound pressure and temperature fields in millisecond bubble nucleation. Ultrason. Sonochemistry 2019, 55, 262–272. [Google Scholar] [CrossRef]
- Acord, M.; Kaovasia, T.P.; Gamo, N.J.; Xiong, T.; Curry, E.; Aghabaglou, F.; Morrison, K.; Tyler, B.; Luciano, M.; Manbachi, A. Design and Fabrication of a Focused Ultrasound Device for Minimallyinvasive Neurosurgery: Reporting a Second, Miniaturized and Mrcompatible Prototype with Steering Capabilities. In Proceedings of the 2021 Design of Medical Devices Conference, Minneapolis, MN, USA, 12–15 April 2021. [Google Scholar] [CrossRef]
- Belzberg, M.; Mahapatra, S.; Perdomo-Pantoja, A.; Chavez, F.; Morrison, K.; Xiong, K.T.; Gamo, N.J.; Restaino, S.; Thakor, N.; Yazdi, Y.; et al. Minimally invasive therapeutic ultrasound: Ultrasound-guided ultrasound ablation in neuro-oncology. Ultrasonics 2020, 108, 106210. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, K.; Canney, M.; Bouchoux, G.; Desseaux, C.; Grill, J.; Heimberger, A.B.; Carpentier, A. Ultrasound-induced blood-brain barrier disruption for the treatment of gliomas and other primary CNS tumors. Cancer Lett. 2020, 479, 13–22. [Google Scholar] [CrossRef]
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front. Oncol. 2022, 12, 903059. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hsieh, H.Y.; Huang, C.Y.; Lin, C.Y.; Wei, K.C.; Liu, H.L. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: A preclinical feasibility study. J. Transl. Med. 2015, 13, 93. [Google Scholar] [CrossRef]
- Bunevicius, A.; McDannold, N.J.; Golby, A.J. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper Neurosurg. (Hagerstown) 2020, 19, 9–18. [Google Scholar] [CrossRef]
- Park, J.; Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J. Control. Release 2017, 250, 77–85. [Google Scholar] [CrossRef]
- Hersh, D.S.; Kim, A.J.; Winkles, J.A.; Eisenberg, H.M.; Woodworth, G.F.; Frenkel, V. Emerging Applications of Therapeutic Ultrasound in Neuro-oncology: Moving Beyond Tumor Ablation. Neurosurgery 2016, 79, 643–654. [Google Scholar] [CrossRef]
- Paun, L.; Moiraghi, A.; Jannelli, G.; Nouri, A.; DiMeco, F.; Pallud, J.; Meling, T.R.; Momjian, S.; Schaller, K.; Prada, F.; et al. From Focused Ultrasound Tumor Ablation to Brain Blood Barrier Opening for High Grade Glioma: A Systematic Review. Cancers 2021, 13, 5614. [Google Scholar] [CrossRef] [PubMed]
- Toccaceli, G.; Delfini, R.; Colonnese, C.; Raco, A.; Peschillo, S. Emerging Strategies and Future Perspective in Neuro-Oncology Using Transcranial Focused Ultrasonography Technology. World Neurosurg. 2018, 117, 84–91. [Google Scholar] [CrossRef]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Prada, F.; Kalani, M.Y.S.; Yagmurlu, K.; Norat, P.; Del Bene, M.; DiMeco, F.; Kassell, N.F. Applications of Focused Ultrasound in Cerebrovascular Diseases and Brain Tumors. Neurotherapeutics 2019, 16, 67–87. [Google Scholar] [CrossRef]
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef]
- Schoen, S., Jr.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv. Drug Deliv. Rev. 2022, 180, 114043. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T.; Chai, W.Y.; Lin, Y.J.; Lin, C.J.; Chen, P.Y.; Tsai, H.C.; Huang, C.Y.; Kuo, J.S.; Liu, H.L.; Wei, K.C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, 6. [Google Scholar] [CrossRef]
- Wei, H.J.; Upadhyayula, P.S.; Pouliopoulos, A.N.; Englander, Z.K.; Zhang, X.; Jan, C.I.; Guo, J.; Mela, A.; Zhang, Z.; Wang, T.J.C.; et al. Focused Ultrasound-Mediated Blood-Brain Barrier Opening Increases Delivery and Efficacy of Etoposide for Glioblastoma Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Pople, C.B.; Suppiah, S.; Llinas, M.; Huang, Y.; Sahgal, A.; Perry, J.; Keith, J.; Davidson, B.; Hamani, C.; et al. MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro Oncol. 2021, 23, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J.; Miller, A.E. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Fry, W.J.; Mosberg, W.H., Jr.; Barnard, J.W.; Fry, F.J. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 1954, 11, 471–478. [Google Scholar] [CrossRef]
- Lindstrom, P.A. Prefrontal ultrasonic irradiation-a substitute for lobotomy. AMA Arch. Neurol. Psychiatry 1954, 72, 399–425. [Google Scholar] [CrossRef]
- Meyers, R.; Fry, W.J.; Fry, F.J.; Dreyer, L.L.; Schultz, D.F.; Noyes, R.F. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg. 1959, 16, 32–54. [Google Scholar] [CrossRef]
- Fry, W.J.; Fry, F.J. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans. Med. Electron. 1960, Me-7, 166–181. [Google Scholar] [CrossRef]
- Clement, G.T.; Sun, J.; Giesecke, T.; Hynynen, K. A hemisphere array for non-invasive ultrasound brain therapy and surgery. Phys. Med. Biol. 2000, 45, 3707–3719. [Google Scholar] [CrossRef]
- Seip, R.; Vanbaren, P.; Ebbini, E.S. Dynamic focusing in ultrasound hyperthermia treatments using implantable hydrophone arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1994, 41, 706–713. [Google Scholar] [CrossRef]
- Hynynen, K.; Jolesz, F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Clement, G.T.; Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 2002, 47, 1219–1236. [Google Scholar] [CrossRef]
- Guthkelch, A.N.; Carter, L.P.; Cassady, J.R.; Hynynen, K.H.; Iacono, R.P.; Johnson, P.C.; Obbens, E.A.; Roemer, R.B.; Seeger, J.F.; Shimm, D.S.; et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial. J. Neuro-Oncol. 1991, 10, 271–284. [Google Scholar] [CrossRef]
- Hynynen, K.; Vykhodtseva, N.I.; Chung, A.H.; Sorrentino, V.; Colucci, V.; Jolesz, F.A. Thermal effects of focused ultrasound on the brain: Determination with MR imaging. Radiology 1997, 204, 247–253. [Google Scholar] [CrossRef]
- Chung, A.H.; Jolesz, F.A.; Hynynen, K. Thermal dosimetry of a focused ultrasound beam in vivo by magnetic resonance imaging. Med. Phys. 1999, 26, 2017–2026. [Google Scholar] [CrossRef]
- Hynynen, K.; Clement, G.T.; McDannold, N.; Vykhodtseva, N.; King, R.; White, P.J.; Vitek, S.; Jolesz, F.A. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn. Reson. Med. 2004, 52, 100–107. [Google Scholar] [CrossRef]
- Ram, Z.; Cohen, Z.R.; Harnof, S.; Tal, S.; Faibel, M.; Nass, D.; Maier, S.E.; Hadani, M.; Mardor, Y. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 2006, 59, 949–955. [Google Scholar] [CrossRef]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010, 66, 323–332. [Google Scholar] [CrossRef]
- Coluccia, D.; Fandino, J.; Schwyzer, L.; O'Gorman, R.; Remonda, L.; Anon, J.; Martin, E.; Werner, B. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 2014, 2, 17. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- Burgess, A.; Ayala-Grosso, C.A.; Ganguly, M.; Jordão, J.F.; Aubert, I.; Hynynen, K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE 2011, 6, e27877. [Google Scholar] [CrossRef]
- Jordão, J.F.; Ayala-Grosso, C.A.; Markham, K.; Huang, Y.; Chopra, R.; McLaurin, J.; Hynynen, K.; Aubert, I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS ONE 2010, 5, e10549. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef]
- Sheeran, P.S.; Luois, S.H.; Mullin, L.B.; Matsunaga, T.O.; Dayton, P.A. Design of ultrasonically-activatable nanoparticles using low boiling point perfluorocarbons. Biomaterials 2012, 33, 3262–3269. [Google Scholar] [CrossRef]
- Chen, C.C.; Sheeran, P.S.; Wu, S.Y.; Olumolade, O.O.; Dayton, P.A.; Konofagou, E.E. Targeted drug delivery with focused ultrasound-induced blood-brain barrier opening using acoustically-activated nanodroplets. J. Control. Release 2013, 172, 795–804. [Google Scholar] [CrossRef]
- Canney, M.S.; Chavrier, F.; Tsysar, S.; Chapelon, J.Y.; Lafon, C.; Carpentier, A. A multi-element interstitial ultrasound applicator for the thermal therapy of brain tumors. J. Acoust. Soc. Am. 2013, 134, 1647–1655. [Google Scholar] [CrossRef]
- MacDonell, J.; Patel, N.; Rubino, S.; Ghoshal, G.; Fischer, G.; Burdette, E.C.; Hwang, R.; Pilitsis, J.G. Magnetic resonance-guided interstitial high-intensity focused ultrasound for brain tumor ablation. Neurosurg. Focus 2018, 44, E11. [Google Scholar] [CrossRef]
- Ultrasound-based Blood-brain Barrier Opening and Albumin-Bound Paclitaxel and Carboplatin for Recurrent Glioblastoma. Available online: https://ClinicalTrials.gov/show/NCT04528680 (accessed on 1 March 2023).
- Efficacy and Safety of NaviFUS System Add-on Bevacizumab (BEV) in Recurrent GBM Patients. Available online: https://ClinicalTrials.gov/show/NCT04446416 (accessed on 1 March 2023).
- Evaluate the Safety and Preliminary Efficacy of the Combination of NaviFUS System With Re-irradiation for rGBM Patients. Available online: https://ClinicalTrials.gov/show/NCT04988750 (accessed on 1 March 2023).
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Vendel, E.; Rottschäfer, V.; de Lange, E.C.M. The need for mathematical modelling of spatial drug distribution within the brain. Fluids Barriers CNS 2019, 16, 12. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- McKeage, K.; Perry, C.M. Trastuzumab: A review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 2002, 62, 209–243. [Google Scholar] [CrossRef]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; Pople, C.B.; Davidson, B.; et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Kerbel, R.; Wels, W.S.; Hynynen, K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 2016, 18, 974–981. [Google Scholar] [CrossRef]
- Ye, D.; Yuan, J.; Yue, Y.; Rubin, J.B.; Chen, H. Focused Ultrasound-Enhanced Delivery of Intranasally Administered Anti-Programmed Cell Death-Ligand 1 Antibody to an Intracranial Murine Glioma Model. Pharmaceutics 2021, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cheng, G.; Ye, D.; Nazeri, A.; Yue, Y.; Liu, W.; Wang, X.; Dunn, G.P.; Petti, A.A.; Leuthardt, E.C.; et al. Focused Ultrasound-enabled Brain Tumor Liquid Biopsy. Sci. Rep. 2018, 8, 6553. [Google Scholar] [CrossRef]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, E.H.; Han, M.; An, S.H.; Park, J. Diminished Expression of P-glycoprotein Using Focused Ultrasound Is Associated with JNK-Dependent Signaling Pathway in Cerebral Blood Vessels. Front. NeuroSci. 2019, 13, 1350. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.Y.; Han, M.; Choi, J.R.; Ahn, S.; Lee, T.; Chang, Y.; Park, J. Localized Down-regulation of P-glycoprotein by Focused Ultrasound and Microbubbles induced Blood-Brain Barrier Disruption in Rat Brain. Sci. Rep. 2016, 6, 31201. [Google Scholar] [CrossRef]
- Chen, H.; Konofagou, E.E. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab. 2014, 34, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Jung, H.H.; Zadicario, E.; Rachmilevitch, I.; Tlusty, T.; Vitek, S.; Chang, J.W. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: Efficiency of acoustic energy delivery through the skull. J. Neurosurg. 2016, 124, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Medel, R.; Monteith, S.J.; Elias, W.J.; Eames, M.; Snell, J.; Sheehan, J.P.; Wintermark, M.; Jolesz, F.A.; Kassell, N.F. Magnetic resonance-guided focused ultrasound surgery: Part 2: A review of current and future applications. Neurosurgery 2012, 71, 755–763. [Google Scholar] [CrossRef]
- Monteith, S.; Snell, J.; Eames, M.; Kassell, N.F.; Kelly, E.; Gwinn, R. Transcranial magnetic resonance-guided focused ultrasound for temporal lobe epilepsy: A laboratory feasibility study. J. Neurosurg. 2016, 125, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; Chung, A.H.; Colucci, V.; Jolesz, F.A. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med. Biol. 1996, 22, 193–201. [Google Scholar] [CrossRef]
- Christian, E.; Yu, C.; Apuzzo, M.L. Focused ultrasound: Relevant history and prospects for the addition of mechanical energy to the neurosurgical armamentarium. World Neurosurg. 2014, 82, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Fishman, P.S.; Frenkel, V. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapeutics 2017, 14, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; Damianou, C.; Darkazanli, A.; Unger, E.; Schenck, J.F. The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery. Ultrasound Med. Biol. 1993, 19, 91–92. [Google Scholar] [CrossRef]
- Coleman, D.J.; Rondeau, M.J.; Silverman, R.H.; Lizzi, F.L. Computerized ultrasonic biometry and imaging of intraocular tumors for the monitoring of therapy. Trans. Am. Ophthalmol. Soc. 1987, 85, 49–81. [Google Scholar] [PubMed]
- Ebbini, E.S.; ter Haar, G. Ultrasound-guided therapeutic focused ultrasound: Current status and future directions. Int. J. Hyperthermia 2015, 31, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.; Benedict, S.; Diederich, C.; Gedroyc, W.; Klibanov, A.; Larner, J. MR-guided focused ultrasound surgery, present and future. Med. Phys. 2013, 40, 080901. [Google Scholar] [CrossRef]
- A Pilot Study: Focused Ultrasound Thalamotomy for the Prevention of Secondary Generalization in Focal Onset Epilepsy. Available online: https://ClinicalTrials.gov/show/NCT03417297 (accessed on 1 March 2023).
- MR-HIFU Treatment of Painful Osteoid Osteoma. Available online: https://ClinicalTrials.gov/show/NCT04658771 (accessed on 1 March 2023).
- Magnetic Resonance Guided High Intensity Focused Ultrasound in Advanced Pancreatic Adenocarcinoma Treatment. Available online: https://ClinicalTrials.gov/show/NCT04298242 (accessed on 1 March 2023).
- Minimally Invasive Treatment of Primary Great Saphenous Vein (GSV) Insufficiency Using High Intensity Focused Ultrasound (HIFU). Available online: https://ClinicalTrials.gov/show/NCT05193643 (accessed on 1 March 2023).
- Treatment of Breast Fibroadenoma Targeted Tissue with HIFU. Available online: https://ClinicalTrials.gov/show/NCT03044054 (accessed on 1 March 2023).
- Medical Imaging and Thermal Treatment for Breast Tumors Using Harmonic Motion Imaging (HMI). Available online: https://ClinicalTrials.gov/show/NCT05219695 (accessed on 1 March 2023).
- A Phase I Study of Lyso-thermosensitive Liposomal Doxorubicin and MR-HIFU for Pediatric Refractory Solid Tumors. Available online: https://ClinicalTrials.gov/show/NCT02536183 (accessed on 1 March 2023).
- Jolesz, F.A. MRI-Guided Focused Ultrasound Surgery. Annu. Rev. Med. 2009, 60, 417–430. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, G.R. High intensity focused ultrasound for the treatment of tumors. Echocardiography 2001, 18, 317–322. [Google Scholar] [CrossRef]
- Mahmoud, M.Z.; Alkhorayef, M.; Alzimami, K.S.; Aljuhani, M.S.; Sulieman, A. High-Intensity Focused Ultrasound (HIFU) in Uterine Fibroid Treatment: Review Study. Pol. J. Radiol. 2014, 79, 384–390. [Google Scholar] [CrossRef]
- Leslie, T.; Ritchie, R.; Illing, R.; Ter Haar, G.; Phillips, R.; Middleton, M.; Bch, B.; Wu, F.; Cranston, D. High-intensity focused ultrasound treatment of liver tumours: Post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br. J. Radiol. 2012, 85, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Gelet, A.; Chapelon, J.Y.; Bouvier, R.; Rouvière, O.; Lyonnet, D.; Dubernard, J.M. Transrectal high intensity focused ultrasound for the treatment of localized prostate cancer: Factors influencing the outcome. Eur. Urol. 2001, 40, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Shoji, S.; Nakano, M.; Hongo, S.; Nitta, M.; Murota, A.; Nagata, Y. Transrectal high-intensity focused ultrasound for the treatment of localized prostate cancer: Eight-year experience. Int. J. Urol. 2009, 16, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Lafon, C.; Melodelima, D.; Salomir, R.; Chapelon, J.Y. Interstitial devices for minimally invasive thermal ablation by high-intensity ultrasound. Int. J. Hyperth. 2007, 23, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bessiere, F.; N'Djin, W.A.; Colas, E.C.; Chavrier, F.; Greillier, P.; Chapelon, J.Y.; Chevalier, P.; Lafon, C. Ultrasound-Guided Transesophageal High-Intensity Focused Ultrasound Cardiac Ablation in a Beating Heart: A Pilot Feasibility Study in Pigs. Ultrasound Med. Biol. 2016, 42, 1848–1861. [Google Scholar] [CrossRef] [PubMed]
- Barba, D.; Saris, S.C.; Holder, C.; Rosenberg, S.A.; Oldfield, E.H. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J. Neurosurg. 1989, 70, 175–182. [Google Scholar] [CrossRef]
- Hayes, R.L.; Koslow, M.; Hiesiger, E.M.; Hymes, K.B.; Hochster, H.S.; Moore, E.J.; Pierz, D.M.; Chen, D.K.; Budzilovich, G.N.; Ransohoff, J. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer 1995, 76, 840–852. [Google Scholar] [CrossRef]
- Liu, Y.; Ehtesham, M.; Samoto, K.; Wheeler, C.J.; Thompson, R.C.; Villarreal, L.P.; Black, K.L.; Yu, J.S. In situ adenoviral interleukin 12 gene transfer confers potent and long-lasting cytotoxic immunity in glioma. Cancer Gene Ther. 2002, 9, 9–15. [Google Scholar] [CrossRef] [PubMed]
- McHale, A.P.; Callan, J.F.; Nomikou, N.; Fowley, C.; Callan, B. Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. Adv. Exp. Med. Biol. 2016, 880, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy--a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Bunevicius, A.; Pikis, S.; Padilla, F.; Prada, F.; Sheehan, J. Sonodynamic therapy for gliomas. J. Neuro-Oncol. 2022, 156, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Lu, M.; Zhang, L.; Liu, Y.; Han, D.; Wang, X.; Geng, Y.; Wan, M. Histotripsy Using Fundamental and Second Harmonic Superposition Combined with Hundred-Microsecond Ultrasound Pulses. Ultrasound Med. Biol. 2018, 44, 2089–2104. [Google Scholar] [CrossRef] [PubMed]
- Movahed, P.; Kreider, W.; Maxwell, A.D.; Hutchens, S.B.; Freund, J.B. Cavitation-induced damage of soft materials by focused ultrasound bursts: A fracture-based bubble dynamics model. J. Acoust. Soc. Am. 2016, 140, 1374. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Ruger, L.; Yang, E.; Gannon, J.; Sheppard, H.; Coutermarsh-Ott, S.; Ziemlewicz, T.J.; Dervisis, N.; Allen, I.C.; Daniel, G.B.; Tuohy, J.; et al. Mechanical High-Intensity Focused Ultrasound (Histotripsy) in Dogs with Spontaneously Occurring Soft Tissue Sarcomas. IEEE Trans. Biomed. Eng. 2022, 70, 768–779. [Google Scholar] [CrossRef]
- Dubinsky, T.J.; Khokhlova, T.D.; Khokhlova, V.; Schade, G.R. Histotripsy: The Next Generation of High-Intensity Focused Ultrasound for Focal Prostate Cancer Therapy. J. Ultrasound Med. 2020, 39, 1057–1067. [Google Scholar] [CrossRef]
- Zhu, L.; Nazeri, A.; Pacia, C.P.; Yue, Y.; Chen, H. Focused ultrasound for safe and effective release of brain tumor biomarkers into the peripheral circulation. PLoS ONE 2020, 15, e0234182. [Google Scholar] [CrossRef]
- Pacia, C.P.; Zhu, L.; Yang, Y.; Yue, Y.; Nazeri, A.; Michael Gach, H.; Talcott, M.R.; Leuthardt, E.C.; Chen, H. Feasibility and safety of focused ultrasound-enabled liquid biopsy in the brain of a porcine model. Sci. Rep. 2020, 10, 7449. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Alli, S.; Bondoc, A.; Golbourn, B.; Sabha, N.; Mikloska, K.; Krumholtz, S.; Srikanthan, D.; Fujita, N.; Luck, A.; et al. MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma. J. Control Release 2021, 330, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
| Logistic Hurdles in the Development of MRgFUS | Solutions |
|---|---|
| Insufficient power at the target | Using multiple transducers in an array |
| Significant scalp damage | Using lower transducer frequencies |
| Hemispheric transducer array Running degassed water through headpiece | |
| Ensuring high-fidelity transmission from US transducer to tissue | Degassed saline/water |
| Need for craniotomy | Phased correction circuitry |
| Surrounding tissue damage | MR thermometry |
| Inability to achieve thermal ablation | Increasing maximum power output of ExAblate |
| Large tumors | Interstitial transducer applicators |
| Inability to undergo intraoperative MRI | Neuronavigation |
| Clinical Study | Conditions Being Treated | HIFU Interventions |
|---|---|---|
| A Pilot Study: Focused Ultrasound Thalamotomy for the Prevention of Secondary Generalization in Focal Onset Epilepsy [75] | Partial Seizures with Secondary Generalization | Magnetic Resonance Guided High-Intensity Focused Ultrasound |
| MR-HIFU Treatment of Painful Osteoid Osteoma [76] | Osteoid Osteoma | Magnetic Resonance Guided High-Intensity Focused Ultrasound |
| Magnetic Resonance Guided High-Intensity Focused Ultrasound in Advanced Pancreatic Adenocarcinoma Treatment [77] | Pancreatic Adenocarcinoma | ExAblate 2100 (Magnetic Resonance Guided High-Intensity Focused Ultrasound) |
| Minimally Invasive Treatment of Primary Great Saphenous Vein (GSV) Insufficiency Using High-Intensity Focused Ultrasound (HIFU) [78] | Great Saphenous Vein Insufficiency | Sonovein (Ultrasound Guided Focused Ultrasound) |
| Treatment of Breast Fibroadenoma Targeted Tissue with HIFU [79] | Breast Fibroadenoma | ECHOPULSE (Ultrasound Guided Focused Ultrasound) |
| Medical Imaging and Thermal Treatment for Breast Tumors Using Harmonic Motion Imaging (HMI) [80] | Fibroadenoma Breast Cancer Stage I | Harmonic Motion Imaging Guided Focused Ultrasound |
| A Phase I Study of Lyso-thermosensitive Liposomal Doxorubicin and MR-HIFU for Pediatric Refractory Solid Tumors [81] | Pediatric Cancer Solid Tumors Rhabdomyosarcoma Ewing Sarcoma Soft Tissue Sarcomas Osteosarcoma Neuroblastoma Wilms Tumor Hepatic Tumor Germ Cell Tumors | Magnetic Resonance Guided High-Intensity Focused Ultrasound Lyso-thermosensitive liposomal doxorubicin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehkri, Y.; Pierre, K.; Woodford, S.J.; Davidson, C.G.; Urhie, O.; Sriram, S.; Hernandez, J.; Hanna, C.; Lucke-Wold, B. Surgical Management of Brain Tumors with Focused Ultrasound. Curr. Oncol. 2023, 30, 4990-5002. https://doi.org/10.3390/curroncol30050377
Mehkri Y, Pierre K, Woodford SJ, Davidson CG, Urhie O, Sriram S, Hernandez J, Hanna C, Lucke-Wold B. Surgical Management of Brain Tumors with Focused Ultrasound. Current Oncology. 2023; 30(5):4990-5002. https://doi.org/10.3390/curroncol30050377
Chicago/Turabian StyleMehkri, Yusuf, Kevin Pierre, Samuel Joel Woodford, Caroline Grace Davidson, Ogaga Urhie, Sai Sriram, Jairo Hernandez, Chadwin Hanna, and Brandon Lucke-Wold. 2023. "Surgical Management of Brain Tumors with Focused Ultrasound" Current Oncology 30, no. 5: 4990-5002. https://doi.org/10.3390/curroncol30050377
APA StyleMehkri, Y., Pierre, K., Woodford, S. J., Davidson, C. G., Urhie, O., Sriram, S., Hernandez, J., Hanna, C., & Lucke-Wold, B. (2023). Surgical Management of Brain Tumors with Focused Ultrasound. Current Oncology, 30(5), 4990-5002. https://doi.org/10.3390/curroncol30050377







