Exercise Programming Modelling a Standard of Care Approach Improves Physical Health and Patient-Reported Outcomes in Individuals Living with Breast Cancer: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Procedures
2.2. Participants
2.3. Exercise Intervention
2.4. Measurements
2.4.1. Body Composition
2.4.2. Physical Fitness Measures
2.4.3. Patient-Reported Outcomes
2.5. Statistical Analysis
3. Results
3.1. Implementation
3.1.1. Reach
3.1.2. Participant Description
3.2. Effectiveness
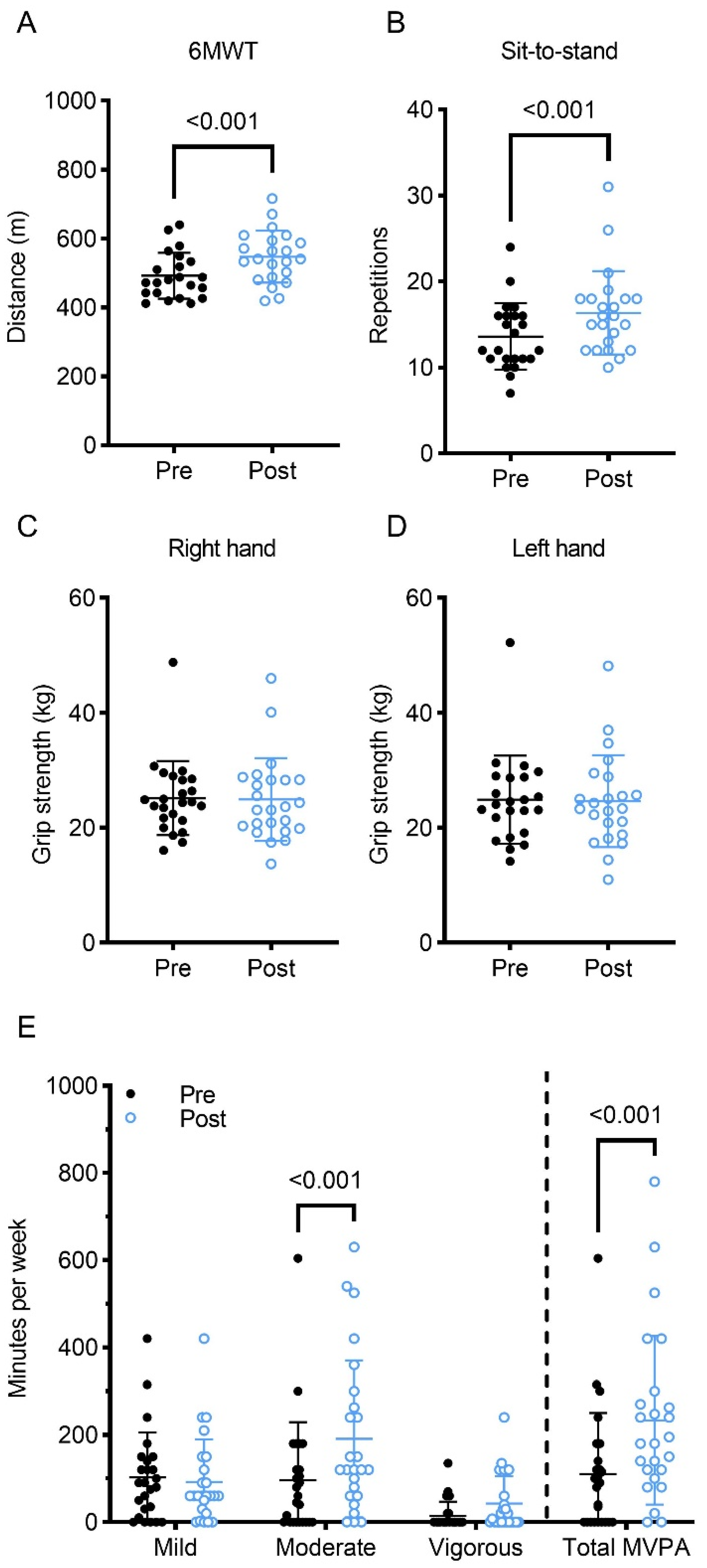
3.2.1. Physical Fitness and Body Composition
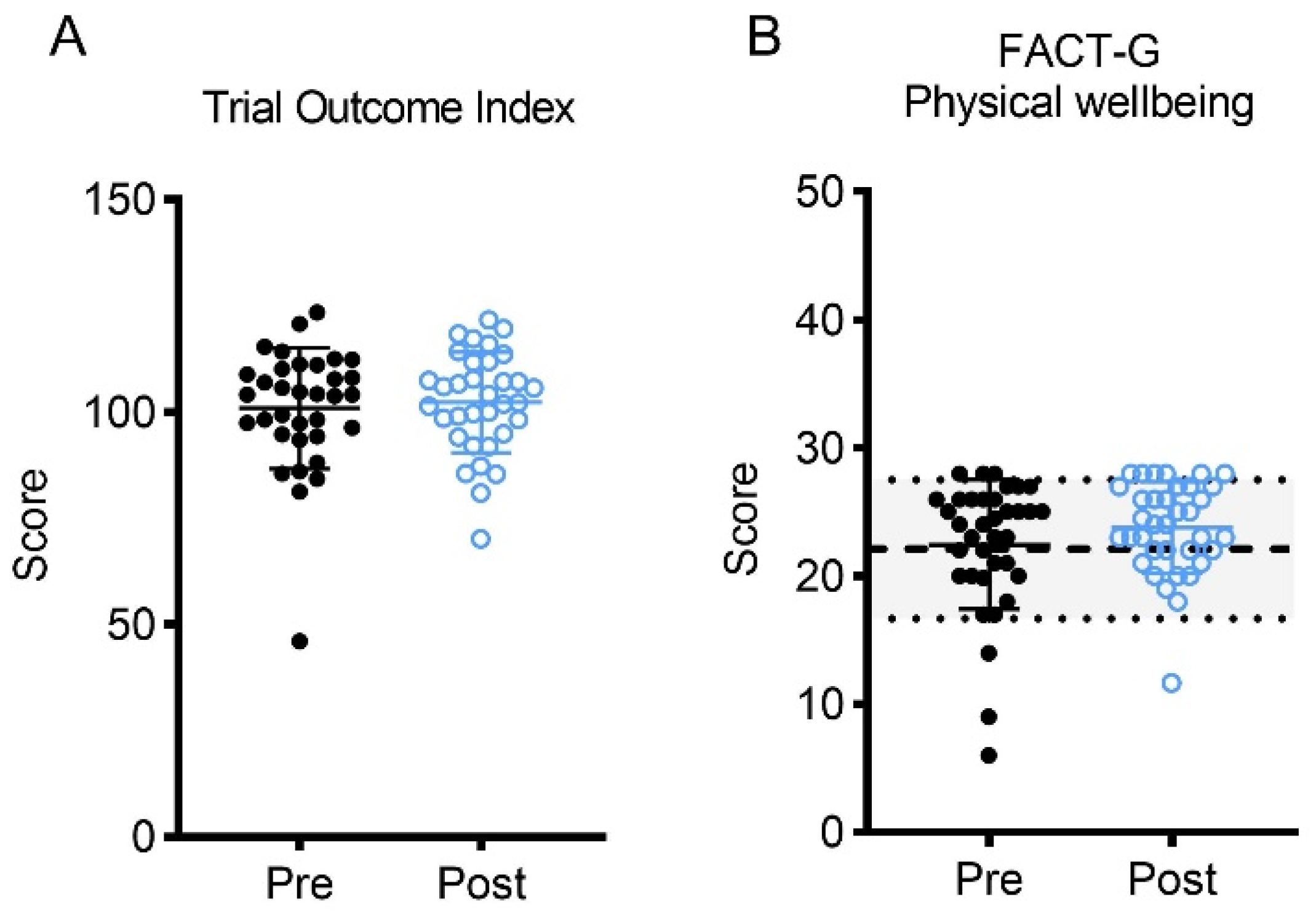
3.2.2. Patient-Reported Outcomes
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolan, L.B.; Barry, D.; Petrella, T.; Davey, L.; Minnes, A.; Yantzi, A.; Marzolini, S.; Oh, P. The Cardiac Rehabilitation Model Improves Fitness, Quality of Life, and Depression in Breast Cancer Survivors. J. Cardiopulm. Rehabil. Prev. 2018, 38, 246–252. [Google Scholar] [CrossRef]
- Baumann, F.T.; Bieck, O.; Oberste, M.; Kuhn, R.; Schmitt, J.; Wentrock, S.; Zopf, E.; Bloch, W.; Schüle, K.; Reuss-Borst, M. Sustainable impact of an individualized exercise program on physical activity level and fatigue syndrome on breast cancer patients in two German rehabilitation centers. Support. Care Cancer 2016, 25, 1047–1054. [Google Scholar] [CrossRef]
- Dittus, K.L.; Lakoski, S.G.; Savage, P.D.; Kokinda, N.; Toth, M.; Stevens, D.; Woods, K.; O’Brien, P.; Ades, P.A. Exercise-Based Oncology Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2015, 35, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, P.; Baumann, F.T. Physical Activity, Exercise and Breast Cancer—What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. BRC 2018, 13, 92–100. [Google Scholar] [CrossRef]
- Do, J.; Cho, Y.; Jeon, J. Effects of a 4-Week Multimodal Rehabilitation Program on Quality of Life, Cardiopulmonary Function, and Fatigue in Breast Cancer Patients. J. Breast Cancer 2015, 18, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Leclerc, A.-F.; Foidart-Dessalle, M.; Tomasella, M.; Coucke, P.; Devos, M.; Bruyère, O.; Bury, T.; Deflandre, D.; Jerusalem, G.; Lifrange, E.; et al. Multidisciplinary Rehabilitation Program after Breast Cancer: Benefits on Physical Function, Anthropometry and Quality of Life. Eur. J. Phys. Rehabil. Med. 2017, 53, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Durstine, J.L. Physical Activity, Exercise, and Chronic Diseases: A Brief Review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Avancini, A.; Pala, V.; Trestini, I.; Tregnago, D.; Mariani, L.; Sieri, S.; Krogh, V.; Boresta, M.; Milella, M.; Pilotto, S.; et al. Exercise Levels and Preferences in Cancer Patients: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 5351. [Google Scholar] [CrossRef] [PubMed]
- Coletta, A.M.; Basen-Engquist, K.M.; Schmitz, K.H. Exercise across the Cancer Care Continuum: Why It Matters, How to Implement It, and Motivating Patients to Move. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.; Coyne, Z.; Houlihan, E.; Leonard, G. Exercise Oncology: An Emerging Discipline in the Cancer Care Continuum. Postgrad. Med. 2022, 134, 26–36. [Google Scholar] [CrossRef]
- Lavallée, J.F.; Abdin, S.; Faulkner, J.; Husted, M. Barriers and Facilitators to Participating in Physical Activity for Adults with Breast Cancer Receiving Adjuvant Treatment: A Qualitative Metasynthesis. Psychooncology 2019, 28, 468–476. [Google Scholar] [CrossRef]
- Keogh, J.W.L.; Olsen, A.; Climstein, M.; Sargeant, S.; Jones, L. Benefits and Barriers of Cancer Practitioners Discussing Physical Activity with Their Cancer Patients. J. Cancer Educ. 2017, 32, 11–15. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise Is Medicine in Oncology: Engaging Clinicians to Help Patients Move through Cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, I.; Norrie, J. Pragmatic Trials. N. Engl. J. Med. 2016, 375, 454–463. [Google Scholar] [CrossRef]
- Pawson, R. Pragmatic Trials and Implementation Science: Grounds for Divorce? BMC Med. Res. Methodol. 2019, 19, 176. [Google Scholar] [CrossRef] [Green Version]
- Patsopoulos, N.A. A Pragmatic View on Pragmatic Trials. Dialog-Clin. Neurosci. 2011, 13, 217–224. [Google Scholar] [CrossRef]
- McNeely, M.L.; Sellar, C.; Williamson, T.; Shea-Budgell, M.; Joy, A.A.; Lau, H.Y.; Easaw, J.C.; Murtha, A.D.; Vallance, J.; Courneya, K.; et al. Community-Based Exercise for Health Promotion and Secondary Cancer Prevention in Canada: Protocol for a Hybrid Effectiveness-Implementation Study. BMJ Open 2019, 9, e029975. [Google Scholar] [CrossRef] [PubMed]
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-Implementation Hybrid Designs. Med. Care 2012, 50, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Cormie, P.; Trevaskis, M.; Thornton-Benko, E.; Zopf, E. Exercise Medicine in Cancer Care. Aust. J. Gen. Pract. 2020, 49, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezenwankwo, E.F.; Nnate, D.A.; Usoro, G.D.; Onyeso, C.P.; Anieto, I.B.; Ibeneme, S.C.; Albertus, Y.; Lambert, V.E.; Ezeukwu, A.O.; Abaraogu, U.O.; et al. A Scoping Review Examining the Integration of Exercise Services in Clinical Oncology Settings. BMC Health Serv. Res. 2022, 22, 236. [Google Scholar] [CrossRef]
- Ehrman, J.K. Inserting Clinical Exercise Physiology into the Continuum of Cancer Care. J. Clin. Exerc. Physiol. 2022, 11, 78–79. [Google Scholar] [CrossRef]
- Thrive Health Services. Available online: https://thrivehealthservices.com/ (accessed on 28 November 2022).
- Canadian Society for Exercise Physiology. Canadian Society for Exercise Physiology—Physical Activity Training for Health (CSEP-PATH); Canadian Society for Exercise Physiology: Ottawa, ON, Canada, 2013. [Google Scholar]
- The American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Rikli, R.; Jones, J.C. Senior Fitness Test Manual, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2013; ISBN 978-1-4504-1118-9. [Google Scholar]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy Scale: Development and Validation of the General Measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.-S.; Stone, A. Self-Reported Fatigue: One Dimension or More? Lessons from the Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-F) Questionnaire. Support. Care Cancer 2011, 19, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Godin, G.; Jobin, J.; Bouillon, J. Assessment of Leisure Time Exercise Behavior by Self-Report: A Concurrent Validity Study. Can. J. Public Health 1986, 77, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire Classification Coding System Using Accelerometer Assessment among Breast Cancer Survivors. J. Cancer Surviv. 2015, 9, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Beck, S.L.; Schwartz, A.L.; Towsley, G.; Dudley, W.; Barsevick, A. Psychometric Evaluation of the Pittsburgh Sleep Quality Index in Cancer Patients. J. Pain Symptom Manag. 2004, 27, 140–148. [Google Scholar] [CrossRef]
- Lovibond, S.; Lovibond, P. Manual for the Depression Anxiety Stress Scales, 2nd ed.; Psychology Foundation: Sydney, NSW, Australia, 1995; ISBN 7334-1423-0. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Brucker, P.S.; Yost, K.; Cashy, J.; Webster, K.; Cella, D. General Population and Cancer Patient Norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval. Health Prof. 2005, 28, 192–211. [Google Scholar] [CrossRef]
- Montan, I.; Löwe, B.; Cella, D.; Mehnert, A.; Hinz, A. General Population Norms for the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue Scale. Value Health 2018, 21, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Maginador, G.; Lixandrão, M.E.; Bortolozo, H.I.; Vechin, F.C.; Sarian, L.O.; Derchain, S.; Telles, G.D.; Zopf, E.; Ugrinowitsch, C.; Conceição, M.S. Aerobic Exercise-Induced Changes in Cardiorespiratory Fitness in Breast Cancer Patients Receiving Chemotherapy: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 2240. [Google Scholar] [CrossRef] [PubMed]
- Asato, C.; Vance, A.; Cheng, S.; Lim, S.; Yamada, P.; Teranishi-Hashimoto, C.; Shepherd, J.; Fukui, J.A. Body Composition Changes in a 12-Week Exercise Intervention for Breast Cancer Patients. JCO 2022, 40, e12500. [Google Scholar] [CrossRef]
- Schneider, A.K.; Leemaqz, S.Y.; Dalton, J.; Verburg, P.E.; Mol, B.W.; Dekker, G.A.; Roberts, C.T.; Grieger, J.A. The Interaction between Metabolic Syndrome and Physical Activity, and Risk for Gestational Diabetes Mellitus. Acta Diabetol. 2021, 58, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Wiskemann, J.; Ulrich, C.M.; Schneeweiss, A.; Steindorf, K. Self-Reported Physical Activity Behavior of Breast Cancer Survivors during and after Adjuvant Therapy: 12 Months Follow-up of Two Randomized Exercise Intervention Trials. Acta Oncol. 2017, 56, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Huedo-Medina, T.B.; Pescatello, L.S.; Ryan, S.M.; Pescatello, S.M.; Moker, E.; LaCroix, J.M.; Ferrer, R.A.; Johnson, B.T. The Efficacy of Exercise in Reducing Depressive Symptoms among Cancer Survivors: A Meta-Analysis. PLoS ONE 2012, 7, e30955. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Postigo-Martin, P.; Granger, C.L.; Waterland, J.; Galiano-Castillo, N.; Denehy, L. The Minimal Clinically Important Difference in the Treadmill Six-Minute Walk Test in Active Women with Breast Cancer during and after Oncological Treatments. Disabil. Rehabil. 2022, 45, 871–878. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and Validation of Criterion-Referenced Clinically Relevant Fitness Standards for Maintaining Physical Independence in Later Years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef]
- Brennan, A.M.; Day, A.G.; Cowan, T.E.; Clarke, G.J.; Lamarche, B.; Ross, R. Individual Response to Standardized Exercise: Total and Abdominal Adipose Tissue. Med. Sci. Sports Exerc. 2020, 52, 490–497. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; DiGuiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular Disease Competes with Breast Cancer as the Leading Cause of Death for Older Females Diagnosed with Breast Cancer: A Retrospective Cohort Study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Ramin, C.; Schaeffer, M.L.; Zheng, Z.; Connor, A.E.; Hoffman-Bolton, J.; Lau, B.; Visvanathan, K. All-Cause and Cardiovascular Disease Mortality among Breast Cancer Survivors in CLUE II, a Long-Standing Community-Based Cohort. J. Natl. Cancer Inst. 2020, 113, 137–145. [Google Scholar] [CrossRef]
- Cornette, T.; Vincent, F.; Mandigout, S.; Antonini, M.T.; Leobon, S.; Labrunie, A.; Venat, L.; Lavau-Denes, S.; Tubiana-Mathieu, N. Effects of Home-Based Exercise Training on VO2 in Breast Cancer Patients under Adjuvant or Neoadjuvant Chemotherapy (SAPA): A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 223–232. [Google Scholar]
- Ibrahim, M.; Muanza, T.; Smirnow, N.; Sateren, W.; Fournier, B.; Kavan, P.; Palumbo, M.; Dalfen, R.; Dalzell, M.-A. A Pilot Randomized Controlled Trial on the Effects of a Progressive Exercise Program on the Range of Motion and Upper Extremity Grip Strength in Young Adults with Breast Cancer. Clin. Breast Cancer 2018, 18, e55–e64. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, D.K.; DuBois, K.; Salerno, E.A. The Effects of Exercise on Cancer-Related Fatigue in Breast Cancer Patients during Primary Treatment: A Meta-Analysis and Systematic Review. Expert Rev. Anticancer Ther. 2020, 20, 865–877. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of Supervised Exercise on Cancer-Related Fatigue in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Troxel, A.B.; Ky, B.; Damjanov, N.; Zemel, B.S.; Rickels, M.R.; Rhim, A.D.; Rustgi, A.K.; Courneya, K.S.; Schmitz, K.H. Dose-Response Effects of Aerobic Exercise among Colon Cancer Survivors: A Randomized Phase II Trial. Clin. Color. Cancer 2018, 17, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Wagoner, C.W.; Dreger, J.; Keats, M.R.; Santa Mina, D.; McNeely, M.L.; Cuthbert, C.; Capozzi, L.C.; Francis, G.J.; Trinh, L.; Sibley, D.; et al. First-Year Implementation of the EXercise for Cancer to Enhance Living Well (EXCEL) Study: Building Networks to Support Rural and Remote Community Access to Exercise Oncology Resources. Int. J. Environ. Res. Public Health 2023, 20, 1930. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agencey of Canada. Canadian Cancer Statistics 2021; Candian Cancer Society: Toronto, ON, Canada, 2021. [Google Scholar]




| Variable | n | Pre: ± SD (Range) | Post: ± SD (Range) | Effect Size |
|---|---|---|---|---|
| Age (years) | 25 | 57.3 ± 10.1 | NA | NA |
| Height (cm) | 23 | 162.1 ± 6.8 | NA | NA |
| 6-MWT (m) | 22 | 491.8 ± 66.5 | 547.3 ± 75.4 * | 1.59 |
| rHR (bpm) | 22 | 78 ± 12 | 81 ± 12 | 0.30 |
| SBP (mmHg) | 22 | 120 ± 17 | 121 ± 19 | 0.01 |
| DBP (mmHg) | 22 | 80 ± 11 | 81 ± 13 | 0.01 |
| Weight (kg) | 23 | 71.2 ± 13.8 | 71.1 ± 14.1 | −0.06 |
| BMI (kg/m2) | 20 | 27.4 ± 5.1 | 27.0 ± 5.4 | −0.20 |
| Waist Circumference (cm) | 23 | 92.2 ± 13.2 | 89.7 ± 12.6 * | −0.67 |
| Hip Circumference (cm) | 23 | 104.9 ± 10.7 | 103.0 ± 9.7 * | −0.65 |
| Hand Grip (R and L) (kg) | 23 | 50.1 ± 7.1 | 49.6 ± 7.6 | −0.04 |
| Balance (R) (s) | 23 | 38.0 ± 14.6 | 37.1 ± 15.4 | −0.16 |
| Balance (L) (s) | 23 | 38.4 ± 13.7 | 38.7 ± 14.3 | 0.02 |
| Balance (Closed, R) (s) | 20 | 11.9 ± 13.3 | 10.1 ± 10.8 | −0.20 |
| Balance (Closed, L) (s) | 20 | 12.6 ± 13.8 | 13.5 ± 14.4 | 0.08 |
| Shoulder (R) (degrees) | 23 | −6.1 ± 7.9 | −5.0 ± 7.2 | 0.31 |
| Shoulder (L) (degrees) | 22 | −9.1 ± 8.1 | −7.9 ± 8.0 | 0.33 |
| Chair Stand | 23 | 14 ± 4 | 16 ± 5 * | 0.90 |
| Sit and Reach (cm) | 23 | 4.0 ± 12.1 | 7.2 ± 8.7 | 0.42 |
| Mild PA/week (min) | 29 | 105 ± 100 (0–420) | 101 ± 93 (0–420) | −0.04 |
| Moderate PA/week (min) | 33 | 91 ± 123 (0–605) | 161 ± 168 * (0–630) | 0.63 |
| Vigorous PA/week (min) | 29 | 14 ± 31 (0–135) | 55 ± 70 * (0–240) | 0.65 |
| MVPA/week (min) | 28 | 91 ± 98 (0–605) | 200 ± 180 * (0–780) | 0.76 |
| LSI | 28 | 26 ± 24 (0–131) | 36 ± 21 * (0–82) | 0.78 |
| Physical Wellbeing | 36 | 23 ± 5 (4–28) | 24 ± 4 * (12–28) | 0.30 |
| Social Wellbeing | 36 | 21 ± 6 (0–28) | 22 ± 5 (11–28) | 0.14 |
| Emotional Wellbeing | 36 | 18 ± 3 (7–24) | 19 ± 4 (10–24) | 0.21 |
| Functional Wellbeing | 36 | 19 ± 7 (0–28) | 20 ± 6 (0–28) | 0.40 |
| Fatigue | 34 | 35 ± 11 (9–52) | 40 ± 9 * (22–52) | 0.52 |
| TOI | 34 | 100 ± 14 (46–124) | 102 ± 12 * (70–122) | 0.12 |
| Stress | 36 | 7 ± 6 (0–34) | 5 ± 6 (0–24) | −0.32 |
| Anxiety | 32 | 3 ± 2 (0–22) | 2 ± 3 (0–10) | −0.23 |
| Depression | 35 | 5 ± 5 (0–37) | 3 ± 3 * (0–10) | −0.43 |
| DASS-42 score | 32 | 13 ± 10 (0–87) | 10 ± 10 (0–37) | −0.40 |
| PSQI score | 26 | 13 ± 3 (8–19) | 12 ± 3 (7–18) | −0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kendall, S.J.; Heinze, S.; Blanchard, C.; Chiekwe, J.C.; Melvin, J.; Culos-Reed, N.; McNeely, M.L.; Keats, M.R.; Grandy, S.A. Exercise Programming Modelling a Standard of Care Approach Improves Physical Health and Patient-Reported Outcomes in Individuals Living with Breast Cancer: A Pilot Study. Curr. Oncol. 2023, 30, 7203-7217. https://doi.org/10.3390/curroncol30080522
Kendall SJ, Heinze S, Blanchard C, Chiekwe JC, Melvin J, Culos-Reed N, McNeely ML, Keats MR, Grandy SA. Exercise Programming Modelling a Standard of Care Approach Improves Physical Health and Patient-Reported Outcomes in Individuals Living with Breast Cancer: A Pilot Study. Current Oncology. 2023; 30(8):7203-7217. https://doi.org/10.3390/curroncol30080522
Chicago/Turabian StyleKendall, Stephanie J., Stefan Heinze, Chris Blanchard, Joy C. Chiekwe, Jennifer Melvin, Nicole Culos-Reed, Margaret L. McNeely, Melanie R. Keats, and Scott A. Grandy. 2023. "Exercise Programming Modelling a Standard of Care Approach Improves Physical Health and Patient-Reported Outcomes in Individuals Living with Breast Cancer: A Pilot Study" Current Oncology 30, no. 8: 7203-7217. https://doi.org/10.3390/curroncol30080522





