Myeloproliferative Neoplasm Driven by ETV6-ABL1 in an Adolescent with Recent History of Burkitt Leukemia
Abstract
1. Introduction
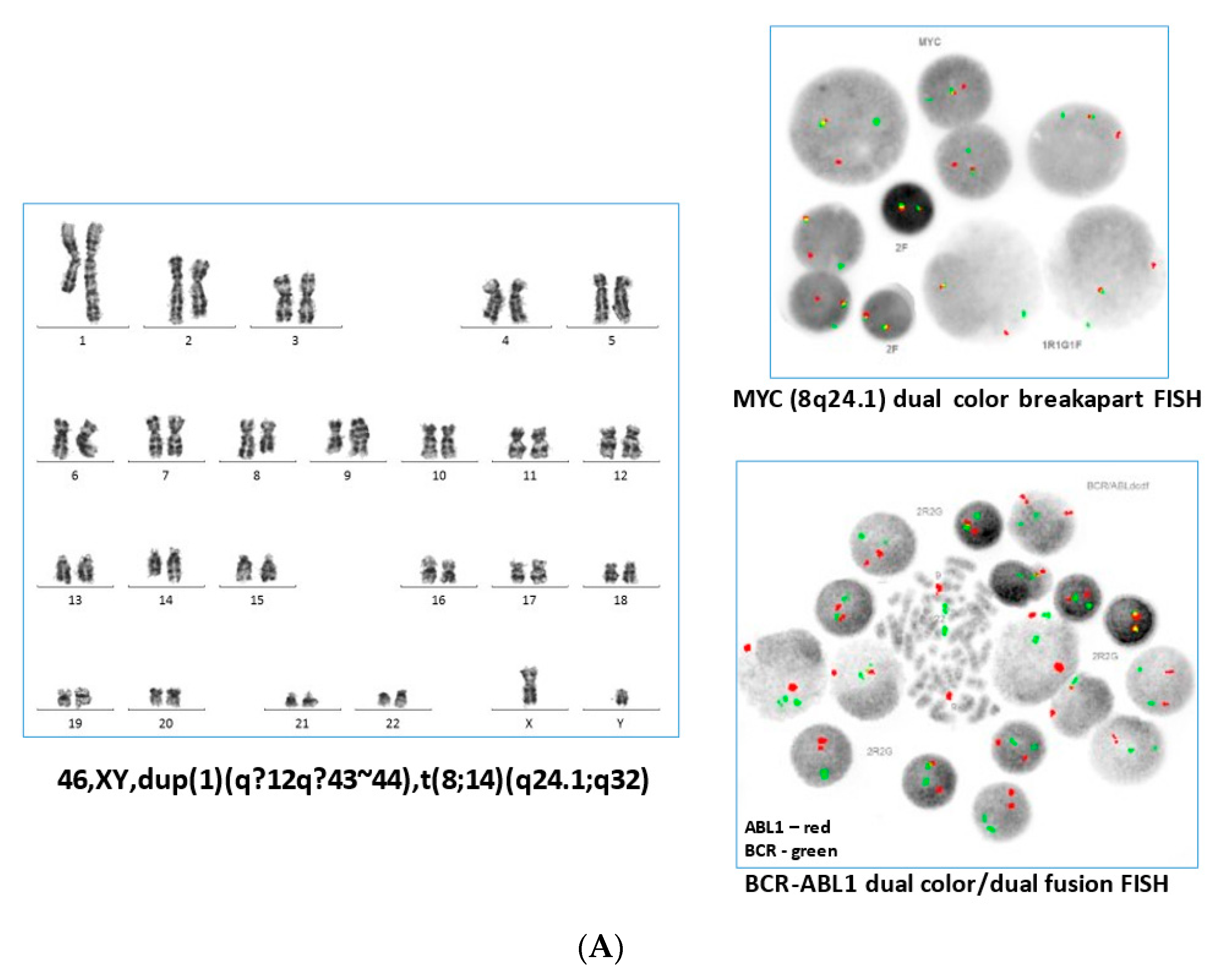
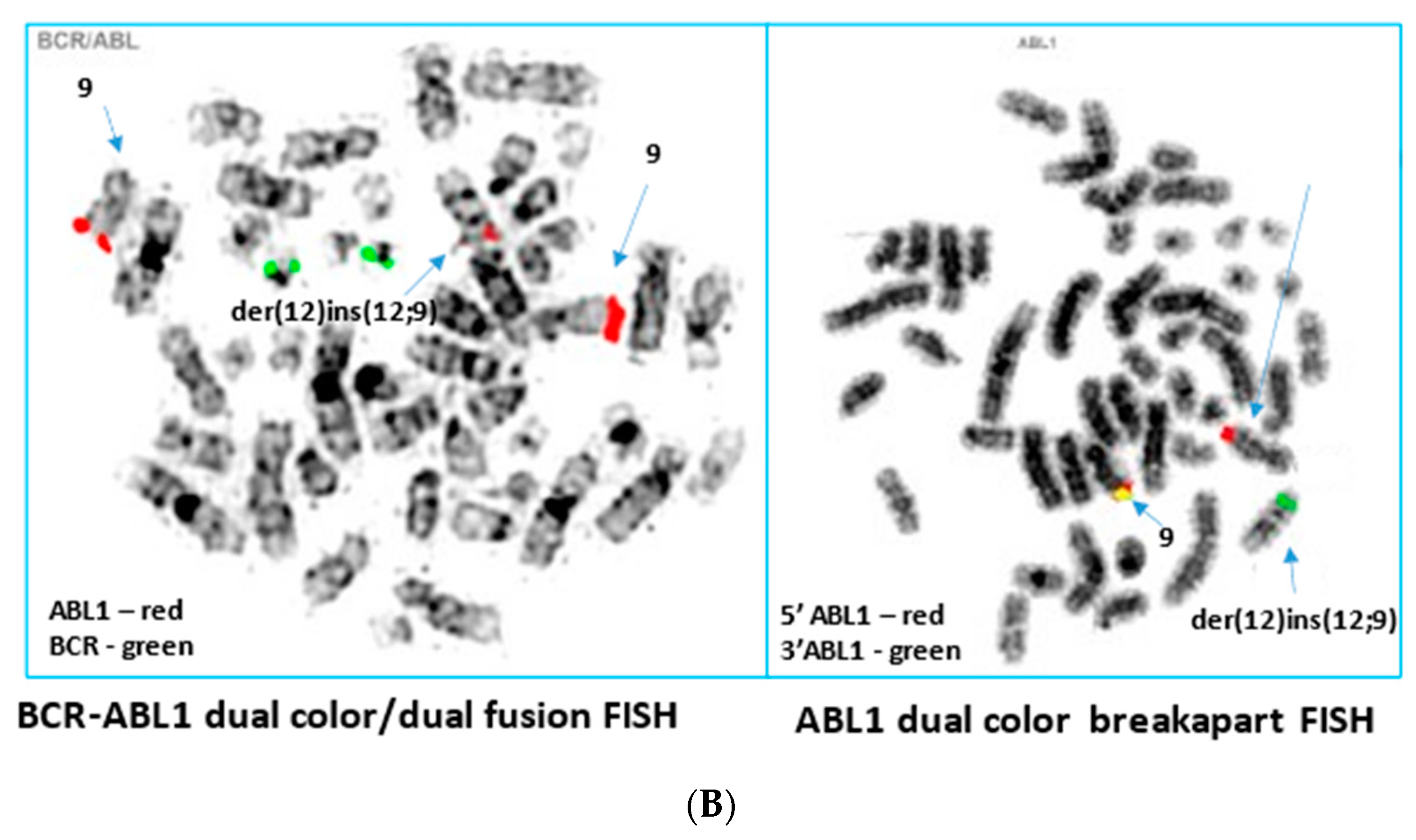
2. Case Report
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaliova, M.; Moorman, A.V.; Cazzaniga, G.; Stanulla, M.; Harvey, R.C.; Roberts, K.G.; Heatley, S.L.; Loh, M.L.; Konopleva, M.; Chen, I.M.; et al. Characterization of leukemias with ETV6-ABL1 fusion. Haematologica 2016, 101, 1082–1093. [Google Scholar] [CrossRef]
- Song, J.S.; Shin, S.Y.; Lee, S.T.; Kim, H.J.; Kim, S.H. A cryptic ETV6/ABL1 rearrangement represents a unique fluorescence in situ hybridization signal pattern in a patient with B acute lymphoblastic leukemia. Ann. Lab. Med. 2014, 34, 475–477. [Google Scholar] [CrossRef]
- Malinge, S.; Monni, R.; Bernard, O.; Penard-Lacronique, V. Activation of the NF-kappaB pathway by the leukemogenic TEL-Jak2 and TEL-Abl fusion proteins leads to the accumulation of antiapoptotic IAP proteins and involves IKKalpha. Oncogene 2006, 25, 3589–3597. [Google Scholar] [CrossRef]
- Choi, S.I.; Jang, M.A.; Jeong, W.J.; Jeon, B.R.; Lee, Y.W.; Shin, H.B.; Hong, D.S.; Lee, Y.K. A Case of Chronic Myeloid Leukemia With Rare Variant ETV6/ABL1 Rearrangement. Ann. Lab. Med. 2017, 37, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kakadia, P.M.; Schmidmaier, R.; Völkl, A.; Schneider, I.; Huk, N.; Schneider, S.; Panzner, G.; Neidel, U.; Fritz, B.; Spiekermann, K.; et al. An ETV6-ABL1 fusion in a patient with chronic myeloproliferative neoplasm: Initial response to Imatinib followed by rapid transformation into ALL. Leuk Res. Rep. 2016, 6, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Ridge, S.A.; Boucher, C.A.; Stocking, C.; Wiedemann, L.M. The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res. 1995, 55, 34–38. [Google Scholar] [PubMed]
- Golub, T.R.; Goga, A.; Barker, G.F.; Afar, D.E.; McLaughlin, J.; Bohlander, S.K.; Rowley, J.D.; Witte, O.N.; Gilliland, D.G. Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia. Mol. Cell. Biol. 1996, 16, 4107–4116. [Google Scholar] [CrossRef]
- Andreasson, P.; Johansson, B.; Carlsson, M.; Jarlsfelt, I.; Fioretos, T.; Mitelman, F.; Hoglund, M. BCR/ABL-negative chronic myeloid leukemia with ETV6/ABL fusion. Genes Chromosom. Cancer 1997, 20, 299–304. [Google Scholar] [CrossRef]
- Curtis, C.E.; Grand, F.H.; Waghorn, K.; Sahoo, T.P.; George, J.; Cross, N.C. A novel ETV6-PDGFRB fusion transcript missed by standard screening in a patient with an imatinib responsive chronic myeloproliferative disease. Leukemia 2007, 21, 1839–1841. [Google Scholar] [CrossRef]
- La Starza, R.; Trubia, M.; Testoni, N.; Ottaviani, E.; Belloni, E.; Crescenzi, B.; Martelli, M.; Flandrin, G.; Pelicci, P.G.; Mecucci, C. Clonal eosinophils are a morphologic hallmark of ETV6/ABL1 positive acute myeloid leukemia. Haematologica 2002, 87, 789–794. [Google Scholar]
- Turcotte, L.M.; Neglia, J.P.; Reulen, R.C.; Ronckers, C.M.; van Leeuwen, F.E.; Morton, L.M.; Hodgson, D.C.; Yasui, Y.; Oeffinger, K.C.; Henderson, T.O. Risk, Risk Factors, and Surveillance of Subsequent Malignant Neoplasms in Survivors of Childhood Cancer: A Review. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Zuna, J.; Zaliova, M.; Muzikova, K.; Meyer, C.; Lizcova, L.; Zemanova, Z.; Brezinova, J.; Votava, F.; Marschalek, R.; Stary, J.; et al. Acute leukemias with ETV6/ABL1 (TEL/ABL) fusion: Poor prognosis and prenatal origin. Genes Chromosom. Cancer 2010, 49, 873–884. [Google Scholar] [CrossRef]
- Kawamata, N.; Dashti, A.; Lu, D.; Miller, B.; Koeffler, H.P.; Schreck, R.; Moore, S.; Ogawa, S. Chronic phase of ETV6-ABL1 positive CML responds to imatinib. Genes Chromosom. Cancer 2008, 47, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Nand, R.; Bryke, C.; Kroft, S.H.; Divgi, A.; Bredeson, C.; Atallah, E. Myeloproliferative disorder with eosinophilia and ETV6-ABL gene rearrangement: Efficacy of second-generation tyrosine kinase inhibitors. Leuk. Res. 2009, 33, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- Tiribelli, M.; Barraco, D.; Medeot, M.; Marin, L.; Ottaviani, E.; De Marchi, F.; Damiani, D.; Fanin, R. Long-term efficacy and safety of nilotinib therapy after imatinib failure in eosinophilic myeloproliferative neoplasm and ETV6-ABL rearrangement. Ann. Hematol. 2015, 94, 1423–1424. [Google Scholar] [CrossRef]
- Zhang, Y.; Nguyen, L.; Lu, C.M.; Wang, E.; Lauw, M.I.S.; Ball, S.; Dong, N.; Moscinski, L.; Chan, O.; Yun, S.; et al. Clinical response to Upfront Targeted Tyrosine Kinase Inhibitors among Patients with Myeloid/Lymphoid Neoplasms with Eosinophilia and Tyrosine Kinase Gene Fusions. Clin. Lymphoma Myeloma Leuk. 2023, 23, e150–e163. [Google Scholar] [CrossRef]
- Brien, S.G.; Vieira, S.; Connors, S.; Bown, N.; Chang, J.; Capdeville, R.; Melo, J.V. Transient response to imatinib mesylate (STI571) in a patient with the ETV6-ABL t(9;12) translocation. Blood 2022, 99, 3465–3467. [Google Scholar] [CrossRef]
- Zimmermannova, O.; Doktorava, E.; Stuchly, J.; Kanderova, V.; Kuzilkova, D.; Strnad, H.; Starkova, J.; Alberich-Jorda, M.; Falkenburg, J.H.F.; Trka, J.; et al. An activating mutation of GNB1 is associated with resistance to tyrosine kinase inhibitors in ETV6-ABL1-positive leukemia. Oncogene 2017, 36, 5985–5994. [Google Scholar] [CrossRef]
- Patel, A.; O’Hare, T.; Deninger, M.W. Mechanisms of resistance to ABL kinase inhibition in CML and the development of next generation ABL kinase inhibitors. Hematol. Oncol. Clin. N. Am. 2017, 31, 589–612. [Google Scholar] [CrossRef]
- Alves, R.; Goncalves, A.C.; Rutella, S.; Almeida, A.M.; De Las Rivas, J.; Trougakos, I.P.; Sarmento Ribeiro, A.B. Resistance to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia—From Molecular Mechanisms to Clinical Revelance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef]
- Loscocco, F.; Visani, G.; Galimberti, S.; Curti, A.; Isidori, A. BCR-ABL Indipendent Mechanisms of Resistance in Chronic Myeloid Leukemia. Front. Oncol. 2019, 9, 939. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzi, S.; Algawahmed, F.; Davidson, S.; Langenberg, K.P.S.; Fuligni, F.; Ali, S.; Anderson, N.; Brunga, L.; Bartram, J.; Abdelhaleem, M.; et al. Myeloproliferative Neoplasm Driven by ETV6-ABL1 in an Adolescent with Recent History of Burkitt Leukemia. Curr. Oncol. 2023, 30, 5946-5952. https://doi.org/10.3390/curroncol30070444
Renzi S, Algawahmed F, Davidson S, Langenberg KPS, Fuligni F, Ali S, Anderson N, Brunga L, Bartram J, Abdelhaleem M, et al. Myeloproliferative Neoplasm Driven by ETV6-ABL1 in an Adolescent with Recent History of Burkitt Leukemia. Current Oncology. 2023; 30(7):5946-5952. https://doi.org/10.3390/curroncol30070444
Chicago/Turabian StyleRenzi, Samuele, Fatimah Algawahmed, Scott Davidson, Karin P. S. Langenberg, Fabio Fuligni, Salah Ali, Nathaniel Anderson, Ledia Brunga, Jack Bartram, Mohamed Abdelhaleem, and et al. 2023. "Myeloproliferative Neoplasm Driven by ETV6-ABL1 in an Adolescent with Recent History of Burkitt Leukemia" Current Oncology 30, no. 7: 5946-5952. https://doi.org/10.3390/curroncol30070444
APA StyleRenzi, S., Algawahmed, F., Davidson, S., Langenberg, K. P. S., Fuligni, F., Ali, S., Anderson, N., Brunga, L., Bartram, J., Abdelhaleem, M., Naqvi, A., Beimnet, K., Schuh, A., Tierens, A., Malkin, D., Shlien, A., Shago, M., & Villani, A. (2023). Myeloproliferative Neoplasm Driven by ETV6-ABL1 in an Adolescent with Recent History of Burkitt Leukemia. Current Oncology, 30(7), 5946-5952. https://doi.org/10.3390/curroncol30070444





