Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Review
Abstract
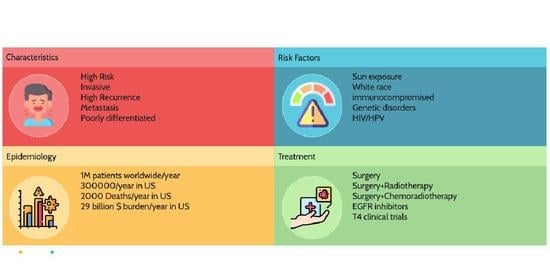
:1. Introduction and Epidemiology
2. Risk Factors
3. Immunosuppression
4. Clinical, Histological, and Pathological Features
5. Lymph Node Metastases
6. TNM Staging
7. Diagnostic Examination and Imaging
8. Prognostic Factors
9. Treatment
9.1. Surgery Alone
9.2. Surgery with Radiotherapy
9.3. Surgery with Adjuvant Chemo Radiotherapy
9.4. Epidermal Growth Factor Receptor (EGFR) Inhibitors
9.5. T4 Clinical Trials
10. Complications
11. Future Implications
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weinberg, A.S.; Ogle, C.A.; Shim, E.K. Metastatic cutaneous squamous cell carcinoma: An update. Dermatol. Surg. 2007, 33, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Arndt, R.; Nindl, I.; Ulrich, C.; Christophers, E.; Stockfleth, E. Association of human papillomavirus infections with cutaneous tumors in immunosuppressed patients. Transpl. Int. 2003, 16, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kwa, R.E.; Campana, K.; Moy, R.L. Biology of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 26, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Hill, D.; Richards, S.; Paver, R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J. Am. Acad. Dermatol. 2005, 53, 253–260. [Google Scholar] [CrossRef]
- Spencer, J.M.; Kahn, S.M.; Jiang, W.; DeLeo, V.A.; Weinstein, I.B. Activated ras genes occur in human actinic keratoses, premalignant precursors to squamous cell carcinomas. Arch. Dermatol. 1995, 131, 796–800. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- D’Souza, J.; Clark, J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 99–105. [Google Scholar] [CrossRef]
- Martorell-Calatayud, A.; Jimenez, O.S.; Mojarrieta, J.C.; Barona, C.G. Cutaneous squamous cell carcinoma: Defining the high-risk variant. Actas Dermo-Sifiliográficas (Engl. Ed.) 2013, 104, 367–379. [Google Scholar] [CrossRef]
- Skulsky, S.L.; O’Sullivan, B.; McArdle, O.; Leader, M.; Roche, M.; Conlon, P.J.; O’Neill, J.P. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines in Oncology. Head Neck 2017, 39, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Oddone, N.; Morgan, G.J.; Palme, C.E.; Perera, L.; Shannon, J.; Wong, E.; Gebski, V.; Veness, M.J. Metastatic cutaneous squamous cell carcinoma of the head and neck. Cancer 2009, 115, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.-D.; Johnson, L.B.; Weiner, M.; Li, K.-P.; Suchindran, C. Epidermolysis bullosa and the risk of life-threatening cancers: The National EB Registry experience, 1986–2006. J. Am. Acad. Dermatol. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Veness, M.J.; Palme, C.E.; Morgan, G.J. High-risk cutaneous squamous cell carcinoma of the head and neck. Cancer 2006, 106, 2389–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef]
- O’Hara, J.; Ferlito, A.; Takes, R.P.; Rinaldo, A.; Strojan, P.; Shaha, A.R.; Rodrigo, J.P.; Paleri, V. Cutaneous squamous cell carcinoma of the head and neck metastasizing to the parotid gland—A review of current recommendations. Head Neck 2011, 33, 1789–1795. [Google Scholar] [CrossRef]
- Jensen, P.; Hansen, S.; Møller, B.; Leivestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef]
- Frierson, H.F.; Deutsch, B.D.; Levine, P.A. Clinicopathologic features of cutaneous squamous cell carcinomas of the head and neck in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hum. Pathol. 1988, 19, 1397–1402. [Google Scholar] [CrossRef]
- Nguyen, P.; Vin-Christian, K.; Ming, M.E.; Berger, T. Aggressive squamous cell carcinomas in persons infected with the human immunodeficiency virus. Arch. Dermatol. 2002, 138, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Southwell, K.E.; Chaplin, J.M.; Eisenberg, R.L.; McIvor, N.P.; Morton, R.P. Effect of immunocompromise on metastatic cutaneous squamous cell carcinoma in the parotid and neck. Head Neck 2006, 28, 244–248. [Google Scholar] [CrossRef]
- Gurudutt, V.V.; Genden, E.M. Cutaneous squamous cell carcinoma of the head and neck. J. Ski. Cancer 2011, 2011, 502723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeny, L.; Zimmerman, T.; Carroll, W.R.; Schmalbach, C.E.; Day, K.E.; Rosenthal, E.L. Head and neck cutaneous squamous cell carcinoma requiring parotidectomy: Prognostic indicators and treatment selection. Otolaryngol.-Head Neck Surg. 2014, 150, 610–617. [Google Scholar] [CrossRef]
- Lai, S.Y.; Weinstein, G.S.; Chalian, A.A.; Rosenthal, D.I.; Weber, R.S. Parotidectomy in the treatment of aggressive cutaneous malignancies. Arch. Otolaryngol.-Head Neck Surg. 2002, 128, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancaccio, G.; Briatico, G.; Pellegrini, C.; Rocco, T.; Moscarella, E.; Fargnoli, M.C. Risk factors and diagnosis of advanced cutaneous squamous cell carcinoma. Dermatol. Pract. Concept. 2021, 11, e2021166S. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.M.; Sinha, Y.; Jaffe, W. Marjolin’s ulcer: A rare entity with a call for early diagnosis. Case Rep. 2015, 2015, bcr2014208176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauterin, T.J.; Veness, M.J.; Morgan, G.J.; Poulsen, M.G.; O’Brien, C.J. Patterns of lymph node spread of cutaneous squamous cell carcinoma of the head and neck. Head Neck J. Sci. Spec. Head Neck 2006, 28, 785–791. [Google Scholar] [CrossRef]
- Ch’ng, S.; Maitra, A.; Lea, R.; Brasch, H.; Tan, S. Parotid metastasis—An independent prognostic factor for head and neck cutaneous squamous cell carcinoma. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 1288–1293. [Google Scholar] [CrossRef]
- O’Brien, C.J.; McNeil, E.B.; McMahon, J.D.; Pathak, I.; Lauer, C.S.; Jackson, M.A. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002, 24, 417–422. [Google Scholar] [CrossRef]
- Moore, B.A.; Weber, R.S.; Prieto, V.; El-Naggar, A.; Holsinger, F.C.; Zhou, X.; Lee, J.J.; Lippman, S.; Clayman, G.L. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 2005, 115, 1561–1567. [Google Scholar] [CrossRef]
- Kraus, D.H.; Carew, J.F.; Harrison, L.B. Regional lymph node metastasis from cutaneous squamous cell carcinoma. Arch. Otolaryngol.-Head Neck Surg. 1998, 124, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Veness, M.J.; Porceddu, S.; Palme, C.E.; Morgan, G.J. Cutaneous head and neck squamous cell carcinoma metastatic to parotid and cervical lymph nodes. Head Neck 2007, 29, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.I.; Clark, J.J.; Veness, M.J.; Milross, C. N1S3: A revised staging system for head and neck cutaneous squamous cell carcinoma with lymph node metastases. Cancer 2010, 116, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Khurana, V.G.; Mentis, D.H.; O’Brien, C.J.; Hurst, T.L.; Stevens, G.N.; Packham, N.A. Parotid and neck metastases from cutaneous squamous cell carcinoma of the head and neck. Am. J. Surg. 1995, 170, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Farasat, S.; Siegrid, S.Y.; Neel, V.A.; Nehal, K.S.; Lardaro, T.; Mihm, M.C.; Byrd, D.R.; Balch, C.M.; Califano, J.A.; Chuang, A.Y. A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: Creation and rationale for inclusion of tumor (T) characteristics. J. Am. Acad. Dermatol. 2011, 64, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- AJCC Cancer Staging Manual Seventh Edition. Available online: https://www.facs.org/media/kcal20pk/ajcc-7e-complete-ajcc-cancer-staging-manual.pdf (accessed on 25 March 2023).
- Hurrell, M.J.; Low, T.-H.; Ebrahimi, A.; Veness, M.; Ashford, B.; Porceddu, S.; Clark, J.R. Evolution of Head and Neck Cutaneous Squamous Cell Carcinoma Nodal Staging—An Australian Perspective. Cancers 2022, 14, 5101. [Google Scholar] [CrossRef]
- Cañueto, J.; Román-Curto, C. Novel Additions to the AJCC’s New Staging Systems for Skin Cancer. Actas Dermo-Sifiliogr. 2017, 108, 818–826. [Google Scholar] [CrossRef]
- Liu, J.; Ebrahimi, A.; Low, T.H.; Gao, K.; Palme, C.E.; Sydney, C.; Ashford, B.G.; Iyer, N.G.; Clark, J.R.; Gupta, R. Predictive value of the 8th edition American Joint Commission Cancer (AJCC) nodal staging system for patients with cutaneous squamous cell carcinoma of the head and neck. J. Surg. Oncol. 2018, 117, 765–772. [Google Scholar] [CrossRef]
- Luk, P.P.; Ebrahimi, A.; Veness, M.J.; McDowell, L.; Magarey, M.; Gao, K.; Palme, C.E.; Clark, J.R.; Gupta, R. Prognostic value of the 8th edition American Joint Commission Cancer nodal staging system for patients with head and neck cutaneous squamous cell carcinoma: A multi-institutional study. Head Neck 2021, 43, 558–567. [Google Scholar] [CrossRef]
- Sood, A.; Wykes, J.; Roshan, D.; Wang, L.Y.; McGuinness, J.; Fowler, A.; Ebrahimi, A. A critical analysis of the prognostic performance of the 8th edition American Joint Committee on Cancer staging for metastatic cutaneous squamous cell carcinoma of the head and neck. Head Neck 2019, 41, 1591–1596. [Google Scholar] [CrossRef]
- Watts, F.; Palme, C.E.; Porceddu, S.; Sundaresan, P.; Clark, J.R.; Gupta, R. Clinician perspectives on the factors influencing prognostic stratification by the American Joint Commission on Cancer Head and Neck Cutaneous Squamous Cell Carcinoma Staging. Surgery 2021, 170, 1467–1473. [Google Scholar] [CrossRef]
- Yousem, D.M.; Som, P.M.; Hackney, D.B.; Schwaibold, F.; Hendrix, R. Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology 1992, 182, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, L.E. MR imaging of perineural tumor spread. Neuroimaging Clin. N. Am. 2004, 14, 663–677. [Google Scholar] [CrossRef]
- Williams, L.S.; Mancuso, A.A.; Mendenhall, W.M. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Bachar, G.; Mizrachi, A.; Rabinovics, N.; Guttman, D.; Shpitzer, T.; Ad-El, D.; Hadar, T. Prognostic factors in metastatic cutaneous squamous cell carcinoma of the head and neck. Ear Nose Throat J. 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Dean, N.R.; Sweeny, L.; Magnuson, J.S.; Carroll, W.R.; Robinson, D.; Desmond, R.A.; Rosenthal, E.L. Outcomes of recurrent head and neck cutaneous squamous cell carcinoma. J. Ski. Cancer 2011, 2011, 972497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, T.S.; Kriesel, K.J.; Hartig, G.K.; Harari, P.M. Parotid area lymph node metastases from cutaneous squamous cell carcinoma: Implications for diagnosis, treatment, and prognosis. Head Neck 2005, 27, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Veness, M.J.; Palme, C.E.; Smith, M.; Cakir, B.; Morgan, G.J.; Kalnins, I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): A better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003, 113, 1827–1833. [Google Scholar] [CrossRef]
- Hinerman, R.W.; Indelicato, D.J.; Amdur, R.J.; Morris, C.G.; Werning, J.W.; Vaysberg, M.; Kirwan, J.; Mendenhall, W.M. Cutaneous Squamous Cell Carcinoma Metastatic to Parotid-Area Lymph Nodes. Laryngoscope 2008, 118, 1989–1996. [Google Scholar] [CrossRef]
- Givi, B.; Andersen, P.E.; Diggs, B.S.; Wax, M.K.; Gross, N.D. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck 2011, 33, 999–1004. [Google Scholar] [CrossRef]
- Audet, N.; Palme, C.E.; Gullane, P.J.; Gilbert, R.W.; Brown, D.H.; Irish, J.; Neligan, P. Cutaneous metastatic squamous cell carcinoma to the parotid gland: Analysis and outcome. Head Neck 2004, 26, 727–732. [Google Scholar] [CrossRef]
- Clayman, G.L.; Lee, J.J.; Holsinger, F.C.; Zhou, X.; Duvic, M.; El-Naggar, A.K.; Prieto, V.G.; Altamirano, E.; Tucker, S.L.; Strom, S.S. Mortality risk from squamous cell skin cancer. J. Clin. Oncol. 2005, 23, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Clark, J.R.; Lorincz, B.B.; Milross, C.G.; Veness, M.J. Metastatic head and neck cutaneous squamous cell carcinoma: Defining a low-risk patient. Head Neck 2012, 34, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Andruchow, J.L.; Veness, M.J.; Morgan, G.J.; Gao, K.; Clifford, A.; Shannon, K.F.; Poulsen, M.; Kenny, L.; Palme, C.E.; Gullane, P. Implications for clinical staging of metastatic cutaneous squamous carcinoma of the head and neck based on a multicenter study of treatment outcomes. Cancer 2006, 106, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Palme, C.E.; O’Brien, C.J.; Veness, M.J.; McNeil, E.B.; Bron, L.P.; Morgan, G.J. Extent of parotid disease influences outcome in patients with metastatic cutaneous squamous cell carcinoma. Arch. Otolaryngol.-Head Neck Surg. 2003, 129, 750–753. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.L.M.; Johnson, J.T.; Myers, E.N. Squamous cell carcinoma of the parotid gland. Head Neck 2006, 28, 626–632. [Google Scholar] [CrossRef]
- Barzilai, G.; Greenberg, E.; Cohen-Kerem, R.; Doweck, I. Pattern of regional metastases from cutaneous squamous cell carcinoma of the head and neck. Otolaryngol. -Head Neck Surg. 2005, 132, 852–856. [Google Scholar] [CrossRef]
- Bron, L.P.; Traynor, S.J.; McNeil, E.B.; O’Brien, C.J. Primary and metastatic cancer of the parotid: Comparison of clinical behavior in 232 cases. Laryngoscope 2003, 113, 1070–1075. [Google Scholar] [CrossRef]
- Dona, E.; Veness, M.J.; Cakir, B.; Morgan, G.J. Metastatic cutaneous squamous cell carcinoma to the parotid: The role of surgery and adjuvant radiotherapy to achieve best outcome. ANZ J. Surg. 2003, 73, 692–696. [Google Scholar] [CrossRef]
- Chua, M.S.; Veness, M.J.; Morgan, G.; Shakespeare, T.; Hehir, A.; Gebski, V.; Cakir, B.; Tiver, K.W. Parotid lymph-node metastases from cutaneous squamous-cell carcinomas: Treatment outcome and prognostic factors following surgery and adjuvant radiotherapy. Australas. Radiol. 2002, 46, 174–179. [Google Scholar] [CrossRef]
- Jol, J.; Van Velthuysen, M.; Hilgers, F.; Keus, R.; Neering, H.; Balm, A. Treatment results of regional metastasis from cutaneous head and neck squamous cell carcinoma. Eur. J. Surg. Oncol. (EJSO) 2003, 29, 81–86. [Google Scholar] [CrossRef]
- Gooris, P.J.; Vermey, A.; de Visscher, J.G.; Burlage, F.R.; Roodenburg, J.L. Supraomohyoid neck dissection in the management of cervical lymph node metastases of squamous cell carcinoma of the lower lip. Head Neck 2002, 24, 678–683. [Google Scholar] [CrossRef] [PubMed]
- DelCharco, J.O.; Mendenhall, W.M.; Parsons, J.T.; Stringer, S.P.; Cassisi, N.J.; Mendenhall, N.P. Carcinoma of the skin metastatic to the parotid area lymph nodes. Head Neck 1998, 20, 369–373. [Google Scholar] [CrossRef]
- O’Bryan, K.; Sherman, W.; Niedt, G.W.; Taback, B.; Manolidis, S.; Wang, A.; Ratner, D. An evolving paradigm for the workup and management of high-risk cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2013, 69, 595–602.e1. [Google Scholar] [CrossRef]
- Schmidt, C.; Martin, J.M.; Khoo, E.; Plank, A.; Grigg, R. Outcomes of nodal metastatic cutaneous squamous cell carcinoma of the head and neck treated in a regional center. Head Neck 2015, 37, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Motley, R.; Kersey, P.; Lawrence, C. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br. J. Plast. Surg. 2003, 56, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Hill, D.; Richards, S.; Paver, R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. Perineural invasion. J. Am. Acad. Dermatol. 2005, 53, 261–266. [Google Scholar] [CrossRef]
- Cherpelis, B.S.; Turner, L.; Ladd, S.; Glass, L.; Fenske, N.A. Innovative 19-Minute Rapid Cytokeratin Immunostaining of Nonmelanoma Skin Cancer in Mohs Micrographic Surgery. Dermatol. Surg. 2009, 35, 1050–1056. [Google Scholar] [CrossRef]
- Zachary, C.B.; Rest, E.B.; Furlong, S.M.; Arcedo, P.N.; Mcgeorge, B.C.; Kist, D.A. Rapid cytokeratin stains enhance the sensitivity of Mohs micrographic surgery for squamous cell carcinoma. J. Dermatol. Surg. Oncol. 1994, 20, 530–535. [Google Scholar] [CrossRef]
- Veness, M.J.; Morgan, G.J.; Palme, C.E.; Gebski, V. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: Combined treatment should be considered best practice. Laryngoscope 2005, 115, 870–875. [Google Scholar] [CrossRef]
- Shin, D.M.; Khuri, F.R.; Murphy, B.; Garden, A.S.; Clayman, G.; Francisco, M.; Liu, D.; Glisson, B.S.; Ginsberg, L.; Papadimitrakopoulou, V. Combined interferon-alfa, 13-cis-retinoic acid, and alpha-tocopherol in locally advanced head and neck squamous cell carcinoma: Novel bioadjuvant phase II trial. J. Clin. Oncol. 2001, 19, 3010–3017. [Google Scholar] [CrossRef]
- Cartei, G.; Cartei, F.; Interlandi, G.; Meneghini, G.; Jop, A.; Zingone, G.; Tabaro, G.; Mazzoleni, F. Oral 5-fluorouracil in squamous cell carcinoma of the skin in the aged. Am. J. Clin. Oncol. 2000, 23, 181–184. [Google Scholar] [CrossRef]
- Bentzen, J.D.; Hansen, H.S. Phase II analysis of paclitaxel and capecitabine in the treatment of recurrent or disseminated squamous cell carcinoma of the head and neck region. Head Neck 2007, 29, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Glisson, B.S.; Feng, L.; Wan, F.; Tang, X.; Wistuba, I.I.; El-Naggar, A.K.; Rosenthal, D.I.; Chambers, M.S.; Lustig, R.A. A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2012, 18, 1435–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Izumi, H.; Oga, A.; Furumoto, H.; Murakami, T.; Ofuji, R.; Muto, M.; Sasaki, K. Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous-cell carcinoma of the skin. Dermatology 2001, 202, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Nanney, L.B.; Magid, M.; Stoscheck, C.M.; King, L.E., Jr. Comparison of epidermal growth factor binding and receptor distribution in normal human epidermis and epidermal appendages. J. Investig. Dermatol. 1984, 83, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ch’ng, S.; Low, I.; Ng, D.; Brasch, H.; Sullivan, M.; Davis, P.; Tan, S.T. Epidermal growth factor receptor: A novel biomarker for aggressive head and neck cutaneous squamous cell carcinoma. Hum. Pathol. 2008, 39, 344–349. [Google Scholar] [CrossRef]
- Bumpous, J. Metastatic cutaneous squamous cell carcinoma to the parotid and cervical lymph nodes: Treatment and outcomes. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 122–125. [Google Scholar] [CrossRef]
- Suen, J.K.; Bressler, L.; Shord, S.S.; Warso, M.; Villano, J.L. Cutaneous squamous cell carcinoma responding serially to single-agent cetuximab. Anti-Cancer Drugs 2007, 18, 827–829. [Google Scholar] [CrossRef]
- Bauman, J.E.; Eaton, K.D.; Martins, R.G. Treatment of recurrent squamous cell carcinoma of the skin with cetuximab. Arch. Dermatol. 2007, 143, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Jalili, A.; Pinc, A.; Pieczkowski, F.; Karlhofer, F.M.; Stingl, G.; Wagner, S.N. Combination of an EGFR blocker and a COX-2 inhibitor for the treatment of advanced cutaneous squamous cell carcinoma. JDDG J. Der Dtsch. Dermatol. Ges. 2008, 6, 1066–1069. [Google Scholar] [CrossRef]
- Bachaud, J.-M.; Cohen-Jonathan, E.; Alzieu, C.; David, J.-M.; Serrano, E.; Daly-Schveitzer, N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 999–1004. [Google Scholar] [CrossRef]
- Adelstein, D.J.; Lavertu, P.; Saxton, J.P.; Secic, M.; Wood, B.G.; Wanamaker, J.R.; Eliachar, I.; Strome, M.; Larto, M.A. Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer 2000, 88, 876–883. [Google Scholar] [CrossRef]
- Palmer, J.D.; Schneider, C.J.; Hockstein, N.; Hanlon, A.L.; Silberg, J.; Strasser, J.; Mauer, E.A.; Dzeda, M.; Witt, R.; Raben, A. Combination of post-operative radiotherapy and cetuximab for high-risk cutaneous squamous cell cancer of the head and neck: A propensity score analysis. Oral Oncol. 2018, 78, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.H.; Deep, N.L.; Nabell, L.; Carroll, W.R.; Desmond, R.; Clemons, L.; Spencer, S.; Magnuson, J.S.; Rosenthal, E.L. Phase 1 study of erlotinib plus radiation therapy in patients with advanced cutaneous squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1275–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Duggan, S.; Deeks, E.D. Cemiplimab: A review in advanced cutaneous squamous cell carcinoma. Drugs 2020, 80, 813–819. [Google Scholar] [CrossRef]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.-J.; Dréno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Martinez, J.C.; Otley, C.C.; Okuno, S.H.; Foote, R.L.; Kasperbauer, J.L. Chemotherapy in the management of advanced cutaneous squamous cell carcinoma in organ transplant recipients: Theoretical and practical considerations. Dermatol. Surg. 2004, 30, 679–686. [Google Scholar] [CrossRef]
- Civantos, F.J.; Moffat, F.L.; Goodwin, W.J. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: Contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006, 116, 1–15. [Google Scholar] [CrossRef]
- Ilmonen, S.; Sollamo, E.; Juteau, S.; Koljonen, V. Sentinel lymph node biopsy in high-risk cutaneous squamous cell carcinoma of the head and neck. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 210–216. [Google Scholar] [CrossRef]
- Gore, S.M.; Shaw, D.; Martin, R.C.; Kelder, W.; Roth, K.; Uren, R.; Gao, K.; Davies, S.; Ashford, B.G.; Ngo, Q. Prospective study of sentinel node biopsy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck 2016, 38, E884–E889. [Google Scholar] [CrossRef]
| Author | Period of Study (Year) | n | Surgery Alone | Surgery + RT | Surgery + RT + CT | Disease-Specific Survival Rate (%) | Overall Survival Rate (%) | Loco Regional Recurrence (%) | Percent Distant Metastasis (%) |
|---|---|---|---|---|---|---|---|---|---|
| Hong et al. [47] | 1989–1999 | 20 | 6 | 14 | - | - | 60 | 15 | 15 |
| Moore et al. [29] | 1996–2001 | 40 | 7 | 29 | 2 | 58.2 | 46.7 | 18 | 17.5 |
| Oddone et al. [12] | 1980–2005 | 250 | 28 | 222 | - | - | 78 | 72 | <20 |
| Veness et al. [48] | 1980–2000 | 167 | 21 | 146 | - | 50 | 58 | 72 | 6 |
| Hinerman et al. [49] | 1969–2005 | 121 | 34 | 87 | 1.65 | 70 | 54 | 26 | - |
| Babak Givi et al. [50] | 1993–2007 | 51 | 11 | 40 | - | - | 30 | 84 | - |
| Audet et al. [51] | 1970–2001 | 56 | 7 | 37 | - | 72 | 53 | 21.5 | - |
| Clayman et al. [52] | 1996–2001 | 210 | 158 | 39 | - | 85 | 70 | 82.38 | 6.19 |
| S.Ch’ng et al. [27] | 1990–2004 | 67 | 28 | 39 | - | 54 | 44 | 52 | 6 |
| Khurana et al. [33] | 1983–1994 | 75 | 14 | 50 | - | 60 | 61 | 43 | 18 |
| Ebrahimi et al. [53] | 1980–2007 | 168 | 33 | 135 | - | 92 | - | 11 | - |
| Andru-Chow et al. [54] | 1960–2003 | 322 | 55 | 267 | - | 74 | - | - | - |
| Palme et al. [55] | 1987–1999 | 126 | 12 | 93 | - | 68 | - | 33 | 4.68 |
| Ying et al. [56] | 1982–2003 | 41 | 14 | 25 | - | 32 | 64 | 49 | 9.76 |
| Barzilai et al. [57] | 1994–2002 | 22 | 10 | 12 | - | - | 56 | 50 | - |
| Bron et al. [58] | 1988–1999 | 101 | 25 | 86 | - | 65 | - | 23 | 7 |
| Dona et al. [59] | 1983–2000 | 74 | 0 | 74 | - | 72 | 58 | 73 | 5 |
| O’Brien et al. [28] | 1987–1999 | 87 | 12 | 75 | - | 63 | - | 68 | 8 |
| Chua et al. [60] | 1980–1997 | 52 | 0 | 52 | - | 65 | 55 | 45 | 8 |
| Jol et al. [61] | 1977–1997 | 41 | 9 | 24 | - | - | 40 | 16 | 7 |
| Gooris et al. [62] | 1975–1998 | 44 | 12 | 32 | - | - | - | 9 | - |
| del Charco et al. [63] | 1966–1994 | 79 | - | 90 | - | 68 | 54 | - | 14 |
| O’Bryan et al. [64] | 2000–2011 | 22 | 4 | 11 | 1 | - | - | - | - |
| Schmidt et al. [65] | 1998–2011 | 113 | 13 | 100 | - | 83 | 80 | 16 | 7 |
| Dean et al. [46] | 1998–2007 | 72 | 24 | 48 | - | 47.2 | - | 65.27 | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, N.; Divatia, M.K.; Jadhav, A.; Wagh, A. Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Review. Curr. Oncol. 2023, 30, 6634-6647. https://doi.org/10.3390/curroncol30070487
Desai N, Divatia MK, Jadhav A, Wagh A. Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Review. Current Oncology. 2023; 30(7):6634-6647. https://doi.org/10.3390/curroncol30070487
Chicago/Turabian StyleDesai, Neha, Mukul K. Divatia, Aniket Jadhav, and Aditya Wagh. 2023. "Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Review" Current Oncology 30, no. 7: 6634-6647. https://doi.org/10.3390/curroncol30070487
APA StyleDesai, N., Divatia, M. K., Jadhav, A., & Wagh, A. (2023). Aggressive Cutaneous Squamous Cell Carcinoma of the Head and Neck: A Review. Current Oncology, 30(7), 6634-6647. https://doi.org/10.3390/curroncol30070487