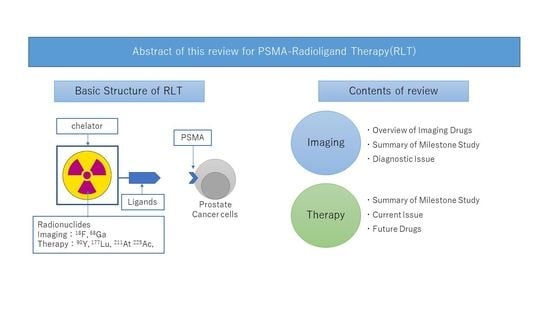
PSMA Targeted Molecular Imaging and Radioligand Therapy for Prostate Cancer: Optimal Patient and Treatment Issues
Abstract
:1. Introduction
2. PSMA-PET Imaging in Prostate Cancer
2.1. PSMA-Targeted Monoclonal Antibody
2.2. Low Molecular Weight (LMW) PSMA Agents
2.2.1. 68Ga-PSMA-11
2.2.2. 18F-PSMA-1007
2.2.3. 18F-DCFPyL
2.3. Reading and Diagnosis of PSMA-PET
2.3.1. Diagnostic Ability of PSMA-PET
2.3.2. Development of Standardized Image Interpretation
2.3.3. PSMA-PET Imaging in Metastatic Prostate Cancer
3. PSMA-Radioligand Therapy (PSMA-RLT)
3.1. 177Lu-PSMA-617
3.1.1. Summary Clinical Trials of 177Lu-PSMA-617
3.1.2. Predictors of Treatment Effectiveness of 177Lu-PSMA-617 and Limitations
3.2. Future Application of PSMA-RLT Agents
3.2.1. PSMA-RLT with β-ray Emitting Radionuclides
3.2.2. PSMA-RLT with α-ray Emitting Radionuclides
3.2.3. Combination Therapies
3.2.4. PSMA-Targeted Nanoparticles
3.3. Clinical Problems in PSMA-RLT
3.3.1. Off-Target Effects
3.3.2. Supply of α- and β-Emitters
3.3.3. Safety and Radiation Protection
3.3.4. Patients with Better Indications for PSMA-RLT Agents
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987, 7, 927–935. [Google Scholar]
- Akhtar, N.H.; Pail, O.; Saran, A.; Tyrell, L.; Tagawa, S.T. Prostate-specific membrane antigen-based therapeutics. Adv. Urol. 2012, 2012, 973820. [Google Scholar] [CrossRef] [Green Version]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Durack, J.C.; Lyashchenko, S.K.; Cheal, S.M.; Beylergil, V.; Lefkowitz, R.A.; Carrasquillo, J.A.; Martinez, D.F.; Fung, A.M.; et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5277–5285. [Google Scholar] [CrossRef] [Green Version]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 1288–1298. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Morigi, J.J.; Giesel, F.; Ceci, F.; Uprimny, C.; Hofman, M.S.; Eiber, M.; Schwarzenbock, S.; Castellucci, P.; et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1622–1635. [Google Scholar] [CrossRef]
- Donswijk, M.L.; Wondergem, M.; de Wit-van der Veen, L.; Bruin, N.M.; van Leeuwen, P.J.; van der Poel, H.G.; Stokkel, M.P.M.; Vogel, W.V. Effects of furosemide and tracer selection on urinary activity and peri-bladder artefacts in PSMA PET/CT: A single-centre retrospective study. EJNMMI Res. 2022, 12, 42. [Google Scholar] [CrossRef]
- Uprimny, C.; Bayerschmidt, S.; Kroiss, A.S.; Fritz, J.; Nilica, B.; Svirydenka, A.; Decristoforo, C.; di Santo, G.; von Guggenberg, E.; Horninger, W.; et al. Impact of forced diuresis with furosemide and hydration on the halo artefact and intensity of tracer accumulation in the urinary bladder and kidneys on [68Ga]Ga-PSMA-11-PET/CT in the evaluation of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 123–133. [Google Scholar] [CrossRef]
- Uprimny, C.; Bayerschmidt, S.; Kroiss, A.S.; Fritz, J.; Nilica, B.; Svirydenka, H.; Decristoforo, C.; von Guggenberg, E.; Horninger, W.; Virgolini, I.J. Early Injection of Furosemide Increases Detection Rate of Local Recurrence in Prostate Cancer Patients with Biochemical Recurrence Referred for 68Ga-PSMA-11 PET/CT. J. Nucl. Med. 2021, 62, 1550–1557. [Google Scholar] [CrossRef]
- Hansen, S.B.; Bender, D. Advancement in Production of Radiotracers. Semin. Nucl. Med. 2022, 52, 266–275. [Google Scholar] [CrossRef]
- Evangelista, L.; Maurer, T.; van der Poel, H.; Alongi, F.; Kunikowska, J.; Laudicella, R.; Fanti, S.; Hofman, M.S. [68Ga]Ga-PSMA Versus [(18)F]PSMA Positron Emission Tomography/Computed Tomography in the Staging of Primary and Recurrent Prostate Cancer. A Systematic Review of the Literature. Eur. Urol. Oncol. 2022, 5, 273–282. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef]
- Alberts, I.; Bütikofer, L.; Rominger, A.; Afshar-Oromieh, A. A randomised, prospective and head-to-head comparison of [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 for the detection of recurrent prostate cancer in PSMA-ligand PET/CT-Protocol design and rationale. PLoS ONE 2022, 17, e0270269. [Google Scholar] [CrossRef]
- Hope, T.A.; Eiber, M.; Armstrong, W.R.; Juarez, R.; Murthy, V.; Lawhn-Heath, C.; Behr, S.C.; Zhang, L.; Barbato, F.; Ceci, F.; et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021, 7, 1635–1642. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Domachevsky, L.; Bernstine, H.; Goldberg, N.; Nidam, M.; Catalano, O.A.; Groshar, D. Comparison between pelvic PSMA-PET/MR and whole-body PSMA-PET/CT for the initial evaluation of prostate cancer: A proof of concept study. Eur. Radiol. 2020, 30, 328–336. [Google Scholar] [CrossRef]
- Park, S.Y.; Zacharias, C.; Harrison, C.; Fan, R.E.; Kunder, C.; Hatami, N.; Giesel, F.; Ghanouni, P.; Daniel, B.; Loening, A.M.; et al. Gallium 68 PSMA-11 PET/MR Imaging in Patients with Intermediate-or High-Risk Prostate Cancer. Radiology 2018, 288, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Corfield, J.; Perera, M.; Bolton, D.; Lawrentschuk, N. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: A systematic review. World J. Urol. 2018, 36, 519–527. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The Impact of 68Ga-PSMA PET/CT on Management Intent in Prostate Cancer: Results of an Australian Prospective Multicenter Study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habl, G.; Sauter, K.; Schiller, K.; Dewes, S.; Maurer, T.; Eiber, M.; Combs, S.E. 68Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate 2017, 77, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.; Joy, A.; Nanabala, R.; Pillai, M.R.A.; Hari, T.A. 68Ga-PSMA PET/CT False-Positive Tracer Uptake in Paget Disease. Clin. Nucl. Med. 2016, 41, e454–e455. [Google Scholar] [CrossRef]
- Ribeiro, A.M.B.; Lima, E.N.P.; Rocha, M.M. Fibrous dysplasia as a possible false-positive finding in 68Ga-labeled prostate-specific membrane antigen positron emission tomography/computed tomography study in the follow-up of prostate cancer. World J. Nucl. Med. 2019, 18, 409–412. [Google Scholar] [CrossRef]
- Bilgin, R.; Ergül, N.; Çermik, T.F. Incidental Meningioma Mimicking Metastasis of Prostate Adenocarcinoma in 68Ga-Labeled PSMA Ligand PET/CT. Clin. Nucl. Med. 2016, 41, 956–958. [Google Scholar] [CrossRef]
- Bertagna, F.; Albano, D.; Cerudelli, E.; Gazzilli, M.; Giubbini, R.; Treglia, G. Potential of Radiolabeled PSMA PET/CT or PET/MRI Diagnostic Procedures in Gliomas/Glioblastomas. Curr. Radiopharm. 2020, 13, 94–98. [Google Scholar] [CrossRef]
- Hermann, R.M.; Djannatian, M.; Czech, N.; Nitsche, M. Prostate-Specific Membrane Antigen PET/CT: False-Positive Results due to Sarcoidosis? Case Rep. Oncol. 2016, 9, 457–463. [Google Scholar] [CrossRef]
- Noto, B.; Vrachimis, A.; Schäfers, M.; Stegger, L.; Rahbar, K. Subacute Stroke Mimicking Cerebral Metastasis in 68Ga-PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2016, 41, e449–e451. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. PSMA-RADS Version 1.0: A Step Towards Standardizing the Interpretation and Reporting of PSMA-targeted PET Imaging Studies. Eur. Urol. 2018, 73, 485–487. [Google Scholar] [CrossRef]
- Toriihara, A.; Nobashi, T.; Baratto, L.; Duan, H.; Moradi, F.; Park, S.; Hatami, N.; Aparici, C.M.; Davidzon, G.; Iagaru, A. Comparison of 3 Interpretation Criteria for 68Ga-PSMA11 PET Based on Inter- and Intrareader Agreement. J. Nucl. Med. 2020, 61, 533–539. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Poyet, C.; Kroeze, S.G.C.; Kranzbühler, B.; Schüler, H.I.G.; Guckenberger, M.; Kaufmann, P.A.; Hermanns, T.; Burger, I.A. Current and potential future role of PSMA-PET in patients with castration-resistant prostate cancer. World J. Urol. 2019, 37, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Fanti, S.; Hadaschik, B.; Herrmann, K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J. Nucl. Med. 2020, 61, 678–682. [Google Scholar] [CrossRef]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Hetzheim, H.; Kratochwil, C.; Benesova, M.; Eder, M.; Neels, O.C.; Eisenhut, M.; Kübler, W.; Holland-Letz, T.; Giesel, F.L.; et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J. Nucl. Med. 2015, 56, 1697–1705. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy With 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef]
- Buteau, J.P.; Martin, A.J.; Emmett, L.; Iravani, A.; Sandhu, S.; Joshua, A.M.; Francis, R.J.; Zhang, A.Y.; Scott, A.M.; Lee, S.T.; et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): A biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022, 23, 1389–1397. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Ravi Kumar, A.S.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef]
- Privé, B.M.; Janssen, M.J.R.; van Oort, I.M.; Muselaers, C.H.J.; Jonker, M.A.; de Groot, M.; Mehra, N.; Verzijlbergen, J.F.; Scheenen, T.W.J.; Zámecnik, P.; et al. Lutetium-177-PSMA-I&T as metastases directed therapy in oligometastatic hormone sensitive prostate cancer, a randomized controlled trial. BMC Cancer 2020, 20, 884. [Google Scholar] [CrossRef]
- Rathke, H.; Flechsig, P.; Mier, W.; Bronzel, M.; Mavriopoulou, E.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Kratochwil, C. Dosimetry Estimate and Initial Clinical Experience with 90Y-PSMA-617. J. Nucl. Med. 2019, 60, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Bruchertseifer, F. Supply and Clinical Application of Actinium-225 and Bismuth-213. Semin. Nucl. Med. 2020, 50, 119–123. [Google Scholar] [CrossRef]
- Ling, S.W.; de Blois, E.; Hooijman, E.; van der Veldt, A.; Brabander, T. Advances in 177Lu-PSMA and 225Ac-PSMA Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Pharmaceutics 2022, 14, 2166. [Google Scholar] [CrossRef]
- Lee, H. Relative Efficacy of 225Ac-PSMA-617 and 177Lu-PSMA-617 in Prostate Cancer Based on Subcellular Dosimetry. Mol. Imaging Radionucl. Ther. 2022, 31, 1–6. [Google Scholar] [CrossRef]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Li, W.; Bai, H.; Zhang, Z. PARP inhibitors in ovarian cancer: Sensitivity prediction and resistance mechanisms. J. Cell. Mol. Med. 2019, 23, 2303–2313. [Google Scholar] [CrossRef] [Green Version]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Burdak-Rothkamm, S.; Mansour, W.Y.; Rothkamm, K. DNA Damage Repair Deficiency in Prostate Cancer. Trends Cancer 2020, 6, 974–984. [Google Scholar] [CrossRef]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.T.; et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Teng, P.C.; Huang, S.P.; Liu, C.H.; Lin, T.Y.; Cho, Y.C.; Lai, Y.L.; Wang, S.C.; Yeh, H.C.; Chuu, C.P.; Chen, D.N.; et al. Identification of DNA Damage Repair-Associated Prognostic Biomarkers for Prostate Cancer Using Transcriptomic Data Analysis. Int. J. Mol. Sci. 2021, 22, 11771. [Google Scholar] [CrossRef]
- Teyssonneau, D.; Margot, H.; Cabart, M.; Anonnay, M.; Sargos, P.; Vuong, N.S.; Soubeyran, I.; Sevenet, N.; Roubaud, G. Prostate cancer and PARP inhibitors: Progress and challenges. J. Hematol. Oncol. 2021, 14, 51. [Google Scholar] [CrossRef]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Kodet, O.; Němejcova, K.; Strnadová, K.; Havlínová, A.; Dundr, P.; Krajsová, I.; Štork, J.; Smetana, K., Jr.; Lacina, L. The Abscopal Effect in the Era of Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 7204. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Meher, N.; VanBrocklin, H.F.; Wilson, D.M.; Flavell, R.R. PSMA-Targeted Nanotheranostics for Imaging and Radiotherapy of Prostate Cancer. Pharmaceuticals 2023, 16, 315. [Google Scholar] [CrossRef]
- Moon, S.H.; Yang, B.Y.; Kim, Y.J.; Hong, M.K.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Jeong, J.M. Development of a complementary PET/MR dual-modal imaging probe for targeting prostate-specific membrane antigen (PSMA). Nanomed. Nanotechnol. Biol. Med. 2016, 12, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef]
- Hrkach, J.; Von Hoff, D.; Mukkaram Ali, M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Cheng, M.H.Y.; Overchuk, M.; Rajora, M.A.; Lou, J.W.H.; Chen, Y.; Pomper, M.G.; Chen, J.; Zheng, G. Targeted Theranostic 111In/Lu-Nanotexaphyrin for SPECT Imaging and Photodynamic Therapy. Mol. Pharm. 2021, 19, 1803–1813. [Google Scholar] [CrossRef]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 42–51. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of 177Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Sahoo, R.K.; Tripathi, M.; Dwivedi, S.N.; Bal, C. 225Ac-PSMA-617-targeted alpha therapy for the treatment of metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Prostate 2021, 81, 580–591. [Google Scholar] [CrossRef]
- Lawal, I.O.; Morgenstern, A.; Vorster, M.; Knoesen, O.; Mahapane, J.; Hlongwa, K.N.; Maserumule, L.C.; Ndlovu, H.; Reed, J.D.; Popoola, G.O.; et al. Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3581–3592. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S. A review of advances in the last decade on targeted cancer therapy using 177Lu: Focusing on 177Lu produced by the direct neutron activation route. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 443–475. [Google Scholar]
- Vogel, W.V.; van der Marck, S.C.; Versleijen, M.W.J. Challenges and future options for the production of lutetium-177. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2329–2335. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; Rasmussen, G.; Morgenstern, A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal. Chem. 2005, 77, 6288–6291. [Google Scholar] [CrossRef]
- Boll, R.A.; Malkemus, D.; Mirzadeh, S. Production of actinium-225 for alpha particle mediated radioimmunotherapy. Appl. Radiat. Isot. 2005, 62, 667–679. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]

| (A) | |
|---|---|
| Regional Classification of PSMA-PET Findings | |
| Class Description | |
| Local tumor (T) | |
| miT0 | No local tumor |
| miT2 | Organ-confined tumor |
| miT3a | Non-organ-confined tumor (extracapsular extension) |
| miT3b | Non-organ-confined tumor (seminal vesicles invasion) |
| miT4 | Tumor invading adjacent structures (other than seminal vesicles) |
| miTr | Presence of local recurrence after radical prostatectomy |
| Regional nodes (N) | |
| miN0 | No positive regional lymph nodes |
| miN1 | Positive regional lymph nodes |
| Distant metastases (M) | |
| miM0 | No distant metastases |
| miM1a | Extra-pelvic lymph nodes |
| miM1b | Bone metastasis |
| miM1c | Non-nodal visceral metastasis: report involved organ(s) |
| (B) | |
| Regional Classification of PSMA-PET Findings | |
| Class Description | |
| Local tumor (T) | |
| miT0 | No local tumor |
| miT2 | Organ-confined tumor |
| miT3a | Non-organ-confined tumor (extracapsular extension) |
| miT3b | Non-organ-confined tumor (seminal vesicles invasion) |
| miT4 | Tumor invading adjacent structures (other than seminal vesicles) |
| miTr | Presence of local recurrence after radical prostatectomy |
| Regional nodes (N) | |
| miN0 | No positive regional lymph nodes |
| miN1 | Positive regional lymph nodes |
| Distant metastases (M) | |
| miM0 | No distant metastases |
| miM1a | Extra-pelvic lymph nodes |
| miM1b | Bone metastasis |
| miM1c | Non-nodal visceral metastasis: report involved organ(s) |
| Alpha Emitters: Physical Properties | |||
|---|---|---|---|
| Radionuclide | Eaverage (MeV) | Range (µm) | Half-life |
| 211At | 6.79 | 60 | 7.2 h |
| 213Bi | 8.32 | 84 | 46 min |
| 223Ra | 5.64 | 45 | 11.43 d |
| 225Ac | 6.83 | 61 | 10 d |
| Beta emitters: physical properties | |||
| Radionuclide | Energymax (keV) | Range (mm) | Half-life |
| 177Lu | 497 | 1.8 | 6.7 d |
| 67Cu | 575 | 2.1 | 61.9 h |
| 131I | 606 | 2.3 | 8.0 d |
| 90Y | 2284 | 11.3 | 64.1 h |
| 1 | Time since diagnosis (years) |
| 2 | Chemotherapy status previous chemotherapy (yes/no) |
| 3 | Haemoglobin (g/dL) |
| 4 | Tumor SUVmean of PSMA-PET |
| 5 | Number of lesions (<20/≥20) |
| 6 | Bone metastases (yes/no) |
| 7 | Liver metastases (yes/no) |
| Clinical Trial Identifier | Brief Description of the Trials | Phase |
|---|---|---|
| Trials for Localized Prostate Cancer | ||
| NCT04297410 | 177Lu-PSMA-I&T prior to prostatectomy (NALuPROST) | 1/2 |
| NCT04430192 | 177Lu-PSMA-617 prior to prostatectomy (LuTectomy) | 1/2 |
| Trials for mHSPC | ||
| NCT04343885 | 177Lu-PSMA-617 + docetaxel vs. docetaxel in mHSPC (UpFrontPSMA) | 2 |
| NCT04443062 | 177Lu-PSMA-617 in oligometastatic metachronous HSPC (Bullseye) | 2 |
| NCT04506567 | 225Ac-J591 antibody + SBRT or 225Ac-J591 Antibody + ADT in oligometastatic metachronous HSPC (ACTION) | 1/2 |
| NCT04720157 | 177Lu-PSMA-617 + SOC vs. SOC alone in mHSPC (PSMAddition) | 3 |
| NCT05079698 | 177Lu-PSMA-617 + SBRT in oligometastatic metachronous HSPC | 1 |
| NCT05162573 | 177Lu-PSMA-617 + EBRT in N1M0 mHSPC (PROQURE-1) | 1 |
| NCT05560659 | 177Lu-PSMA-617 + SBRT vs. SBRT in oligometastatic metachronous HSPC (POPSTAR II) | 2 |
| Trials for mCRPC | ||
| ACTRN12615000912583 | 177Lu-PSMA-617 in progressive mCRPC (LuPSMA) | 2 |
| NCT00538668 | 177Lu-J591 antibody in progressive mCRPC | 1 |
| NCT03392428 | 177Lu-PSMA-617 vs. cabazitaxel in progressive mCRPC (TheraP) | 2 |
| NCT03490838 | 177Lu-PSMA-R2 in progressive mCRPC(PROter) | 1/2 |
| NCT03511664 | 177Lu-PSMA-617 + SOC vs. SOC in progressive mCRPC (VISION) | 3 |
| NCT03658447 | 177Lu-PSMA-617 + pembrolizumab in progressive mCRPC (PRINCE) | 1/2 |
| NCT03724747 | 227Th-BAY2315497 antibody in progressive mCRPC | 1 |
| NCT03874884 | 177Lu-PSMA-617 + olaparib in progressive mCRPC (LuPARP) | 1 |
| NCT04419402 | Enzalutamide + 177Lu-PSMA-617 vs. enzalutamide alone in mCRPC (ENZA-P) | 2 |
| NCT04506567 | 225Ac-J591 antibody in progressive mCRPC | 1/2 |
| NCT04597411 | 225Ac-PSMA-617 in progressive mCRPC | 1 |
| NCT04647526 | 177Lu-PSMA-I&T vs. ARAT in progressive mCRPC (SPLASH) | 3 |
| NCT04663997 | 177Lu-PSMA-617 vs. docetaxel in progressive mCRPC | 2 |
| NCT04886986 | 225Ac-J591 antibody + 177Lu-PSMA-I&T in progressive mCRPC | 1/2 |
| NCT04946370 | 225Ac-J591 antibody + pembrolizumab in progressive mCRPC | 1/2 |
| NCT04996602 | 177Lu-EB-PSMA in progressive mCRPC | 1 |
| NCT05150236 | 177Lu-PSMA-617 + ipilimumab and nivolumab vs. 177Lu-PSMA-617 in progressive mCRPC (ANZUP2001) | 2 |
| NCT05113537 | 177Lu-PSMA-617 + abemaciclib in progressive mCRPC (UPLIFT) | 1/2 |
| NCT05521412 | 161Tb-PSMA-I&T in progressive mCRPC (VIOLET) | 1/2 |
| NCT05219500 | 225Ac-PSMA-I&T in progressive mCRPC (TATCIST) | 2 |
| NCT05340374 | 177Lu-PSMA-617 + cabazitaxel in mCRPC (LuCAB) | 1/2 |
| NCT05383079 | 177Lu-PSMA-I&T + radium-223 in progressive mCRPC (AlphaBet) | 1/2 |
| NCT05570994 | 177Lu-HTK03170 in progressive mCRPC | 1/2 |
| NCT05725070 | 212Pb-NG001 in progressive mCRPC | 1 |
| 1 | Life expectancy is less than 6 months (ECOG performance status > 2); unless the main objective is alleviating suffering from disease-related symptoms. |
| 2 | Unacceptable medical or radiation safety risk for isolation on a nuclear medicine therapy unit (if required by national regulations). |
| 3 | Unmanageable urinary tract obstruction or hydronephrosis; in patients with diagnosed or who are at high risk of urinary retention, 99mTc-MAG3 or 99mTc-DTPA renal scintigraphy should be considered as a baseline exam. |
| 4 | Progressive deterioration of organ function (GFR < 30 mL/min or creatinine > 2-fold upper limit of normal (ULN); liver enzymes > 5-fold ULN). |
| 5 | Myelosuppression: |
a. Total white cell count less than 2.5 × 109/L | |
b. Platelet count less than 75 × 109/L | |
| 6 | Conditions that require timely interventions (radiation therapy, surgery), e.g., spinal cord compression and unstable fractures, PSMA-RLT might be performed afterward upon the patient’s condition. Borderline cases should be evaluated within the multidisciplinary tumor board for the individual benefit-to-risk ratio. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, S.; Yaginuma, K.; Meguro, S.; Onagi, A.; Matsuoka, K.; Hata, J.; Sato, Y.; Akaihata, H.; Kataoka, M.; Ogawa, S.; et al. PSMA Targeted Molecular Imaging and Radioligand Therapy for Prostate Cancer: Optimal Patient and Treatment Issues. Curr. Oncol. 2023, 30, 7286-7302. https://doi.org/10.3390/curroncol30080529
Hoshi S, Yaginuma K, Meguro S, Onagi A, Matsuoka K, Hata J, Sato Y, Akaihata H, Kataoka M, Ogawa S, et al. PSMA Targeted Molecular Imaging and Radioligand Therapy for Prostate Cancer: Optimal Patient and Treatment Issues. Current Oncology. 2023; 30(8):7286-7302. https://doi.org/10.3390/curroncol30080529
Chicago/Turabian StyleHoshi, Seiji, Kei Yaginuma, Satoru Meguro, Akifumi Onagi, Kanako Matsuoka, Junya Hata, Yuichi Sato, Hidenori Akaihata, Masao Kataoka, Soichiro Ogawa, and et al. 2023. "PSMA Targeted Molecular Imaging and Radioligand Therapy for Prostate Cancer: Optimal Patient and Treatment Issues" Current Oncology 30, no. 8: 7286-7302. https://doi.org/10.3390/curroncol30080529
APA StyleHoshi, S., Yaginuma, K., Meguro, S., Onagi, A., Matsuoka, K., Hata, J., Sato, Y., Akaihata, H., Kataoka, M., Ogawa, S., Uemura, M., & Kojima, Y. (2023). PSMA Targeted Molecular Imaging and Radioligand Therapy for Prostate Cancer: Optimal Patient and Treatment Issues. Current Oncology, 30(8), 7286-7302. https://doi.org/10.3390/curroncol30080529