Lorlatinib Effectiveness and Quality-of-Life in Patients with ALK-Positive NSCLC Who Had Failed Second-Generation ALK Inhibitors: Canadian Real-World Experience
Abstract
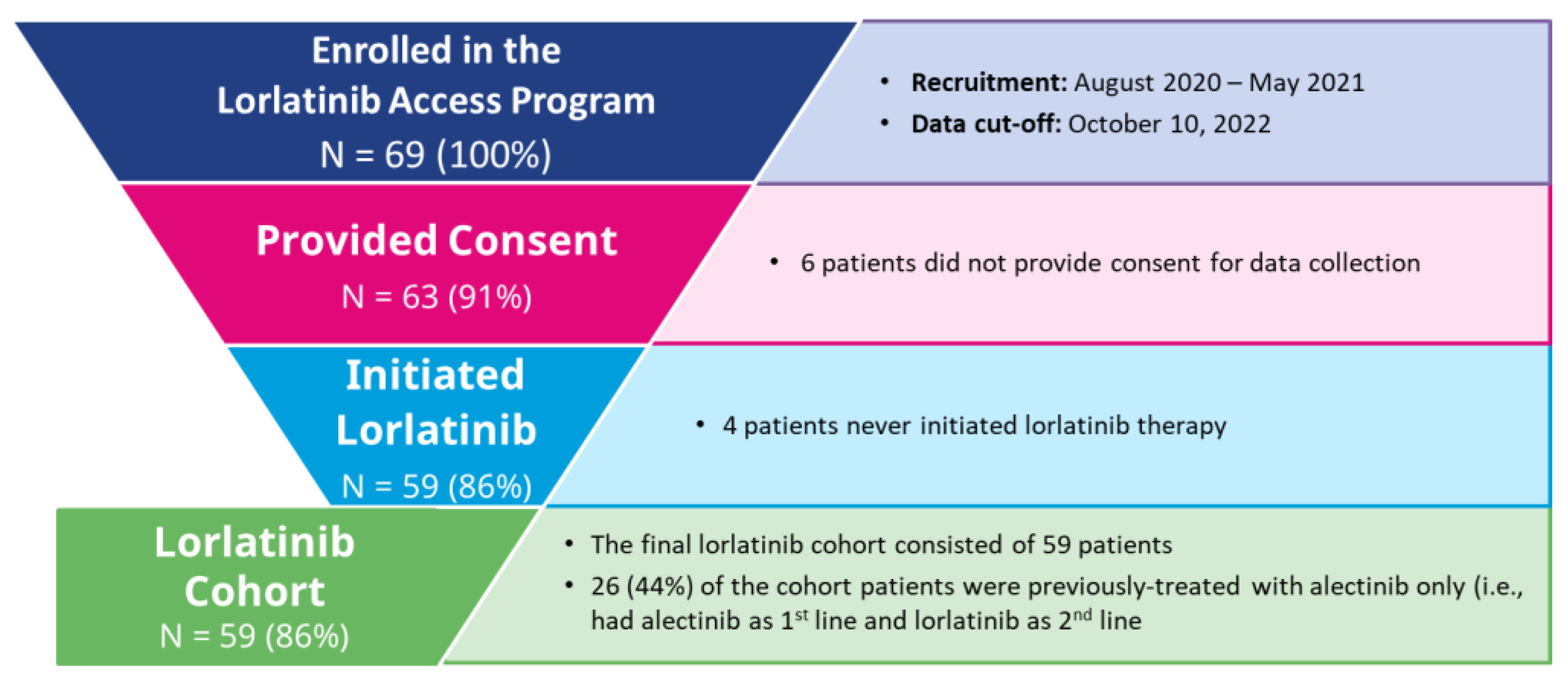
:1. Introduction
| Study Type | n | Median PFS in Months (95% CI) | Median DoT in Months [Range, Unless Otherwise Stated] |
|---|---|---|---|
| Cohort Exp 3B-5, Phase 2 study (NCT01970865)—Multi-country/Global [28] | 139 | 6.6 (5.4–7.4) | 10.1 [0.2–43.2] |
| Cohort 2, Phase 2 study (NCT03909971)—People’s Republic of China [29] | 42 | 5.6 (2.9–9.7) | 8.43 [range: 0.7–14.9] * |
| RWE Study—France [22] | 195 | 9.9 (6.0–12.3) | 11.8 [95% CI: 8.5–18.8] |
| RWE Study—Hong Kong, Singapore, South Korea, Taiwan, Thailand, and the United States [23] | 76 | 9.3 (6.5–not reached) | 5.6 [0–24.7] |
| RWE Study—Taiwan [24] | 22 | 6.2 (4.2–8.3) | Not reported |
| RWE Study—Korea [25] | 10 | 6.5 (1.0–16.5) # | 5.8 [1.3–16.5] # |
| RWE Study—Germany [26] | 37 | 7.1 (4.9–9.3) | 10.4 [95% CI: 6.5–12.8] $ |
| RWE Study—Austria [27] | 37 | Not reported | 4.4 [95% CI: 1.3–7.6] |
| RWE Study—Japan [20] | 51 | Not reported | 11.1 [95% CI, 4.6–13.8] ** |
| RWE Study—Korea [21] | 90 | 7.5 | Not reported |
2. Methods
2.1. Study Design and Study Population
2.2. Follow-Up and Data Sources
2.3. Outcomes and Covariates
2.4. Analysis
2.5. Stratified Analysis
3. Results
3.1. Baseline Characteristics
3.2. Time-to-Treatment Discontinuation (TTD)
3.3. Change in Patients’ Health Utility Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Society: Toronto, ON, Canada, 2020; Available online: cancer.ca/Canadian-Cancer-Statistics-2020-EN (accessed on 14 October 2022).
- Arbour, K.C.; Riely, G.J. Diagnosis and Treatment of Anaplastic Lymphoma Kinase–Positive Non–Small Cell Lung Cancer. Hematol. Clin. N. Am. 2016, 31, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Denault, M.-H.; Labbé, C.; St-Pierre, C.; Fournier, B.; Gagné, A.; Morillon, C.; Joubert, P.; Simard, S.; Martel, S. Wait Times and Survival in Lung Cancer Patients across the Province of Quebec, Canada. Curr. Oncol. 2022, 29, 3187–3199. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’amico, T.A.; et al. Non–Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Hendriks, L.; Kerr, K.; Menis, J.; Mok, T.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors inALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Health Canada. Lorbrena (Lorlatinib) Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00065981.PDF (accessed on 14 October 2022).
- Ignatius Ou, S.H.; Nishio, M.; Ahn, M.J.; Mok, T.; Barlesi, F.; Zhou, C.; Felip, E.; de Marinis, F.; Kim, S.W.; Perol, M.; et al. Efficacy of Brigatinib in Patients With Advanced Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer Who Progressed on Alectinib or Ceritinib: ALTA-2 Study. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Lin, J.J.; Schoenfeld, A.J.; Zhu, V.W.; Yeap, B.Y.; Chin, E.; Rooney, M.; Plodkowski, A.J.; Digumarthy, S.R.; Dagogo-Jack, I.; Gainor, J.F.; et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 258–265. [Google Scholar] [CrossRef]
- Nishio, M.; Yoshida, T.; Kumagai, T.; Hida, T.; Toyozawa, R.; Shimokawaji, T.; Goto, K.; Nakagawa, K.; Ohe, Y.; Seto, T.; et al. Brigatinib in Japanese Patients With ALK-Positive NSCLC Previously Treated With Alectinib and Other Tyrosine Kinase Inhibitors: Outcomes of the Phase 2 J-ALTA Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 452–463. [Google Scholar] [CrossRef]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Myall, N.J.; Sun, F.; Patil, T.; Mushtaq, R.; Yu, C.; Sinha, S.; Pollom, E.L.; Nagpal, S.; Camidge, D.R.; et al. Brain Metastases in EGFR- and ALK-Positive NSCLC: Outcomes of Central Nervous System-Penetrant Tyrosine Kinase Inhibitors Alone Versus in Combination With Radiation. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 116–129. [Google Scholar] [CrossRef]
- Solomon, B.J.; Bauer, T.M.; Ignatius Ou, S.-H.; Liu, G.; Hayashi, H.; Bearz, A.; Penkov, K.; Wu, Y.-L.; Arrieta, O.; Jassem, J.; et al. Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non–Small-Cell Lung Cancer from the Phase III CROWN Study. J. Clin. Oncol. 2022, 40, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.; Sasane, M.; Zhang, J.; Culver, K.W.; Dea, K.; Nitulescu, R.; Wu, E.Q. Brain metastases in patients with ALK+ non-small cell lung cancer: Clinical symptoms, treatment patterns and economic burden. J. Med. Econ. 2015, 18, 312–322. [Google Scholar] [CrossRef]
- Peters, S.; Bexelius, C.; Munk, V.; Leighl, N. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat. Rev. 2016, 45, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.S.; Wong, W.; Ravelo, A.; Miller, P.J.E.; Schwartzberg, L.S. Effect of Brain Metastasis on Patient-Reported Outcomes in Advanced NSCLC Treated in Real-World Community Oncology Settings. Clin. Lung Cancer 2018, 19, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- INESSS: Extrait D’avis au Ministre. Lorbrena—Cancer du Poumon non à Petites Cellules. Available online: https://inesss.algorithmes-onco.info/fr/algorithme-investigation-traitement-suivi-cancer-poumon-5v.22 (accessed on 14 October 2022).
- pCODR Expert Review Committee (pERC). Lorlatinib (Lorbrena) NSCLC—pERC Final Recommendation. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10183LorlatinibNSCLC_FnRec_ApprovedbyChair_Post_30Jan2020_final.pdf (accessed on 14 October 2022).
- Tamiya, M.; Goto, Y.; Kenmotsu, H.; Kurata, T.; Murakami, S.; Yanagitani, N.; Taniguchi, H.; Kuyama, S.; Shimizu, J.; Yokoyama, T.; et al. EP08.02-115. A Retrospective, Multicenter, Observational Study to Evaluate Outcomes with Lorlatinib After Alectinib in ALK+ NSCLC in Japan. J. Thorac. Oncol. 2022, 17, S456. [Google Scholar]
- AHN, B.; Lee, S.; Lim, S.M.; Lee, Y.; Kim, H.R.; Cho, B.C.; Han, J.-Y.; Hong, M.H. EP08.02-112. Real-world Analysis of the Efficacy and Safety of lorlatinib in Patients with ALK-Positive Non-small Cell Lung Cancer in Korea. J. Thorac. Oncol. 2022, 17, S402. [Google Scholar]
- Baldacci, S.; Besse, B.; Avrillon, V.; Mennecier, B.; Mazieres, J.; Dubray-Longeras, P.; Cortot, A.B.; Descourt, R.; Doubre, H.; Quantin, X.; et al. Lorlatinib for advanced anaplastic lymphoma kinase-positive non-small cell lung cancer: Results of the IFCT-1803 LORLATU cohort. Eur. J. Cancer 2022, 166, 51–59. [Google Scholar] [CrossRef]
- Zhu, V.W.; Lin, Y.T.; Kim, D.W.; Loong, H.H.; Nagasaka, M.; To, H.; Ang, Y.L.; Ock, C.Y.; Tchekmedyian, N.; Ou, S.I.; et al. An International Real-World Analysis of the Efficacy and Safety of Lorlatinib Through Early or Expanded Access Programs in Patients With Tyrosine Kinase Inhibitor-Refractory ALK-Positive or ROS1-Positive NSCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1484–1496. [Google Scholar] [CrossRef]
- Lee, P.-H.; Chen, K.-C.; Hsu, K.-H.; Huang, Y.-H.; Tseng, J.-S.; Yang, T.-Y.; Chang, G.-C. Real-world efficacy and safety of lorlatinib in treating advanced ALK-positive non–small cell lung cancer patients. Anti-Cancer Drugs 2021, 32, 1099–1104. [Google Scholar]
- Lee, J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Choi, Y.; Ahn, M.J. Efficacy and Safety of Lorlatinib in Korean Non-Small-Cell Lung Cancer Patients With ALK or ROS1 Rearrangement Whose Disease Failed to Respond to a Previous Tyrosine Kinase Inhibitor. Clin. Lung Cancer 2019, 20, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Frost, N.; Christopoulos, P.; Kauffmann-Guerrero, D.; Stratmann, J.; Riedel, R.; Schaefer, M.; Alt, J.; Gütz, S.; Christoph, D.C.; Laack, E.; et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: Results from the German early access program. Ther. Adv. Med. Oncol. 2021, 13, 1758835920980558. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Fabikan, H.; Illini, O.; Weinlinger, C.; Setinek, U.; Krenbek, D.; Prosch, H.; Rauter, M.; Schumacher, M.; Wöll, E.; et al. Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals 2020, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Shaw, A.T.; Bearz, A.; Camidge, D.R.; Solomon, B.J.; Bauman, J.R.; Bauer, T.M.; Peters, S.; Toffalorio, F.; Abbattista, A.; et al. Intracranial and extracranial efficacy of lorlatinib in patients with ALK-positive non-small-cell lung cancer previously treated with second-generation ALK TKIs. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 620–630. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, Q.; Liu, X.; Du, Y.; Fan, Y.; Cheng, Y.; Fang, J.; Lu, Y.; Huang, C.; Zhou, J.; et al. Lorlatinib for Previously Treated ALK-Positive Advanced NSCLC: Primary Efficacy and Safety From a Phase 2 Study in People’s Republic of China. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 816–826. [Google Scholar] [CrossRef]
- Lau, V.I.; Johnson, J.A.; Bagshaw, S.M.; Rewa, O.G.; Basmaji, J.; Lewis, K.A.; Wilcox, M.E.; Barrett, K.; Lamontagne, F.; Lauzier, F.; et al. Health-related quality-of-life and health-utility reporting in critical care. World J. Crit. Care Med. 2022, 11, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Pullenayegum, E.; Gaebel, K.; Bansback, N.; Bryan, S.; Ohinmaa, A.; Poissant, L.; Johnson, J.A.; Canadian, E.Q.D.L.V.S.G. A Time Trade-off-derived Value Set of the EQ-5D-5L for Canada. Med. Care 2016, 54, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Zhou, T.; Guan, H.; Wang, L.; Zhang, Y.; Rui, M.; Ma, A. Health-Related Quality of Life in Patients With Different Diseases Measured With the EQ-5D-5L: A Systematic Review. Front. Public Health 2021, 9, 675523. [Google Scholar] [CrossRef]
- Pickard, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, G.M.; Gong, Y.; Kehl, K.; Mishra-Kalyani, P.; Goldberg, K.B.; Khozin, S.; Kluetz, P.G.; Oxnard, G.R.; Pazdur, R. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 830–838. [Google Scholar] [CrossRef]
- Gong, Y.; Kehl, K.L.; Oxnard, G.R.; Khozin, S.; Mishra-Kalyani, P.S.; Michael, B.G. Time to treatment discontinuation (TTD) as a pragmatic endpoint in metastatic non-small cell lung cancer (mNSCLC): A pooled analysis of 8 trials. J. Clin. Oncol. 2018, 36, 9064. [Google Scholar]
- Jahanzeb, M.; Lin, H.M.; Pan, X.; Yin, Y.; Wu, Y.; Nordstrom, B.; Socinski, M.A. Real-World Treatment Patterns and Progression-Free Survival Associated with Anaplastic Lymphoma Kinase (ALK) Tyrosine Kinase Inhibitor Therapies for ALK+ Non-Small Cell Lung Cancer. Oncologist 2020, 25, 867–877. [Google Scholar] [CrossRef]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.W.; Perol, M.; Ou, S.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Mazieres, J.; Iadeluca, L.; Shaw, A.T.; Solomon, B.J.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Kim, D.W.; Mok, T.; et al. Patient-reported outcomes from the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive non-small cell lung cancer. Lung Cancer 2022, 174, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Bauer, T.M.; Mok, T.S.K.; Liu, G.; Mazieres, J.; de Marinis, F.; Goto, Y.; Kim, D.W.; Wu, Y.L.; Jassem, J.; et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: Updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir. Med. 2023, 11, 354–366. [Google Scholar] [CrossRef]
- Schmid, S.; Garcia, M.; Cheng, S.; Zhan, L.; Chotai, S.; Balaratnam, K.; Khan, K.; Patel, D.; Catherine Brown, M.; Sachdeva, R.; et al. Treatment patterns and outcomes in early-stage ALK-rearranged non-small cell lung cancer. Lung Cancer 2022, 166, 58–62. [Google Scholar] [CrossRef]
- Government of Canada. Optimizing the Use of Real World Evidence to Inform Regulatory Decision-Making. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html (accessed on 11 November 2022).
- Burns, L.; Roux, N.L.; Kalesnik-Orszulak, R.; Christian, J.; Hukkelhoven, M.; Rockhold, F.; O’Donnell, J. Real-World Evidence for Regulatory Decision-Making: Guidance From Around the World. Clin. Ther. 2022, 44, 420–437. [Google Scholar] [CrossRef]
- INESSS. Evaluation of Drugs for Listing Purposes: A Change of Approach. 2018. Available online: https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=web&cd=&ved=0CAIQw7AJahcKEwjAppGbsfv_AhUAAAAAHQAAAAAQAw&url=https%3A%2F%2Fwww.inesss.qc.ca%2Ffileadmin%2Fdoc%2FINESSS%2FInscription_medicaments%2FProcessus%2FEvaluation_of_drugs_a_change_of_approach.pdf&psig=AOvVaw144NjMqnSWkikZPpRw1PxU&ust=1688777669297227&opi=89978449 (accessed on 2 March 2023).
- Roberts, M.H.; Ferguson, G.T. Real-World Evidence: Bridging Gaps in Evidence to Guide Payer Decisions. Pharmacoecon Open 2021, 5, 3–11. [Google Scholar] [CrossRef]
- MacPhail, C.; Snow, S. Not All Canadian Cancer Patients Are Equal-Disparities in Public Cancer Drug Funding across Canada. Curr. Oncol. 2022, 29, 2064–2072. [Google Scholar] [CrossRef] [PubMed]



| Baseline Characteristic | Lorlatinib Cohort n = 59 |
|---|---|
| Age at Lorlatinib Initiation (Years) | |
| Median (IQR) | 62 (55–69) |
| Minimum–Maximum | 35–89 |
| Age group, n (%) | |
| <65 years | 36 (61.0%) |
| 65+ years | 23 (39.0%) |
| Sex, n (%) | |
| Female | 28 (47.5%) |
| Male | 31 (52.5%) |
| CNS Metastases, n (%) | |
| Yes | 19 (32.2%) |
| No or Not evaluated ^ | 40 (67.8%) |
| ECOG Performance Status, n (%) | |
| 0 or 1 | 52 (88.1%) |
| 2+ | 7 (11.9%) |
| Number of Prior ALK TKI Therapies, n (%) | |
| 1 | 29 (49.2%) |
| 2+ | 30 (50.8%) |
| Last ALK TKI Received Before Lorlatinib, n (%) | |
| Alectinib | 43 (72.9%) |
| Brigatinib | 10 (16.9%) |
| Others (ceritinib or crizotinib) # | 6 (10.2%) |
| Lorlatinib Initial dose, n (%) † | |
| 100 mg | 52 (88.1%) |
| 50 mg or 75 mg | 7 (11.9%) |
| Duration of Therapy of the Last ALK TKI Received (Months) | |
| Median (IQR) | 12 (9–16) |
| Minimum–Maximum | 3–44 |
| Not available, n (%) | 32 (54.2%) * |
| Time from Diagnosis of metastatic ALK-positive NSCLC to lorlatinib initiation (Months) | |
| Median (IQR) | 16.7 (11.0–28.0) |
| Minimum–Maximum | 5.0–91.0 |
| Not available, n (%) | 36 (61.0%) * |
| Last Line of Therapy Discontinuation Reason, n (%) | |
| Disease Progression | 23 (39.0%) |
| Tolerability/Adverse Events/Other | 6 (10.2%) |
| Not available | 30 (50.8%) * |
| Total Duration of Therapy for Prior Systemic Therapies (including ALK TKIs) (Months) | |
| Median (IQR) | 15.5 (11.5–26.5) |
| Minimum–Maximum | 3–54 |
| Not available, n (%) | 31 (52.5%) * |
| Stratification | Median TTD (95% CI) | Log-Rank Test |
|---|---|---|
| Number of Prior Lines of ALK TKIs | ||
| 1 prior line (n = 29) | 15.3 months (6.7–not reached) | p = 0.964 |
| 2+ prior lines (n = 30) | 14.5 months (6.7–not reached) | |
| Presence of CNS Metastases | ||
| Yes (n = 19) | Not reached | p = 0.190 |
| No or not evaluated ^ (n = 40) | 12.1 months (6.7–21.2) | |
| ECOG PS | ||
| 0/1 (n = 52) | 15.4 months (9.0–not reached) | p = 0.381 |
| 2+ (n = 7) | 6.3 months (0.7–not reached) | |
| Last ALK TKI Received Before Lorlatinib | ||
| Alectinib (n = 43) | 13.3 months (7.1–not reached) | p = 0.564 |
| Other ALK TKI † (n = 16) | 18.1 months (6.3–not reached) | |
| Stratification | Study Cohort with at Least 3 Months of Follow-Up | Study Cohort with at Least 6 Months of Follow-Up | Study Cohort with at Least 12 Months of Follow-Up | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean HUS at Baseline | Mean HUS at 3 Months | Mean Change from Baseline (95% CI) | Mean HUS at Baseline | Mean HUS at 6 Months | Mean Change from Baseline (95% CI) | Mean HUS at Baseline | Mean HUS at 12 Months | Mean Change from Baseline (95% CI) | |
| Number of Prior Lines of ALK TKIs (% of patients who completed HUS questionnaire) | |||||||||
| 1 prior line | 0.795 (87%) | 0.827 (87%) | +0.032 (−0.023–0.086) | 0.813 (81.8%) | 0.812 (81.8%) | −0.001 (−0.051–0.049) | 0.828 (50%) | 0.857 (50%) | +0.028 (−0.058–0.115) |
| 2+ prior lines | 0.695 (96%) | 0.795 (96%) | +0.100 ‡ (0.020–0.180) (p = 0.016) | 0.730 (82.6%) | 0.747 (82.6%) | +0.016 (−0.092–0.124) | 0.694 (90%) | 0.725 (90%) | +0.030 (−0.078–0.139) |
| Presence of CNS Metastases (% of patients who completed HUS questionnaire) | |||||||||
| No ^ | 0.778 (90.6%) | 0.815 (90.6%) | +0.037 (−0.001–0.074) | 0.812 (83.3%) | 0.789 (83.3%) | −0.023 (−0.091–0.046) | 0.826 (70%) | 0.836 (70%) | +0.010 (−0.067–0.087) |
| Yes | 0.667 (93.8%) | 0.799 (93.8%) | +0.131 ‡ (0.002–0.261) (p = 0.047) | 0.685 (80%) | 0.757 (80%) | +0.071 ‡ (−0.041–0.184) | 0.661 (91.7%) | 0.715 (91.7%) | +0.054 (−0.078–0.185) |
| ECOG Performance Status (% of patients who completed HUS questionnaire) | |||||||||
| 0/1 | 0.796 (>85%) | 0.839 (>85%) | +0.043 (not reported) (p = 0.050) | 0.816 (60–90%) | 0.796 (60–90%) | −0.019 (not reported) | 0.820 (60–90%) | 0.820 (60–90%) | −0.001 (not reported) |
| 2+ | 0.309 (100%) | 0.576 (100%) | +0.267 ‡ (not reported) | 0.262 (60–80%) | 0.579 (60–80%) | +0.317 ‡,# (not reported) | 0.262 (100%) | 0.512 (100%) | +0.250 ‡,# (not reported) |
| Last ALK TKI Received Before Lorlatinib (% of patients who completed HUS questionnaire) | |||||||||
| Alectinib | 0.754 (88.2%) | 0.818 (88.2%) | +0.064 ‡ (0.000–0.128) (p = 0.048) | 0.772 (81.3%) | 0.798 (81.3%) | +0.027 (−0.028–0.081) | 0.761 (73.9%) | 0.807 (73.9%) | +0.046 (−0.033–0.125) |
| Other ALK TKI | 0.712 (100%) | 0.791 (100%) | +0.078 ‡ (−0.006–0.163) | 0.769 (84.6%) | 0.732 (84.6%) | −0.037 (−0.200–0.127) | 0.737 (88.9%) | 0.731 (88.9%) | −0.006 (−0.158–0.147) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupp, M.; Fanton-Aita, F.; Snow, S.; Wheatley-Price, P.; Melosky, B.; Juergens, R.A.; Chu, Q.; Blais, N.; Banerji, S.; Ng, R.; et al. Lorlatinib Effectiveness and Quality-of-Life in Patients with ALK-Positive NSCLC Who Had Failed Second-Generation ALK Inhibitors: Canadian Real-World Experience. Curr. Oncol. 2023, 30, 6559-6574. https://doi.org/10.3390/curroncol30070481
Rupp M, Fanton-Aita F, Snow S, Wheatley-Price P, Melosky B, Juergens RA, Chu Q, Blais N, Banerji S, Ng R, et al. Lorlatinib Effectiveness and Quality-of-Life in Patients with ALK-Positive NSCLC Who Had Failed Second-Generation ALK Inhibitors: Canadian Real-World Experience. Current Oncology. 2023; 30(7):6559-6574. https://doi.org/10.3390/curroncol30070481
Chicago/Turabian StyleRupp, Martin, Fiorella Fanton-Aita, Stephanie Snow, Paul Wheatley-Price, Barbara Melosky, Rosalyn A. Juergens, Quincy Chu, Normand Blais, Shantanu Banerji, Ryan Ng, and et al. 2023. "Lorlatinib Effectiveness and Quality-of-Life in Patients with ALK-Positive NSCLC Who Had Failed Second-Generation ALK Inhibitors: Canadian Real-World Experience" Current Oncology 30, no. 7: 6559-6574. https://doi.org/10.3390/curroncol30070481
APA StyleRupp, M., Fanton-Aita, F., Snow, S., Wheatley-Price, P., Melosky, B., Juergens, R. A., Chu, Q., Blais, N., Banerji, S., Ng, R., Khoudigian, S., Sharma, A., On, P. V., & Liu, G. (2023). Lorlatinib Effectiveness and Quality-of-Life in Patients with ALK-Positive NSCLC Who Had Failed Second-Generation ALK Inhibitors: Canadian Real-World Experience. Current Oncology, 30(7), 6559-6574. https://doi.org/10.3390/curroncol30070481






