Preoperative Breast Magnetic Resonance Imaging: An Ontario Health (Cancer Care Ontario) Clinical Practice Guideline
Abstract
1. Introduction
2. Materials and Methods
2.1. Background
2.2. Guideline Objective
2.3. Research Question
2.4. Target Population
2.5. Development Process
2.6. Literature Search
2.7. Recommendation Development and Review
3. Recommendations and Key Evidence
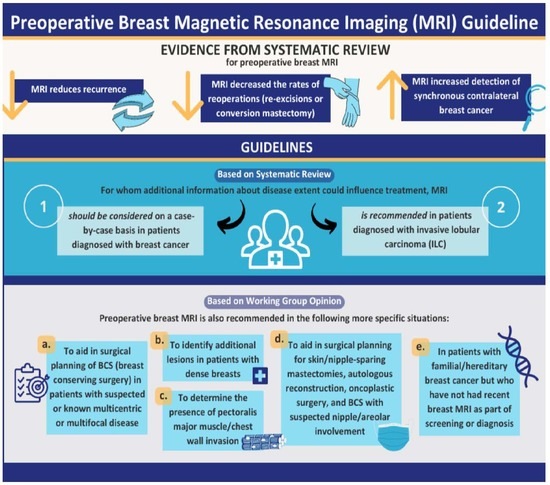
3.1. Recommendation 1
- Preoperative breast MRI should be considered on a case-by-case basis in patients diagnosed with breast cancer for whom additional information about disease extent could influence treatment. The ensuing decision of whether to conduct an MRI should be made in consultation with the patient and must take into account the balance of benefits and risks and patient preferences.
- Stronger recommendations for specific situations are provided in Recommendations 2 and 3.
3.1.1. Qualifying Statements for Recommendation 1
- Benefits and harms (see Key Evidence and Table 1) may vary depending on patient and disease characteristics such as breast density, tumour size, tumour stage, number and distribution of tumours (multicentric or multifocal), subtype of cancer, type of surgery being considered or preferred, adjuvant treatment, and patient factors/comorbidities.
- System issues such as MRI availability may result in treatment delays that may modify the decision.
- “Treatment” in the recommendation includes surgery as well as radiation and systemic treatment.
- In patients with a strong preference for mastectomy or with contraindications to BCS, MRI is unlikely to change surgical planning in the ipsilateral breast. Breast MRI may still impact treatment if mammographically occult CBC is detected.
- Contrast-enhanced mammography (contrast-enhanced spectral mammography, contrast-enhanced digital mammography), diffusion-weighted imaging (DWI) MRI, magnetic resonance spectroscopy, or other advanced imaging techniques are known to provide additional information beyond that of conventional imaging and may be suitable instead of or in addition to CE-MRI. Potential adverse effects due to contrast agent and radiation exposure vary among these techniques, whereas many other potential benefits and harms in Table 1 would be relevant. These are mentioned briefly in the systematic review, but the evaluation was outside of scope. They are less widely available, and there is much less evidence regarding their effect on patient outcomes.
3.1.2. Key Evidence for Recommendation 1
Recurrence
- Use of MRI is associated with a reduction of recurrence of any type ( HR = 0.77, 95% confidence interval [CI] = 0.65 to 0.90) [moderate level of certainty]. Approximate recurrence: 8.2% versus 10.5%; 2.3% less (1% to 3.6% fewer).
Contralateral Cancer
- Use of MRI is associated with an increase in detection of synchronous CBC (prior to initial surgery) (HR = 2.52, 95% CI = 1.75 to 3.62; HR > 1 indicates increased detection with MRI) [moderate level of certainty]. Approximate synchronous CBC detection: 4.7% versus 1.9%; 2.8% more (1.4% to 4.8% more).
- Use of MRI is associated with a slight reduction in metachronous CBC (HR = 0.71, 95% CI = 0.59 to 0.85) [moderate level of certainty]. Approximate metachronous CBC: 1.7% versus 2.4%; 0.7% fewer (0.4% to 1.0% fewer).
Conversion Mastectomy
- Use of MRI is associated with a reduction in the rate of conversion mastectomy OR = 0.76, 95% CI = 0.58 to 0.99) [low level of certainty]. Approximate conversion mastectomy rate: 5.5% versus 7.1%; 1.6% fewer (95% CI = 0.1% to 2.9% fewer).
Positive Margins
- Use of MRI reduced the rate of positive margins in studies with low or low-moderate risk of bias (OR = 0.57, 95% CI = 0.36 to 0.89) [moderate level of certainty]. Approximate rate of positive margins: 6.5% versus 10.9%; 4.4% fewer (95% CI = 1.1% to 6.7% fewer).
Reoperations and Re-Excisions
- Use of MRI is associated with a reduction in the rate of reoperation (OR = 0.73, 95% CI = 0.63 to 0.85) [low level of certainty]. Approximate rate of reoperation: 14.4% versus 18.7%; 4.3% fewer (95% CI = 2.3% to 6.0% fewer).
- Use of MRI is associated with a reduction in the rate of re-excision (OR = 0.63, 95% CI = 0.45 to 0.89) [low level of certainty]. Approximate rate of re-excision: 6.9% versus 10.5%; 3.6% fewer (95% CI = 1.0% to 5.5% fewer).
Mastectomy Rates
- Use of MRI is associated with an increase in the initial mastectomy rate in patients planned (prior to MRI) for BCS (OR = 5.18, 95% CI = 2.37 to 11.29) [very low level of certainty]. Approximate initial mastectomy rate: 5.5% versus 1.1%; 4.4% more (95% CI = 3.6% to 11.5% more). Use of MRI is associated with an increase in the final mastectomy rate (OR = 1.87, 95% CI = 1.23 to 2.85) [very low level of certainty]. Approximate final mastectomy rate: 14% versus 8%; 6% more (95% CI = 1.7% to 11.9% more).
- Studies including all patients diagnosed with breast cancer (not restricted to predetermined BCS) showed that use of MRI is associated with an increase in the initial mastectomy rate (OR = 1.29, 95% CI = 1.09 to 1.35) [low level of certainty]. Approximate initial mastectomy rate: 38.0% versus 32.3%, or 5.8% more (95% CI = 1.9% to 9.9% more). The use of MRI is associated with an increase in the final mastectomy rate (OR = 1.19, 95% CI = 1.06 to 1.33). Approximate final mastectomy rate: 41.8% versus 37.6%, 4.2% more (95% CI = 1.4% to 6.9% more). There was no difference in the final mastectomy rate when the trials using registry data were excluded (OR = 0.98, 95% CI = 0.82 to 1.17).
Other Supporting Studies (Not Part of the Meta-Analysis)
- Two studies that characterized mammographically occult ipsilateral lesions (>2 cm away or in different quadrants than the index tumour) found that they were larger than the index lesion in approximately 20% of cases [29,30]. In the absence of MRI, such tumours, unless detected coincidentally during the operation of the index tumour, would be untreated surgically.
3.1.3. Justification for Recommendation 1
- We consider the significant reduction in recurrence, probable improvement in disease-free survival and metachronous CBC, and reduction in reoperations (re-excisions and conversion mastectomies) evidence of benefit that outweighs the potential negative effects overall. This recommendation places a higher value on treating cancer in a single operation and avoiding recurrence than on avoiding the discomfort of an MRI and potential additional biopsies.
- While the absolute benefit is small for most outcomes and not always statistically significant, the trend is toward MRI being beneficial for each outcome, and therefore this consistency strengthens the conclusion that preoperative MRI has a positive impact in general.
- While MRI use is associated with an increase in mastectomy rate, the reasons are likely to be multifactorial, including the need to encompass additional foci of cancer, a lack of BCS/oncoplastic surgery expertise for more complex cases, and patient preferences. In retrospective studies (and some of the RCTs), MRI was used for clinical reasons that may not have been recorded or adjusted for but that could be related to mastectomy use. As mastectomy rates may vary by country, region, hospital, and surgeon, and due to patient factors such as age, relationship status, and race/ethnicity, the additional effect of MRI on mastectomy outcomes is difficult to assess.
3.2. Recommendation 2
- Preoperative breast MRI is recommended in patients diagnosed with invasive lobular carcinoma (ILC) for whom additional information about disease extent could influence treatment. The decision of whether to conduct an MRI should be made in consultation with the patient and must take into account the balance of benefits and risks and patient preferences.
3.2.1. Qualifying Statements for Recommendation 2
- Risks and benefits will vary depending on patient and disease characteristics.
- System issues such as MRI availability may result in treatment delays that may modify the decision.
3.2.2. Key Evidence for Recommendation 2
- Use of MRI is associated with a reduction in the rate of conversion mastectomy in patients with ILC (OR = 0.38, 95% CI = 0.25 to 0.56) [high certainty of evidence]. Approximate conversion mastectomy rate in ILC: 5.9% versus 14.2%; 8.3% fewer (5.7% to 10.3% fewer).
- Use of MRI is associated with a reduction in the rate of positive margins in patients with ILC (OR = 0.63, 95% CI = 0.49 to 0.82) [moderate level of certainty]. Approximate rate of positive margins: 18.9% versus 27.0%; 8.1% fewer (3.7% to 11.7%).
- Use of MRI is associated with a large reduction in the rate of reoperation in patients with ILC (OR = 0.30, 95% CI = 0.13 to 0.72) [moderate level of certainty]. Approximate rate of reoperation: 12.3% versus 31.9%; 19.6% fewer (6.77% to 26.1% fewer).
- Lobbes et al. [33] found MRI increased the detection of synchronous CBC in ILC (OR = 4.07, 95% CI = 1.73 to 3.61, p < 0.001) (HR > 1 indicates increased detection with MRI).
3.2.3. Justification for Recommendation 2
- We consider the significant reduction in positive margins resulting in a large reduction in reoperations (including conversion mastectomy), in addition to the benefits in survival and recurrence for all patients (see Recommendation 1), to be evidence of a benefit that outweighs the potential negative effects overall. This recommendation places a higher value on treating cancer in a single operation and avoiding recurrence than on avoiding the discomfort of an MRI and potential additional biopsies. The benefit of MRI is consistent with the results of studies that reported that, compared to invasive ductal carcinoma, ILC has been found to be more difficult to detect by mammography, more likely multifocal, more often occurs with synchronous CBC, and has more involved margins after initial resection [36,37,38,39,40,41].
3.3. Recommendation 3
- (a)
- To aid in the surgical planning of BCS in patients with suspected or known multicentric or multifocal disease.
- (b)
- To identify additional lesions in patients with dense breasts.
- (c)
- To determine the presence of pectoralis major muscle/chest wall invasion in patients with posteriorly located tumours or when invasion of the pectoralis major muscle or chest wall is suspected.
- (d)
- To aid in surgical planning for skin/nipple-sparing mastectomies or for autologous reconstruction, oncoplastic surgery, and BCS with suspected nipple/areolar involvement.
- (e)
- Patients with familial/hereditary breast cancer who have not had a recent breast MRI as part of screening or diagnosis.
3.3.1. Qualifying Statement for Recommendation 3
3.3.2. Key Evidence for Recommendation 3
- (a)
- Most studies in the literature review [19] either excluded multicentric and multifocal disease or included these in the list of factors used to adjust results in multivariate analysis, indicating these are known to influence outcomes, but with the result that we did not find a direct comparison of outcomes according to MRI use. The presence of multicentric and multifocal disease increases the complexity of surgical planning and in older guidelines was a contraindication to BCS. When the disease is well-characterized, the possibility of BCS may be increased in some cases and ruled out in others, and the likelihood of an incidental finding during surgery decreases. The consensus of the authors is that the increased sensitivity of MRI justifies its use in suspected/known multicentric or multifocal disease if BCS is desired.
- (b)
- Several studies mentioned in the literature review [19] reported that the sensitivity of mammography decreases as breast density increases, while the sensitivity of MRI is high and independent of breast density. The GEMMA (Gadobutrol-Enhanced MR Mammography) trials studied MRI in patients with newly diagnosed and histologically proven breast cancer. In GEMMA1, MRI sensitivity was 83% (independent of density), while the sensitivity of mammography decreased from 79% to 62% as breast density increased [44]. Corresponding results in the GEMMA2 trial were 91% (independent of density) for MRI and 82% (low density) to 64% (high density) for mammography. The Ottawa study of preoperative MRI found additional lesions changing surgical management in 31% of patients with low density (fat density) and 62% with dense breasts [45]. Screening studies reported similar variations in the sensitivity of mammography based on breast density. The Supplemental MRI Screening for Women with Extremely Dense Breast Tissue (DENSE trial) randomized 40,373 women with extremely dense breast tissue and normal screening mammography to either supplemental MRI or only mammography and found MRI reduced interval cancers by 50% in those offered MRI and 80% in those who agreed to have an MRI [46,47,48]. A systematic review and meta-analysis [49] found that breast density is one of the strongest risk factors for breast cancer.
- (c)
- (d)
- Standard BCS may lead to fair to poor esthetic and functional results [54], and more complex oncoplastic surgery or mastectomy may be more appropriate if the optimal tumour-to-breast ratio for each quadrant is exceeded. Breast MRI or other advanced imaging (e.g., positron emission tomography/computed tomography) may be a prerequisite for extreme oncoplasty [55]. MRI is frequently used prior to nipple-sparing mastectomy, especially in the case of centrally located tumours [56,57,58,59,60]. MRI may rule out nipple involvement such that 2 cm is no longer considered a minimum tumour-to-nipple distance; 5 mm [61] or 1 cm [62,63,64,65,66,67] may be sufficient.
- (e)
- Hereditary cancer patients have a high risk of synchronous and metachronous CBC. A systematic review reported 10-year CBC rates of 25% to 31% for patients with germline mutations, compared to 4% to 8% for sporadic cases [68].
3.4. Technical Factors for MRI Use
4. Discussion
4.1. Limitations
4.2. Review and Update
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mann, R.M.; Kuhl, C.K.; Moy, L. Contrast-enhanced MRI for breast cancer screening. J. Magn. Reson. Imaging 2019, 50, 377–390. [Google Scholar] [CrossRef]
- Schoub, P.K. Understanding indications and defining guidelines for breast magnetic resonance imaging. SA J. Radiol. 2018, 22, a1353. [Google Scholar] [CrossRef]
- Orel, S.G.; Schnall, M.D. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology 2001, 220, 13–30. [Google Scholar] [CrossRef]
- Lehman, C.D. Magnetic resonance imaging in the evaluation of ductal carcinoma in situ. J. Natl. Cancer Inst. Monogr. 2010, 2010, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Causer, P.A.; Wong, J.W.; Wright, F.C.; Jong, R.A.; Hill, K.A.; Messner, S.J.; Yaffe, M.J.; Narod, S.A.; Plewes, D.B. Improvement in DCIS detection rates by MRI over time in a high-risk breast screening study. Breast J. 2011, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Schrading, S.; Bieling, H.B.; Wardelmann, E.; Leutner, C.C.; Koenig, R.; Kuhn, W.; Schild, H.H. MRI for diagnosis of pure ductal carcinoma in situ: A prospective observational study. Lancet 2007, 370, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sickles, E.A.; D’Orsi, C.J. ACR BI-RADS® Atlas—Follow-up and Outcome Monitoring. Section II. The Basic Clinically Relevant Audit. In ACR BI-RADS® Atlas, 5th ed.; D’Orsi, C.J., Sickles, E.A., Mendelson, E.B., Morris, E.A., Eds.; American College of Radiology: Reston, VA, USA, 2013; [modified 5 Feruary 2014]. [Google Scholar]
- Ontario Health (Cancer Care Ontario). Guidelines & Advice: Breast Cancer Screening for People at High Risk. 2017. Available online: https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/screening/breast-cancer-high-risk (accessed on 21 June 2023).
- Warner, E.; Messersmith, H.; Causer, P.; Eisen, A.; Shumak, R.; Plewes, D. Magnetic Resonance Imaging Screening of Women at High Risk for Breast Cancer, Version 3. 2021. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/2051 (accessed on 26 October 2021).
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.T.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjäger, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Lee, C.S.; Destounis, S.V. Breast Cancer Screening for Women at Higher-Than-Average Risk: Updated Recommendations From the ACR. J. Am. Coll. Radiol. 2023, in press. [Google Scholar] [CrossRef]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Kobrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef]
- Institut National D’excellence en Santé et en Services Sociaux (INESSS). Main Indications for Breast MRI in the Context of Investigation and Planning of Breast Cancer Treatment. 2018. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Rapports/Oncologie/IRM_sein/IRM_Cancer-du-sein_EN_VF.pdf (accessed on 26 March 2021).
- American College of Radiology. ACR Practice Parameter for the Performance of Contrast-Enhanced Magnetic Resonance Imaging (MRI) of the Breast. 2018. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Contrast-Breast.pdf?la=en (accessed on 16 March 2020).
- Appavoo, S.; Aldis, A.; Causer, P.; Crystal, P.; Kornecki, A.; Mundt, Y.; Seely, J.; Wadden, N. CAR Practice Guidelines and Technical Standards for Breast Imaging and Intervention. 2012. Available online: https://car.ca/book/breast-imaging-guidelines/ (accessed on 17 March 2020).
- Blue Shield of California. 6.01.29—Magnetic Resonance Imaging for Detection and Diagnosis of Breast Cancer. 2020. Available online: https://www.blueshieldca.com/bsca/bsc/public/common/PortalComponents/provider/StreamDocumentServlet?fileName=PRV_MRI_Breast.pdf (accessed on 9 February 2021).
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burnstein, H.J.; Chew, H.; et al. NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines)®. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf (accessed on 20 June 2023).
- Eisen, A.; Fletcher, G.G.; Fienberg, S.; George, R.; Holloway, C.; Kulkarni, S.; Seely, J.; Muradali, D. Preoperative Breast Magnetic Resonance Imaging. Program in Evidence-Based Care Evidence Summary No.: 1-25. 2021. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/pebc1-25es.pdf (accessed on 27 May 2022).
- Muradali, D.; Fletcher, G.G.; Cordeiro, E.; Fienberg, S.; George, R.; Kulkarni, S.; Seely, J.; Shaheen, R.; Eisen, A. The Preoperative Breast MRI Expert Panel. Preoperative Breast Magnetic Resonance Imaging Guideline. 2023. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/70786 (accessed on 22 June 2023).
- Eisen, A.; Fletcher, G.G.; Fienberg, S.; George, R.; Holloway, C.; Kulkarni, S.; Seely, J.; Muradali, D. Breast magnetic resonance imaging for preoperative evaluation of breast cancer. Can. Assoc. Radiol. J. 2023, in press. [Google Scholar]
- Browman, G.P.; Newman, T.E.; Mohide, E.A.; Graham, I.D.; Levine, M.N.; Pritchard, K.I.; Evans, W.K.; Maroun, J.A.; Hodson, D.I.; Carey, M.S.; et al. Progress of clinical oncology guidelines development using the Practice Guidelines Development Cycle: The role of practitioner feedback. J. Clin. Oncol. 1998, 16, 1226–1231. [Google Scholar] [CrossRef]
- Browman, G.P.; Levine, M.N.; Mohide, E.A.; Hayward, R.S.; Pritchard, K.I.; Gafni, A.; Laupacis, A. The practice guidelines development cycle: A conceptual tool for practice guidelines development and implementation. J. Clin. Oncol. 1995, 13, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, A.; Varela, N.P.; Allarakhia, M.; Grimard, L.; Hey, A.; Lau, J.; Thain, L.; Eisen, A. Baseline staging imaging for distant metastasis in women with stages I, II, and III breast cancer. Curr. Oncol. 2020, 27, e123–e145. [Google Scholar] [CrossRef] [PubMed]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.4. 2020. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download (accessed on 6 May 2021).
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. 2020. Available online: https://training.cochrane.org/handbook (accessed on 13 August 2021).
- Brennan, M.E.; Houssami, N.; Lord, S.; Macaskill, P.; Irwig, L.; Dixon, J.M.; Warren, R.M.; Ciatto, S. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: Systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J. Clin. Oncol. 2009, 27, 5640–5649. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Blackmore, K.M.; Muradali, D.; Done, S.J.; Majpruz, V.; Weerasinghe, A.; Mirea, L.; Eisen, A.; Rabeneck, L.; Warner, E. Performance measures of magnetic resonance imaging plus mammography in the High Risk Ontario Breast Screening Program. J. Natl. Cancer Inst. 2020, 112, 136–144. [Google Scholar] [CrossRef]
- Goodman, S.; Mango, V.; Friedlander, L.; Desperito, E.; Wynn, R.; Ha, R. Are mammographically occult additional tumors identified more than 2 cm away from the primary breast cancer on MRI clinically significant? Acad. Radiol. 2019, 26, 502–507. [Google Scholar] [CrossRef]
- Iacconi, C.; Galman, L.; Zheng, J.; Sacchini, V.; Sutton, E.J.; Dershaw, D.; Morris, E.A. Multicentric cancer detected at breast MR imaging and not at mammography: Important or not? Radiology 2016, 279, 378–384. [Google Scholar] [CrossRef]
- Mann, R.M.; Balleyguier, C.; Baltzer, P.A.; Bick, U.; Colin, C.; Cornford, E.; Evans, A.; Fallenberg, E.; Forrai, G.; Fuchsjager, M.H.; et al. Breast MRI: EUSOBI recommendations for women’s information. Eur. Radiol. 2015, 25, 3669–3678. [Google Scholar] [CrossRef]
- Blue Cross Blue Shield Association. 6.01.29—Magnetic Resonance Imaging for Detection and Diagnosis of Breast Cancer. 2019. Available online: https://www.evidencepositioningsystem.com/BCBSA/html/pol_6.01.29.html (accessed on 31 October 2019).
- Lobbes, M.B.; Vriens, I.J.; van Bommel, A.C.; Nieuwenhuijzen, G.A.; Smidt, M.L.; Boersma, L.J.; van Dalen, T.; Smorenburg, C.; Struikmans, H.; Siesling, S.; et al. Breast MRI increases the number of mastectomies for ductal cancers, but decreases them for lobular cancers. Breast Cancer Res. Treat. 2017, 162, 353–364. [Google Scholar] [CrossRef]
- Mann, R.M.; Hoogeveen, Y.L.; Blickman, J.G.; Boetes, C. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: A review of existing literature. Breast Cancer Res. Treat. 2008, 107, 1–14. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Radiologists. Guidance on Screening and Symptomatic Breast Imaging. 2019. Available online: https://www.rcr.ac.uk/publication/guidance-screening-and-symptomatic-breast-imaging-fourth-edition (accessed on 26 March 2021).
- Biglia, N.; Maggiorotto, F.; Liberale, V.; Bounous, V.E.; Sgro, L.G.; Pecchio, S.; D’Alonzo, M.; Ponzone, R. Clinical-pathologic features, long term-outcome and surgical treatment in a large series of patients with invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC). Eur. J. Surg. Oncol. 2013, 39, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Derias, M.; Subramanian, A.; Allan, S.; Shah, E.; Teraifi, H.E.; Howlett, D. The Role of Magnetic Resonance Imaging in the Investigation and Management of Invasive Lobular Carcinoma-A 3-Year Retrospective Study in Two District General Hospitals. Breast J. 2016, 22, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.F.; Hill, A.D.; Fleming, F.J.; O’Doherty, A.; Quinn, C.M.; McDermott, E.W.; O’Higgins, N. Identifying patients at risk of compromised margins following breast conservation for lobular carcinoma. Am. J. Surg. 2006, 191, 201–205. [Google Scholar] [CrossRef]
- Yeatman, T.J.; Cantor, A.B.; Smith, T.J.; Smith, S.K.; Reintgen, D.S.; Miller, M.S.; Ku, N.N.; Baekey, P.A.; Cox, C.E. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann. Surg. 1995, 222, 549–559; discussion 559–561. [Google Scholar] [CrossRef]
- Krecke, K.N.; Gisvold, J.J. Invasive lobular carcinoma of the breast: Mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am. J. Roentgenol. 1993, 161, 957–960. [Google Scholar] [CrossRef]
- Veltman, J.; Boetes, C.; van Die, L.; Bult, P.; Blickman, J.G.; Barentsz, J.O. Mammographic detection and staging of invasive lobular carcinoma. Clin. Imaging 2006, 30, 94–98. [Google Scholar] [CrossRef]
- Eastern Health Breast Disease Site Group. Indications for Use of Breast Magnetic Resonance Imaging. 2017. Available online: https://cancercare.easternhealth.ca/health-care-professionals/guidelines/breast-cancer/ (accessed on 26 March 2021).
- Ditsch, N.; Untch, M.; Thill, M.; Muller, V.; Janni, W.; Albert, U.S.; Bauerfeind, I.; Blohmer, J.; Budach, W.; Dall, P.; et al. AGO recommendations for the diagnosis and treatment of patients with early breast cancer: Update 2019. Breast Care 2019, 14, 224–245. [Google Scholar] [CrossRef]
- Sardanelli, F.; Newstead, G.M.; Putz, B.; Jirakova Trnkova, Z.; Trimboli, R.M.; Abe, H.; Haverstock, D.; Rosenberg, M. Gadobutrol-enhanced magnetic resonance imaging of the breast in the preoperative setting: Results of 2 prospective international multicenter phase III studies. Investig. Radiol. 2016, 51, 454–461. [Google Scholar] [CrossRef]
- Seely, J.M.; Lamb, L.; Malik, N.; Lau, J. The yield of pre-operative breast MRI in patients according to breast tissue density. Eur. Radiol. 2016, 26, 3280–3289. [Google Scholar] [CrossRef]
- Bakker, M.F.; de Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.H.M.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.H.C.; Lobbes, M.B.I.; et al. Supplemental MRI screening for women with extremely dense breast tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Klinkenbijl, J.H.G.; van Leeuwen, E.; Verkooijen, H.M. Wat zeggen de uitkomsten van de DENSE-studie? Ned. Tijdschr. Geneeskd. 2020, 164, D4822. [Google Scholar] [PubMed]
- Franz, H.B.G. Profitieren frauen mit extrem dichtem brustgewebe von zusatzlicher MRT? Geburtshilfe Frauenheilkd. 2020, 80, 460–462. [Google Scholar] [CrossRef]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Samreen, N.; Lee, C.; Bhatt, A.; Carter, J.; Hieken, T.; Adler, K.; Zingula, S.; Glazebrook, K.N. A Clinical Approach to Diffusion-Weighted Magnetic Resonance Imaging in Evaluating Chest Wall Invasion of Breast Tumors. J. Clin. Imaging Sci. 2019, 9, 11. [Google Scholar] [CrossRef]
- Myers, K.S.; Stern, E.; Ambinder, E.B.; Oluyemi, E.T. Breast cancer abutting the pectoralis major muscle on breast MRI: What are the clinical implications? Br. J. Radiol. 2021, 94, 20201202. [Google Scholar] [CrossRef]
- Kazama, T.; Nakamura, S.; Doi, O.; Suzuki, K.; Hirose, M.; Ito, H. Prospective evaluation of pectoralis muscle invasion of breast cancer by MR imaging. Breast Cancer 2005, 12, 312–316. [Google Scholar] [CrossRef]
- Morris, E.A.; Schwartz, L.H.; Drotman, M.B.; Kim, S.J.; Tan, L.K.; Liberman, L.; Abramson, A.F.; Van Zee, K.J.; Dershaw, D.D. Evaluation of pectoralis major muscle in patients with posterior breast tumors on breast MR images: Early experience. Radiology 2000, 214, 67–72. [Google Scholar] [CrossRef]
- Pukancsik, D.; Kelemen, P.; Ujhelyi, M.; Kovacs, E.; Udvarhelyi, N.; Meszaros, N.; Kenessey, I.; Kovacs, T.; Kasler, M.; Matrai, Z. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: An aesthetic and functional prospective cohort study. Eur. J. Surg. Oncol. 2017, 43, 303–310. [Google Scholar] [CrossRef]
- Silverstein, M.J.; Savalia, N.; Khan, S.; Ryan, J. Extreme oncoplasty: Breast conservation for patients who need mastectomy. Breast J. 2015, 21, 52–59. [Google Scholar] [CrossRef]
- Tousimis, E.; Haslinger, M. Overview of indications for nipple sparing mastectomy. Gland Surg. 2018, 7, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Piato, J.R.; de Andrade, R.D.; Chala, L.F.; de Barros, N.; Mano, M.S.; Melitto, A.S.; Goncalves, R.; Soares Junior, J.M.; Baracat, E.C.; Filassi, J.R. MRI to predict nipple involvement in breast cancer patients. AJR Am. J. Roentgenol. 2016, 206, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Hayashi, N.; Nagura, N.; Kajiura, Y.; Yoshida, A.; Takei, J.; Suzuki, K.; Tsunoda, H.; Yamauchi, H. Long-term oncologic safety of nipple-sparing mastectomy with immediate reconstruction. Clin. Breast Cancer 2021, 21, 352–359. [Google Scholar] [CrossRef]
- Koike-Shimo, A.; Tsugawa, K.; Kawamoto, H.; Kanemaki, Y.; Maeda, I. Oncologic outcome and technical consideration of nipple-sparing mastectomy in breast cancer: The St. Marianna experience with 384 patients. J. Clin. Oncol. 2014, 32, e12024. [Google Scholar] [CrossRef]
- del Riego, J.; Pitarch, M.; Codina, C.; Nebot, L.; Andreu, F.J.; Aparicio, O.; Medina, A.; Martin, A. Multimodality approach to the nipple-areolar complex: A pictorial review and diagnostic algorithm. Insights Imaging 2020, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Ponzone, R.; Maggiorotto, F.; Carabalona, S.; Rivolin, A.; Pisacane, A.; Kubatzki, F.; Renditore, S.; Carlucci, S.; Sgandurra, P.; Marocco, F.; et al. MRI and intraoperative pathology to predict nipple-areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur. J. Cancer 2015, 51, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Nam, S.J.; Kim, S.W.; Lee, S.K.; Bae, S.Y.; Yi, H.W.; Park, S.; Paik, H.J.; Lee, J.E. Feasibility of nipple-sparing mastectomy with immediate breast reconstruction in breast cancer patients with tumor-nipple distance less than 2.0 cm. World J. Surg. 2016, 40, 2028–2035. [Google Scholar] [CrossRef]
- Mariscotti, G.; Durando, M.; Houssami, N.; Berzovini, C.M.; Esposito, F.; Fasciano, M.; Campanino, P.P.; Bosco, D.; Bussone, R.; Ala, A.; et al. Preoperative MRI evaluation of lesion-nipple distance in breast cancer patients: Thresholds for predicting occult nipple-areola complex involvement. Clin. Radiol. 2018, 73, 735–743. [Google Scholar] [CrossRef]
- Gao, Y.; Brachtel, E.F.; Hernandez, O.; Heller, S.L. An analysis of nipple enhancement at breast MRI with radiologic-pathologic correlation. Radiographics 2019, 39, 10–27. [Google Scholar] [CrossRef]
- Seki, H.; Sakurai, T.; Mizuno, S.; Tokuda, T.; Kaburagi, T.; Seki, M.; Karahashi, T.; Nakajima, K.; Shimizu, K.; Jinno, H. A novel nipple-areola complex involvement predictive index for indicating nipple-sparing mastectomy in breast cancer patients. Breast Cancer 2019, 26, 808–816. [Google Scholar] [CrossRef]
- Balci, F.L.; Kara, H.; Dulgeroglu, O.; Uras, C. Oncologic safety of nipple-sparing mastectomy in patients with short tumor-nipple distance. Breast J. 2019, 25, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.D.; Salibian, A.A.; Lee, J.; Harris, K.; Axelrod, D.M.; Guth, A.A.; Shapiro, R.L.; Schnabel, F.R.; Karp, N.S.; Choi, M. Oncologic trends, outcomes, and risk factors for locoregional recurrence: An analysis of tumor-to-nipple distance and critical factors in therapeutic nipple-sparing mastectomy. Plast. Reconstr. Surg. 2019, 143, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Liebens, F.P.; Carly, B.; Pastijn, A.; Rozenberg, S. Management of BRCA1/2 associated breast cancer: A systematic qualitative review of the state of knowledge in 2006. Eur. J. Cancer 2007, 43, 238–257. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. Complete Accreditation Information: Breast MRI. 2020. Available online: https://accreditationsupport.acr.org/support/solutions/articles/11000063266-complete-accreditation-information-breast-mri (accessed on 23 March 2021).
- American College of Radiology. ACR Appropriateness Criteria®: Monitoring Response to Neoadjuvant Systemic Therapy for Breast Cancer. 2017. Available online: https://acsearch.acr.org/list (accessed on 23 March 2021).
- American College of Radiology Committee on Quality Assurance in Magnetic Resonance Imaging. Magnetic Resonance Imaging. Quality Control Manual. 2016. Available online: https://www.acr.org/-/media/ACR/NOINDEX/QC-Manuals/MR_QCManual.pdf (accessed on 23 March 2021).
- American College of Radiology Committee on MR Safety. ACR Manual on MR Safety. Version 1.0. 2020. Available online: https://www.acr.org/Clinical-Resources/Radiology-Safety/MR-Safety (accessed on 23 March 2021).
- American College of Radiology Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. 2021. Available online: https://www.acr.org/Clinical-Resources/Contrast-Manual (accessed on 13 August 2021).
- American College of Radiology. ACR Practice Parameter for the Performance of Magnetic Resonance Imaging-Guided Breast Interventional Procedures. 2016. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Guided-Breast.pdf?la=en (accessed on 16 March 2020).
- American College of Radiology. ACR BI-RADS Atlas. Breast Imaging Reporting and Data System. 2013. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed on 13 November 2019).
- Amurao, M.R.; Einstein, S.A.; Panda, A.; Och, J.G.; Pooley, R.A.; Yanasak, N.E.; American College of Radiology (ACR); American Association of Physicists in Medicine (AAPM). ACR–AAPM Technical Standard for Diagnostic Medical Physics Performance Monitoring of Magnetic Resonance (MR) Imaging Equipment. 2019. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/mr-equip.pdf?la=en (accessed on 20 September 2019).
- DeMartini, W.B.; Rahbar, H. Breast magnetic resonance imaging technique at 1.5 T and 3 T: Requirements for quality imaging and American College of Radiology accreditation. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.D.; Lipson, J.A.; Ikeda, D.M.; Lee, J.M. Updates and revisions to the BI-RADS magnetic resonance imaging lexicon. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 483–493. [Google Scholar] [CrossRef]
- Covington, M.F.; Young, C.A.; Appleton, C.M. American College of Radiology accreditation, performance metrics, reimbursement, and economic considerations in breast MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 303–314. [Google Scholar] [CrossRef]
- Bick, U.; Trimboli, R.M.; Athanasiou, A.; Balleyguier, C.; Baltzer, P.A.T.; Bernathova, M.; Borbely, K.; Brkljacic, B.; Carbonaro, L.A.; Clauser, P.; et al. Image-guided breast biopsy and localisation: Recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 2020, 11, 12. [Google Scholar] [CrossRef]
- Rahbar, H.; Hanna, L.G.; Gatsonis, C.; Mahoney, M.C.; Schnall, M.D.; DeMartini, W.B.; Lehman, C.D. Contralateral Prophylactic Mastectomy in the American College of Radiology Imaging Network 6667 trial: Effect of Breast MR Imaging Assessments and Patient Characteristics. Radiology 2014, 273, 53–60. [Google Scholar] [CrossRef]
- DeMartini, W.B.; Hanna, L.; Gatsonis, C.; Mahoney, M.C.; Lehman, C.D. Evaluation of tissue sampling methods used for MRI-detected contralateral breast lesions in the American College of Radiology Imaging Network 6667 trial. AJR Am. J. Roentgenol. 2012, 199, W386–W391. [Google Scholar] [CrossRef]
- Weinstein, S.P.; Hanna, L.G.; Gatsonis, C.; Schnall, M.D.; Rosen, M.A.; Lehman, C.D. Frequency of malignancy seen in probably benign lesions at contrast-enhanced breast MR imaging: Findings from ACRIN 6667. Radiology 2010, 255, 731–737. [Google Scholar] [CrossRef]
- Lehman, C.D.; Gatsonis, C.; Kuhl, C.K.; Hendrick, R.E.; Pisano, E.D.; Hanna, L.; Peacock, S.; Smazal, S.F.; Maki, D.D.; Julian, T.B.; et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N. Engl. J. Med. 2007, 356, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Rakow-Penner, R.; Murphy, P.M.; Dale, A.; Ojeda-Fournier, H. State of the art diffusion weighted imaging in the breast: Recommended protocol. Curr. Radiol. Rep. 2017, 5, 3. [Google Scholar] [CrossRef]
- Nessim, C.; Winocour, J.; Holloway, D.P.; Saskin, R.; Holloway, C.M. Wait times for breast cancer surgery: Effect of magnetic resonance imaging and preoperative investigations on the diagnostic pathway. J. Oncol. Pract. 2015, 11, e131–e138. [Google Scholar] [CrossRef]
- Hollingsworth, A.B.; Stough, R.G. Preoperative breast MRI: Barking up the wrong endpoints. Breast Dis. 2015, 26, 19–25. [Google Scholar] [CrossRef]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Böhm-Vélez, M.; Mahoney, M.C.; Evans, W.P., 3rd; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Adams, A.M.; Jong, R.A.; Barr, R.G.; Lehrer, D.E.; Pisano, E.D.; Evans, W.P., 3rd; Mahoney, M.C.; Hovanessian Larsen, L.; et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology 2010, 254, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.; Weigel, S.; Schrading, S.; Arand, B.; Bieling, H.; Konig, R.; Tombach, B.; Leutner, C.; Rieber-Brambs, A.; Nordhoff, D.; et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: The EVA trial. J. Clin. Oncol. 2010, 28, 1450–1457. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.D.; Bieling, H.B. Abbreviated breast Magnetic Resonance Imaging (MRI): First postcontrast subtracted images and maximum-intensity projection—A novel approach to breast cancer screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef]
- Mann, R.M.; van Zelst, J.C.M.; Vreemann, S.; Mus, R.D.M. Is Ultrafast or Abbreviated Breast MRI Ready for Prime Time? Curr. Breast Cancer Rep. 2019, 11, 9–16. [Google Scholar] [CrossRef]
- Heacock, L.; Reig, B.; Lewin, A.A.; Toth, H.K.; Moy, L.; Lee, C.S. Abbreviated breast MRI: Road to clinical implementation. J. Breast Imaging 2020, 2, 201–214. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Osorio, S.; Florez, K.; Ospino, A.; Diaz, G.M. Abbreviated magnetic resonance imaging in breast cancer: A systematic review of literature. Eur. J. Radiol. Open 2021, 8, 100307. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. Complete Accreditation Information: MRI and Breast MRI. 2022. Available online: https://accreditationsupport.acr.org/support/solutions/folders/11000012261 (accessed on 14 February 2023).
- Choudhery, S.; Chou, S.H.S.; Chang, K.; Kalpathy-Cramer, J.; Lehman, C.D. Kinetic Analysis of Lesions Identified on a Rapid Abridged Multiphase (RAMP) Breast MRI Protocol. Acad. Radiol. 2020, 27, 672–681. [Google Scholar] [CrossRef] [PubMed]
| Factor | Potential Benefits | Potential Harms |
|---|---|---|
| High Sensitivity |
|
|
| Specificity |
|
|
| Patient Factors |
|
|
| Adverse Effects |
| |
| Delay in Treatment |
| |
| Equity |
|
|
| Cost |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muradali, D.; Fletcher, G.G.; Cordeiro, E.; Fienberg, S.; George, R.; Kulkarni, S.; Seely, J.M.; Shaheen, R.; Eisen, A. Preoperative Breast Magnetic Resonance Imaging: An Ontario Health (Cancer Care Ontario) Clinical Practice Guideline. Curr. Oncol. 2023, 30, 6255-6270. https://doi.org/10.3390/curroncol30070463
Muradali D, Fletcher GG, Cordeiro E, Fienberg S, George R, Kulkarni S, Seely JM, Shaheen R, Eisen A. Preoperative Breast Magnetic Resonance Imaging: An Ontario Health (Cancer Care Ontario) Clinical Practice Guideline. Current Oncology. 2023; 30(7):6255-6270. https://doi.org/10.3390/curroncol30070463
Chicago/Turabian StyleMuradali, Derek, Glenn G. Fletcher, Erin Cordeiro, Samantha Fienberg, Ralph George, Supriya Kulkarni, Jean M. Seely, Rola Shaheen, and Andrea Eisen. 2023. "Preoperative Breast Magnetic Resonance Imaging: An Ontario Health (Cancer Care Ontario) Clinical Practice Guideline" Current Oncology 30, no. 7: 6255-6270. https://doi.org/10.3390/curroncol30070463
APA StyleMuradali, D., Fletcher, G. G., Cordeiro, E., Fienberg, S., George, R., Kulkarni, S., Seely, J. M., Shaheen, R., & Eisen, A. (2023). Preoperative Breast Magnetic Resonance Imaging: An Ontario Health (Cancer Care Ontario) Clinical Practice Guideline. Current Oncology, 30(7), 6255-6270. https://doi.org/10.3390/curroncol30070463