Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease
Abstract
1. Introduction to the Immediate Perioperative Period (IPP)
2. Direct Effects of Perioperative Stress and/or Inflammatory Responses on Malignant Tissue
3. Synergistic Perioperative Deleterious Effects of Stress and Inflammation and Their Blockade
4. Immunosuppression during and following the IPP
5. Immunotherapy—Overview
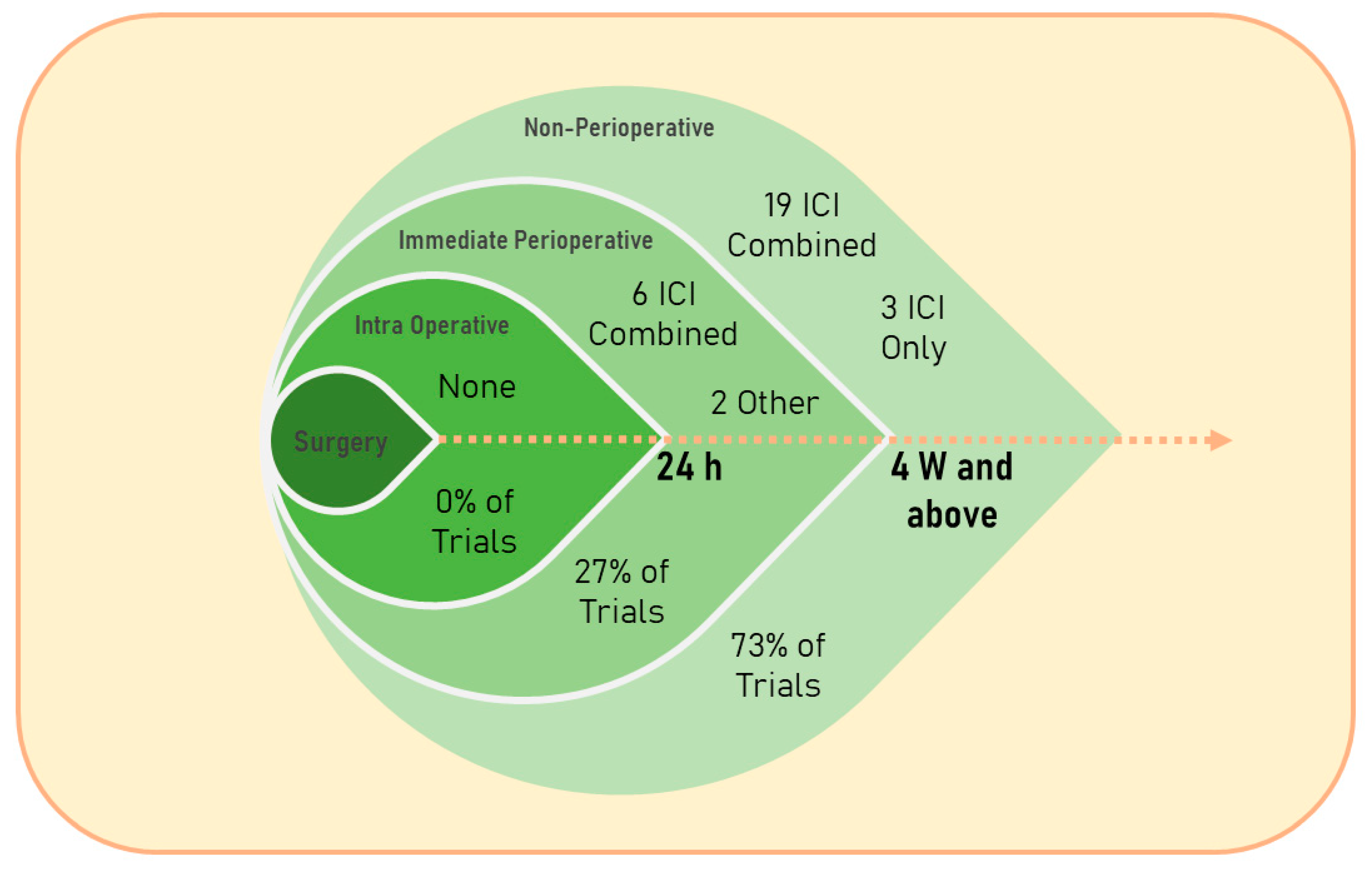
5.1. Immunotherapy during the IPP: Feasibility and Effectiveness
5.1.1. Anti-Stress-Inflammatory Approach—The Inhibition of β-Adrenergic and COX2 Signaling
5.1.2. Oncolytic Virotherapy
5.1.3. Cytokine Therapies
5.1.4. Monoclonal Antibodies
5.1.5. Immune Checkpoint Inhibitors: Treatment Evolvement and Usage within the IPP
5.2. Immunotherapy during the IPP: Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben-Eliyahu, S. Tumor Excision as a Metastatic Russian Roulette: Perioperative Interventions to Improve Long-Term Survival of Cancer Patients. Trends Cancer 2020, 6, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Eckerling, A.; Ricon-Becker, I.; Sorski, L.; Sandbank, E.; Ben-Eliyahu, S. Stress and cancer: Mechanisms, significance and future directions. Nat. Rev. Cancer 2021, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef]
- Matzner, P.; Sandbank, E.; Neeman, E.; Zmora, O.; Gottumukkala, V.; Ben-Eliyahu, S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat. Rev. Clin. Oncol. 2020, 17, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Moretti, S.; Massi, D.; Farini, V.; Baroni, G.; Parri, M.; Innocenti, S.; Cecchi, R.; Chiarugi, P. beta-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab. Investig. 2013, 93, 279–290. [Google Scholar] [CrossRef]
- Yang, E.V.; Kim, S.; Donovan, E.L.; Chen, M.; Gross, A.C.; Webster Marketon, J.I.; Barsky, S.H.; Glaser, R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav. Immun. 2009, 23, 267–275. [Google Scholar] [CrossRef]
- Madden, K.S.; Szpunar, M.J.; Brown, E.B. beta-Adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 2011, 130, 747–758. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Cole, S.; Costanzo, E.; Bradley, S.; Coffin, J.; Jabbari, S.; Rainwater, K.; Ritchie, J.M.; Yang, M.; Sood, A.K. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003, 9, 4514–4521. [Google Scholar]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zeng, H.; Xiao, Y.; Zhao, Y.; Zheng, B.; Deng, Y.; Liu, J.; Huang, B.; Zhang, X.; Yang, K.; et al. Chronic stress promotes EMT-mediated metastasis through activation of STAT3 signaling pathway by miR-337-3p in breast cancer. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, A.S.; Dorniak, P.L.; Sadaoui, N.C.; Kang, Y.; Lin, T.; Armaiz-Pena, G.; Wu, S.Y.; Rupaimoole, R.; Allen, J.K.; Gharpure, K.M.; et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene 2016, 35, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. The Sympathetic Nervous System Induces a Metastatic Switch in Primary Breast Cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Benish, M.; Bartal, I.; Goldfarb, Y.; Levi, B.; Avraham, R.; Raz, A.; Ben-Eliyahu, S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 2008, 15, 2042–2052. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving Survival Rates in Two Models of Spontaneous Postoperative Metastasis in Mice by Combined Administration of a beta-Adrenergic Antagonist and a Cyclooxygenase-2 Inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef]
- Liat, S.; Rivka, M.; Pini, M.; Lavon, H.; Shaashua, L.; Rosenne, E.; Ben-Eliyahu, S. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav. Immun. 2016, 58, 91–98. [Google Scholar]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef]
- Hanalis-Miller, T.; Ricon-Becker, I.; Trachtenberg, E.; Sakis, N.; Magen, A.; Goldzweig, G.; Birnboim-Hazan, Y.; Ohayon, F.; Wadhawker, S.; Sharon, E.; et al. Perioperative psychological intervention in breast cancer patients aiming to reduce stress responses and improve cancer outcomes: A pilot clinical trial. Brain Behav. Immun. 2022, 106, 2. [Google Scholar] [CrossRef]
- Vallejo, R.; Hord, E.D.; Barna, S.A.; Santiago-Palma, J.; Ahmed, S. Perioperative Immunosuppression in Cancer Patients. J. Environ. Pathol. Toxicol. Oncol. 2003, 22, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Neeman, E.; Zmora, O.; Ben-Eliyahu, S. A New Approach to Reducing Postsurgical Cancer Recurrence: Perioperative Targeting of Catecholamines and Prostaglandins. Clin. Cancer Res. 2012, 18, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.V.; Peter, M.B.; Shenoy, H.G.; Horgan, K.; Hughes, T.A. Surgery induced immunosuppression. Surg. 2011, 9, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Zhang, X.; Zhao, L.; Zhao, X.; Li, Z.; Song, T.; Huang, C. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem. Biophys. Res. Commun. 2013, 439, 471–476. [Google Scholar] [CrossRef]
- Bucsek, M.J.; Qiao, G.X.; MacDonald, C.R.; Giridharan, T.; Evans, L.; Niedzwecki, B.; Liu, H.; Kokolus, K.M.; Eng, J.W.-L.; Messmer, M.N.; et al. beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res. 2017, 77, 5639–5651. [Google Scholar] [CrossRef]
- Mohammadpour, H.; MacDonald, C.R.; Qiao, G.; Chen, M.; Dong, B.; Hylander, B.L.; McCarthy, P.L.; Abrams, S.I.; Repasky, E.A. beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Investig. 2019, 129, 5537–5552. [Google Scholar] [CrossRef]
- Xu, P.; He, H.; Gu, Y.; Wang, Y.; Sun, Z.; Yang, L.; Miao, C. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp. Cell Res. 2018, 370, 692–698. [Google Scholar] [CrossRef]
- Saul, A.N.; Oberyszyn, T.M.; Daugherty, C.; Kusewitt, D.; Jones, S.; Jewell, S.; Malarkey, W.B.; Lehman, A.; Lemeshow, S.; Dhabhar, F.S. Chronic Stress and Susceptibility to Skin Cancer. Gynecol. Oncol. 2005, 97, 1760–1767. [Google Scholar] [CrossRef]
- Shakhar, G.; Ben-Eliyahu, S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J. Immunol. 1998, 160, 3251–3258. [Google Scholar] [CrossRef]
- Ben-Eliyahu, S.; Shakhar, G.; Page, G.G.; Stefanski, V.; Shakhar, K. Suppression of NK cell activity and of resistance to metastasis by stress: A role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation 2000, 8, 154–164. [Google Scholar] [CrossRef]
- Melamed, R.; Rosenne, E.; Shakhar, K.; Schwartz, Y.; Abudarham, N.; Ben-Eliyahu, S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: Suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav. Immun. 2005, 19, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Inbar, S.; Neeman, E.; Avraham, R.; Benish, M.; Rosenne, E.; Ben-Eliyahu, S. Do Stress Responses Promote Leukemia Progression? An Animal Study Suggesting a Role for Epinephrine and Prostaglandin-E2 through Reduced NK Activity. PLoS ONE 2011, 6, e19246. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.F.; Jin, F.J.; Li, N.; Guan, H.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor beta 2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Armaiz-Pena, G.N.; Gonzalez-Villasana, V.; Nagaraja, A.S.; Rodriguez-Aguayo, C.; Sadaoui, N.C.; Stone, R.L.; Matsuo, K.; Dalton, H.J.; Previs, R.A.; Jennings, N.B.; et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 2015, 6, 4266–4273. [Google Scholar] [CrossRef]
- Lamkin, D.M.; Ho, H.Y.; Ong, T.H.; Kawanishi, C.K.; Stoffers, V.L.; Ahlawat, N.; Ma, J.C.Y.; Arevalo, J.M.G.; Cole, S.W.; Sloan, E.K. beta-Adrenergic-stimulated macrophages: Comprehensive localization in the M1-M2 spectrum. Brain Behav. Immun. 2016, 57, 338–346. [Google Scholar] [CrossRef]
- Kim, R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J. Transl. Med. 2018, 16, 8. [Google Scholar] [CrossRef]
- Cata, J.P. Research in Perioperative Care of the Cancer Patient: Opportunities and Challenges. Curr. Oncol. 2023, 30, 1186–1195. [Google Scholar] [CrossRef]
- Ballestín, S.S.; Bardaji, A.L.; Continente, C.M.; Bartolomé, M.J.L. Antitumor Anesthetic Strategy in the Perioperatory Period of the Oncological Patient: A Review. Front. Med. 2022, 9, 799355. [Google Scholar] [CrossRef]
- Dubowitz, J.A.; Sloan, E.K.; Riedel, B.J. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin. Exp. Metastasis 2018, 35, 347–358. [Google Scholar] [CrossRef]
- Beilin, B.; Shavit, Y.; Razumovsky, J.; Wolloch, Y.; Zeidel, A.; Bessler, H. Effects of Mild Perioperative Hypothermia on Cellular Immune Responses. Anesthesiology 1998, 89, 1133–1140. [Google Scholar] [CrossRef]
- Wenisch, C.; Narzt, E.; Sessler, D.I.; Parschalk, B.; Lenhardt, R.; Kurz, A.; Graninger, W. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth. Analg. 1996, 82, 810–816. [Google Scholar]
- Nielsen, H.J. Detrimental effects of perioperative blood transfusion. Br. J. Surg. 1995, 82, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Greenfeld, K.; Avraham, R.; Benish, M.; Goldfarb, Y.; Rosenne, E.; Shapira, Y.; Rudich, T.; Ben-Eliyahu, S. Immune suppression while awaiting surgery and following it: Dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav. Immun. 2007, 21, 503–513. [Google Scholar] [CrossRef]
- Ricon, I.; Hanalis-Miller, T.; Haldar, R.; Jacoby, R.; Ben-Eliyahu, S. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of beta-adrenergic and cyclooxygenase 2 signaling. Cancer 2019, 125, 45–56. [Google Scholar] [CrossRef]
- Antoni, M.H.; Dhabhar, F.S. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 2019, 125, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Clevenger, L.; Christensen, D.; DeGeest, K.; Bender, D.; Ahmed, A.; Goodheart, M.J.; Dahmoush, L.; Penedo, F.; Lucci, J.A., III; et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: Relationships with depression, fatigue, and disability. Brain. Behav. Immun. 2013, 30, S126–S134. [Google Scholar] [CrossRef] [PubMed]
- Bartal, I.; Melamed, R.; Greenfeld, K.; Atzil, S.; Glasner, A.; Domankevich, V.; Naor, R.; Beilin, B.; Yardeni, I.Z.; Ben-Eliyahu, S. Immune perturbations in patients along the perioperative period: Alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav. Immun. 2010, 24, 376–386. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, P.; Sun, Z.; Wang, Y.; Chen, J.; Miao, C. Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol. Immunother. 2015, 64, 1383–1392. [Google Scholar] [CrossRef]
- Ohwada, S.; Ogawa, T.; Makita, F.; Tanahashi, Y.; Ohya, T.; Tomizawa, N.; Satoh, Y.; Kobayashi, I.; Izumi, M.; Takeyoshi, I.; et al. Beneficial effects of protein-bound polysaccharide K plus tegafur/uracil in patients with stage II or III colorectal cancer: Analysis of immunological parameters. Oncol. Rep. 2006, 15, 861–868. [Google Scholar] [CrossRef]
- Angka, L.; Martel, A.B.; Kilgour, M.; Jeong, A.; Sadiq, M.; de Souza, C.T.; Baker, L.; Kennedy, M.A.; Kekre, N.; Auer, R.C.; et al. Natural Killer Cell IFN gamma Secretion is Profoundly Suppressed Following Colorectal Cancer Surgery. Ann. Surg. Oncol. 2018, 25, 3747–3754. [Google Scholar] [CrossRef]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef]
- Lee, S.; Margolin, K. Cytokines in Cancer Immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.J.; Lattime, E.C. Virotherapy clinical trials for regional disease: In situ immune modulation using recombinant poxvirus vectors. Cancer Gene Ther. 2002, 9, 1013–1021. [Google Scholar] [CrossRef]
- Gelderman, K.A.; Tomlinson, S.; Ross, G.D.; Gorter, A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004, 25, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Baker, D.; James, N.H.; Ratcliffe, K.; Jenkins, M.; Ashton, S.E.; Sproat, G.; Swann, R.; Gray, N.; Ryan, A.; et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 2010, 16, 3548–3661. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Caprotti, R.; Brivio, F.; Fumagalli, L.; Nobili, C.; Degrate, L.; Lissoni, P.; Parolini, D.; Messina, G.; Colciago, M.; Scotti, M.; et al. Free-from-progression period and overall short preoperative immunotherapy with IL-2 increases the survival of pancreatic cancer patients treated with macroscopically radical surgery. Anticancer. Res. 2008, 28, 1951–1954. [Google Scholar]
- Brivio, F.; Fumagalli, L.; Lissoni, P.; Nardone, A.; Nespoli, L.; Fattori, L.; Denova, M.; Chiarelli, M.; Nespoli, A. Pre-operative immunoprophylaxis with interleukin-2 may improve prognosis in radical surgery for colorectal cancer stage B-C. Anticancer. Res. 2006, 26, 599–603. [Google Scholar]
- Bundred, N.; Porta, N.; Brunt, A.M.; Cramer, A.; Hanby, A.; Shaaban, A.M.; Rakha, E.A.; Armstrong, A.; Cutress, R.I.; Dodwell, D.; et al. Combined Perioperative Lapatinib and Trastuzumab in Early HER2-Positive Breast Cancer Identifies Early Responders: Randomized UK EPHOS-B Trial Long-Term Results. Clin. Cancer Res. 2022, 28, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Gelb, A.B.; Chen, C.C.; Rao, G.; A Reardon, D.; Wen, P.Y.; Bi, W.L.; Peruzzi, P.; Amidei, C.; Triggs, D.; et al. Combined immunotherapy with controlled interleukin-12 gene therapy and immune checkpoint blockade in recurrent glioblastoma: An open-label, multi-institutional phase I trial. Neuro-Oncology 2022, 24, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.W.; Judy, K.D.; Scott, C.B.; Garcia, S.; Harshyne, L.A.; Kenyon, L.; Talekar, K.; Flanders, A.; Atsina, K.-B.; Kim, L.; et al. Phase Ib Clinical Trial of IGV-001 for Patients with Newly Diagnosed Glioblastoma. Clin. Cancer Res. 2021, 27, 1912–1922. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Hazut, O.; Shaashua, L.; Benish, M.; Levi, B.; Sorski, L.; Benjamin, B.; Hoffman, A.; Zmora, O.; Ben-Eliyahu, S. The effect of beta-adrenergic blockade and COX-2 inhibition on healing of colon, muscle, and skin in rats undergoing colonic anastomosis. Int. J. Clin. Pharmacol. Ther. 2011, 49, 545–554. [Google Scholar] [CrossRef]
- Benjamin, B.; Hazut, O.; Shaashua, L.; Benish, M.; Zmora, N.; Barshack, I.; Hoffman, A.; Ben-Eliyahu, S.; Zmora, O. Effect of beta blocker combined with COX-2 inhibitor on colonic anastomosis in rats. Int. J. Color. Dis. 2010, 25, 1459–1464. [Google Scholar] [CrossRef]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving Postoperative Immune Status and Resistance to Cancer Metastasis A Combined Perioperative Approach of Immunostimulation and Prevention of Excessive Surgical Stress Responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Haldar, R.; Shaashua, L.; Lavon, H.; Lyons, Y.A.; Zmora, O.; Sharon, E.; Birnbaum, Y.; Allweis, T.; Sood, A.K.; Barshack, I.; et al. Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav. Immun. 2018, 73, 294–309. [Google Scholar] [CrossRef]
- Haldar, R.; Ricon-Becker, I.; Radin, A.; Gutman, M.; Cole, S.W.; Zmora, O.; Ben-Eliyahu, S. Perioperative COX2 and beta-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: A randomized controlled trial. Cancer 2020, 126, 3991–4001. [Google Scholar] [CrossRef]
- Ricon-Becker, I.; Haldar, R.; Simon, M.S.; Gutman, M.; Cole, S.W.; Ben-Eliyahu, S.; Zmora, O. Effect of perioperative COX-2 and beta-adrenergic inhibition on 5-year disease-free-survival in colorectal cancer: A pilot randomized controlled Colorectal Metastasis PreventIon Trial (COMPIT). Eur. J. Surg. Oncol. 2022, 49, 655–661. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Oncolytic Viruses: Newest Frontier for Cancer Immunotherapy. Cancers 2021, 13, 5452. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Gujar, S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat. Rev. Urol. 2018, 15, 235–250. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Pfankuche, V.M.; Spitzbarth, I.; Lapp, S.; Ulrich, R.; Deschl, U.; Kalkuhl, A.; Baumgärtner, W.; Puff, C. Reduced angiogenic gene expression in morbillivirus-triggered oncolysis in a translational model for histiocytic sarcoma. J. Cell. Mol. Med. 2017, 21, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Y.; Zhang, X.; Wang, H.; Xu, W.; Wang, H.; Xiao, F.; Bai, Z.; Yao, H.; Ma, X.; et al. An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation. Hum. Gene Ther. 2017, 28, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Bartee, E. The use of oncolytic virotherapy in the neoadjuvant setting. J. Immunother. Cancer 2022, 10, e004462. [Google Scholar] [CrossRef]
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.; Berger, A.C.; Conry, R.; Demidov, L.; Sharma, A.; Treichel, S.A.; Radcliffe, H.; Gorski, K.S.; et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: A randomized, open-label, phase 2 trial. Nat. Med. 2021, 27, 1789–1796. [Google Scholar] [CrossRef]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Nagano, H.; Sakon, M.; Eguchi, H.; Kondo, M.; Yamamoto, T.; Ota, H.; Nakamura, M.; Wada, H.; Damdinsuren, B.; Marubashi, S.; et al. Hepatic resection followed by IFN-alpha and 5-FU for advanced hepatocellular carcinoma with tumor thrombus in the major portal branch. Hepato-Gastroenterology 2007, 54, 172–179. [Google Scholar] [PubMed]
- Sedman, P.C.; Ramsden, C.W.; Brennan, T.G.; Giles, G.R.; Guillou, P.J. Effects of low dose perioperative interferon on the surgically induced suppression of antitumour immune responses. Br. J. Surg. 1988, 75, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Ittenson, A.; Röhl, F.-W.; Ecke, M.; Allhoff, E.P.; Böhm, M. Pretreatment with Interferon-a2a Modulates Perioperative Immunodysfunction in Patients with Renal Cell Carcinoma. Onkologie 2008, 31, 28–34. [Google Scholar] [CrossRef]
- Rajala, P.; Kaasinen, E.; Raitanen, M.; Liukkonen, T.; Rintala, E.; Finnbladder Group. Perioperative single dose instillation of epirubicin or interferon-alpha after transurethral resection for the prophylaxis of primary superficial bladder cancer recurrence: A prospective randomized multicenter study—Finnbladder III long-term results. J. Urol. 2002, 168, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Matory, Y.L.; E Ettinghausen, S.; A Rayner, A.; O Sharrow, S.; A Seipp, C.; Custer, M.C.; A Rosenberg, S. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 1985, 135, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef]
- Klatte, T.; Ittenson, A.; Röhl, F.-W.; Ecke, M.; Allhoff, E.P.; Böhm, M. Perioperative immunomodulation with interleukin-2 in patients with renal cell carcinoma: Results of a controlled phase II trial. Br. J. Cancer 2006, 95, 1167–1173. [Google Scholar] [CrossRef]
- Deehan, D.J.; Heys, S.D.; Ashby, J.; Eremin, O. Interleukin-2 (IL-2) augments host cellular immune reactivity in the perioperative period in patients with malignant disease. Eur. J. Surg. Oncol. (EJSO) 1995, 21, 16–22. [Google Scholar] [CrossRef]
- Ackerman, R.S.; Muncey, A.R.; Aldawoodi, N.N.; Kotha, R.; Getting, R.E.G. Cancer Immunotherapies: What the Perioperative Physician Needs to Know. Curr. Oncol. Rep. 2022, 24, 399–414. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Boér, K.; Kahán, Z.; Landherr, L.; Csőszi, T.; Máhr, K.; Ruzsa, Á.; Horváth, Z.; Budai, B.; Rubovszky, G. Pathologic Complete Response Rates After Neoadjuvant Pertuzumab and Trastuzumab with Chemotherapy in Early Stage HER2-Positive Breast Cancer—Increasing Rates of Breast Conserving Surgery: A Real-World Experience. Pathol. Oncol. Res. 2021, 27, 1609785. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.-S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Kroschinsky, F.; on behalf of the Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group; Stölzel, F.; von Bonin, S.; Beutel, G.; Kochanek, M.; Kiehl, M.; Schellongowski, P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Gutierrez, C.; McEvoy, C.; Munshi, L.M.; Stephens, R.S.; Detsky, M.E.; Nates, J.L.M.; Pastores, S.M.M. Critical Care Management of Toxicities Associated With Targeted Agents and Immunotherapies for Cancer. Crit. Care Med. 2020, 48, 10–21. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Lio, J.; O’Donnell, J.S.; Yan, J.M.; Madore, J.; Allen, S.; Smyth, M.J.; Teng, M.W.L. Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome. Oncoimmunology 2019, 8, e1581530. [Google Scholar] [CrossRef]
- Gennari, R.; Menard, S.; Fagnoni, F.; Ponchio, L.; Scelsi, M.; Tagliabue, E.; Castiglioni, F.; Villani, L.; Magalotti, C.; Gibelli, N.; et al. Pilot Study of the Mechanism of Action of Preoperative Trastuzumab in Patients with Primary Operable Breast Tumors Overexpressing HER2. Clin. Cancer Res. 2004, 10, 5650–5655. [Google Scholar] [CrossRef]
- Blanco, E.; Chocarro, L.; Fernández-Rubio, L.; Bocanegra, A.; Arasanz, H.; Echaide, M.; Garnica, M.; Piñeiro-Hermida, S.; Kochan, G.; Escors, D. Leading Edge: Intratumor Delivery of Monoclonal Antibodies for the Treatment of Solid Tumors. Int. J. Mol. Sci. 2023, 24, 2676. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). 2021. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 2 April 2023).
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ Br. Med. J. 2018, 363, k4226. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1792. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Song, Y.X.; Tang, J.Z.; Zhang, B. What is the optimal duration of immune checkpoint inhibitors in malignant tumors? Front. Immunol. 2022, 13, 983581. [Google Scholar] [CrossRef] [PubMed]
- Marron, T.U.; E Ryan, A.; Reddy, S.M.; Kaczanowska, S.; Younis, R.H.; Thakkar, D.; Zhang, J.; Bartkowiak, T.; Howard, R.; Anderson, K.G.; et al. Considerations for treatment duration in responders to immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e001901. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Cortellini, A.; Friedlaender, A.; Banna, G.L.; Porzio, G.; Bersanelli, M.; Cappuzzo, F.; Aerts, J.G.; Giusti, R.; Bria, E.; Cortinovis, D.; et al. Immune-related Adverse Events of Pembrolizumab in a Large Real-world Cohort of Patients With NSCLC With a PD-L1 Expression ≥ 50% and Their Relationship With Clinical Outcomes. Clin. Lung Cancer 2020, 21, 498–508. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Hamid, O.; Daud, A.; Wolchok, J.D.; Joshua, A.M.; Hwu, W.-J.; Weber, J.S.; Gangadhar, T.C.; Joseph, R.W.; et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J. Clin. Oncol. 2018, 36, 1668–1674. [Google Scholar] [CrossRef]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P.A. Neoadjuvant Versus Adjuvant Systemic Treatment in Breast Cancer: A Meta-Analysis. J. Natl. Cancer Institut. 2005, 97, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; de Vere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Hoefsmit, E.P.; Smyth, M.J.; Blank, C.U.; Teng, M.W.L. The Promise of Neoadjuvant Immunotherapy and Surgery for Cancer Treatment. Clin. Cancer Res. 2019, 25, 5743–5751. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Fry, D.E. Article Commentary: Sepsis, Systemic Inflammatory Response, and Multiple Organ Dysfunction: The Mystery Continues. Am. Surg. 2012, 78, 1–8. [Google Scholar] [CrossRef]
- Marik, P.E.; Flemmer, M. The immune response to surgery and trauma: Implications for treatment. J. Trauma Acute Care Surg. 2012, 73, 801–808. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and Beyond in Cancer Immunotherapy. J. Interferon Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef]
- Kottschade, L.A. Incidence and Management of Immune-Related Adverse Events in Patients Undergoing Treatment with Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2018, 20, 24. [Google Scholar] [CrossRef]
- Lewis, A.L.; Chaft, J.; Girotra, M.; Fischer, G.W. Immune checkpoint inhibitors: A narrative review of considerations for the anaesthesiologist. Br. J. Anaesth. 2020, 124, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, N.; Singhal, M.; Augustin, H.G. Preclinical mouse solid tumour models: Status quo, challenges and perspectives. Nat. Rev. Cancer 2017, 17, 751–765. [Google Scholar] [CrossRef]
- Yin, L.; Wang, X.-J.; Chen, D.-X.; Liu, X.-N.; Wang, X.-J. Humanized mouse model: A review on preclinical applications for cancer immunotherapy. Am. J. Cancer Res. 2020, 10, 4568–4584. [Google Scholar]
- Karnik, I.; Her, Z.; Neo, S.H.; Liu, W.N.; Chen, Q. Emerging Preclinical Applications of Humanized Mouse Models in the Discovery and Validation of Novel Immunotherapeutics and Their Mechanisms of Action for Improved Cancer Treatment. Pharmaceutics 2023, 15, 1600. [Google Scholar] [CrossRef]
- Matzner, P.; Sorski, L.; Haldar, R.; Shaashua, L.; Benbenishty, A.; Lavon, H.; Azan, Y.; Sandbank, E.; Melamed, R.; Rosenne, E.; et al. Deleterious synergistic effects of distress and surgery on cancer metastasis: Abolishment through an integrated perioperative immune-stimulating stress-inflammatory-reducing intervention. Brain Behav. Immun. 2019, 80, 170–178. [Google Scholar] [CrossRef]
- Park, C.G.; Hartl, C.A.; Schmid, D.; Carmona, E.M.; Kim, H.-J.; Goldberg, M.S. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci. Transl. Med. 2018, 10, eaar1916. [Google Scholar] [CrossRef]
- Gonzalez-Ericsson, P.I.; Stovgaard, E.S.; Sua, L.F.; Reisenbichler, E.; Kos, Z.; Carter, J.M.; Michiels, S.; Le Quesne, J.; Nielsen, T.O.; Lænkholm, A.V.; et al. The path to a better biomarker: Application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J. Pathol. 2020, 250, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-R.; Wu, X.-L.; Sun, Y.-L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 1–27. [Google Scholar] [CrossRef]
- Luo, R.; Chyr, J.; Wen, J.; Wang, Y.; Zhao, W.; Zhou, X. A novel integrated approach to predicting cancer immunotherapy efficacy. Oncogene 2023, 42, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Chen, Y.; Xiao, N.; Zheng, Z.; Liu, H.; Wan, J. Liquid biopsy on the horizon in immunotherapy of non-small cell lung cancer: Current status, challenges, and perspectives. Cell Death Dis. 2023, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, F.; Valenza, C.; Tarantino, P.; Curigliano, G. Immunotherapy for triple negative breast cancer: How can pathologic responses to experimental drugs in early-stage disease be enhanced? Expert. Opin. Investig. Drugs 2022, 31, 855–874. [Google Scholar] [CrossRef]
- Yue, D.; Liu, W.; Chen, C.; Zhang, T.; Ma, Y.; Cui, L.; Gu, Y.; Bei, T.; Zhao, X.; Zhang, B.; et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl. Lung Cancer Res. 2022, 11, 263–276. [Google Scholar] [CrossRef]
- Hwang, M.; Canzoniero, J.V.; Rosner, S.; Zhang, G.; White, J.R.; Belcaid, Z.; Cherry, C.; Balan, A.; Pereira, G.; Curry, A.; et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J. Immunother. Cancer 2022, 10, e004688. [Google Scholar] [CrossRef]
- Bratman, S.V.; Yang, S.Y.C.; Iafolla, M.A.J.; Liu, Z.; Hansen, A.R.; Bedard, P.L.; Lheureux, S.; Spreafico, A.; Razak, A.A.; Shchegrova, S.; et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 2020, 1, 873–881. [Google Scholar] [CrossRef]
- Lu, M.; Wu, K.-H.H.; Trudeau, S.; Jiang, M.; Zhao, J.; Fan, E. A genomic signature for accurate classification and prediction of clinical outcomes in cancer patients treated with immune checkpoint blockade immunotherapy. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
| Interventions | Period | Specific Timing | Title | Status | Conditions | Phase | Trial Identifier | |
|---|---|---|---|---|---|---|---|---|
| 1 | Combination product: MVA-BN-Brachyury and Atezolizumab | IPP | Three weeks before and after surgery (on average) | Perioperative With MVA-BN-Brachyury and PROSTVAC For Intermediate-Risk And High-Risk Localized Prostate Cancer | Withdrawn | Prostate adenocarcinoma | 2 | NCT04020094 |
| 2 | Drug: QBECO Drug: Placebo | IPP | Days before surgery and 6 weeks after | A Study of QBECO Versus Placebo in the Treatment of Colorectal Cancer That Has Spread to the Liver | Not yet recruiting | Colorectal cancer Liver metastases | 2 | NCT05677113 |
| 3 | Drug: M7824; drug: M9241; radiation: SBRT | IPP | 0–2 weeks before surgery | Immune Checkpoint Inhibitor M7824 and the Immunocytokine M9241 in Combination With Stereotactic Body Radiation Therapy (SBRT) in Adults With Advanced Pancreas Cancer | Terminated | Pancreatic cancer Pancreatic neoplasms Metastatic pancreatic cancer | 1| 2 | NCT04327986 |
| 4 | Drug: Ipilimumab Drug: Nivolumab Procedure: core biopsy/cryoablation Procedure: breast surgery | IPP | 1–2 weeks before surgery and 2 weeks after | Peri-Operative Ipilimumab + Nivolumab and Cryoablation in Women With Triple-negative Breast Cancer | Recruiting | Breast cancer | 2 | NCT03546686 |
| 5 | Drug: Histamine Dihydrochloride (HDC) Drug: Interleukin-2 (IL-2) | IPP | Two weeks daily up to surgery and continuing three days after for a week | Histamine Dihydrochloride and Interleukin-2 in Primary Resectable Pancreatic Cancer | Not yet recruiting | Pancreatic cancer metastasis | 2 | NCT05810792 |
| 6 | Drug: Sintilimab injection Drug: TACE Radiation: radiotherapy | IPP | Up to 3 weeks before surgery and 4 weeks after | Perioperative Therapy for Hepatocellular Carcinoma | Terminated | Hepatocellular carcinoma | 2 | NCT04653389 |
| 7 | Drug: Camrelizumab Drug: Apatinib Mesylate Procedure: postoperative TACE Procedure: radical surgery Procedure: preoperative TACE | IPP | 1 week before surgery at most and 4–8 weeks after | Camrelizumab Combined With Apatinib Mesylate and TACE in the Perioperative Treatment of Hepatocellular Carcinoma | Recruiting | Hepatocellular carcinoma | 3 | NCT05613478 |
| 8 | Drug: Camrelizumab Drug: Apatinib Mesylate Procedure: TACE treatment Procedure: radical surgery | IPP | 2–4 weeks before surgery and 4 weeks after surgery | Camrelizumab Combined With Apatinib Mesylate for Perioperative Treatment of Resectable Hepatocellular Carcinoma | Recruiting | Hepatocellular carcinoma Molecular-targeted therapy | N/A | NCT04521153 |
| 9 | Drug: Nivolumab Drug: Ipilimumab | N/S | Immunotherapy in Patients With Early dMMR Rectal Cancer | Not yet recruiting | Cancer of the rectum | 2 | NCT05732389 | |
| 10 | Drug: Nivolumab Drug: Relatlimab | N/S | Feasibility and Efficacy of Perioperative Nivolumab With or Without Relatlimab for Patients With Potentially Resectable Hepatocellular Carcinoma (HCC) | Recruiting | Hepatocellular carcinoma | 1 | NCT04658147 | |
| 11 | Drug: nab-paclitaxel AUC = 2 and carboplatin 80 mg/m2 Drug: camrelizumab 200 mg Procedure: radiotherapy | Non-IPP | Camrelizumab Combined With Neoadjuvant Concurrent Chemoradiotherapy for Resectable Locally Advanced ESCC | Not yet recruiting | Esophageal squamous cell carcinoma | 2 | NCT05650216 | |
| 12 | Drug: Penpulimab Drug: Anlotinib Hydrochloride Drug: Cadonilimab Drug: chemotherapy | Non-IPP | 6–9 weeks before surgery | An Exploratory Study of Immunotherapy Combined With Anlotinib and Chemotherapy in Perioperative Treatment of LAGC | Not yet recruiting | Gastric cancer | N/A | NCT05800080 |
| 13 | Drug: Pembrolizumab | Non-IPP | Six weeks prior | Immunotherapy in MSI/dMMR Tumors in Perioperative Setting. | Recruiting | MSI/dMMR or EBV-positive gastric cancers | 2 | NCT04795661 |
| 14 | Drug: Recombinant intravesical BCG (Bacillus Calmette-Guֳ©rin VPM1002BC); drug: Atezolizumab; drug: Cisplatin; drug: Gemcitabine | Non-IPP | 7–11 weeks before surgery and 4 weeks after | Intravesical Recombinant BCG Followed by Perioperative Chemo-immunotherapy for Patients With MIBC | Suspended | Bladder Cancer | 2 | NCT04630730 |
| 15 | Drug: sintilimab Drug: anlotinib | Non-IPP | At least 4–6 weeks before and 3 weeks after surgery | Sintilimab Combined With Anlotinib for Perioperative Non-small Cell Lung Cancer Based on MRD Evaluation | Recruiting | Non-small-Cell Lung Cancer | 2 | NCT05460195 |
| 16 | Drug: XELOX or SOX Drug: JS001 + XELOX or SOX | Non-IPP | Five weeks before surgery and five weeks after | Neoadjuvant Immunotherapy and Chemotherapy for Locally Advanced Esophagogastric Junction and Gastric Cancer Trial | Recruiting | Gastric cancer; stomach neoplasm | 2 | NCT04744649 |
| 17 | Drug: Toripalimab | Non-IPP | 6–8 weeks before surgery and 4–6 weeks after | Neoadjuvant Chemoradiotherapy Combined With Perioperative Toripalimab in Locally Advanced Esophageal Cancer | Recruiting | Advanced esophageal squamous cell Cancer | 2 | NCT04437212 |
| 18 | Drug: Camrelizumab; drug: Oxaliplatin; drug: S1 | Non-IPP | 3–9 weeks before surgery | Efficacy and Safety of Perioperative Chemotherapy Plus PD-1 Antibody in Gastric Cancer | Unknown status | Gastric cancer | 2 | NCT04367025 |
| 19 | Drug: Nivolumab and Ipilimumab; other: chemotherapy | Non-IPP | Postoperative Immunotherapy vs Standard Chemotherapy for Gastric Cancer With High Risk for Recurrence (VESTIGE) | Active, not recruiting | Gastric and esophagogastric junction adenocarcinoma | 2 | NCT03443856 | |
| 20 | Drug: nivolumab 4.5 mg/kg + Paclitaxel (albumin-bound-type) 260 mg/m2+ Carboplatin AUC5 | Non-IPP | Seven weeks before surgery (extrapolated from outcome measures) | Clinical Study of Neoadjuvant Chemotherapy and Immunotherapy Combined With Probiotics in Patients With Potential/Resectable NSCLC | Active, not recruiting | Non-small-cell lung cancer stage III | 1 | NCT04699721 |
| 21 | Drug: teripalimab plus chemotherapy; drug: chemotherapy plus teripalimab | Non-IPP | 8–10 weeks before surgery | Teripalimab Plus Chemotherapy in Local Advanced Esophageal Cancer | Unknown status | Squamous cell carcinoma esophageal cancer | 2 | NCT03985670 |
| 22 | Drug: Atezolizumab Procedure: conventional surgery Drug: Fluorouracil Drug: Oxaliplatin | Non-IPP | 6–8 weeks before surgery and 6 weeks after | Atezolizumab, Oxaliplatin, and Fluorouracil in Treating Patients With Esophageal or Gastroesophageal Cancer | Recruiting | Esophageal; gastroesophageal junction; adenocarcinoma AJCC | 2 | NCT03784326 |
| 23 | Drug: Lenvatinib; drug: Lenvatinib Mesylate; drug: Pembrolizumab | Non-IPP | At least 4 weeks before surgery | Perioperative Lenvatinib With Pembrolizumab in Patients With Locally Advanced Nonmetastatic Clear Cell Renal Cell Carcinoma | Recruiting | Kidney cancer Renal cell cancer | 2 | NCT04393350 |
| 24 | Drug: Tislelizumab; drug: pemetrexed; drug: cis-platinum; or drug: carboplatin | Non-IPP | 7–9 weeks before surgery | A Single-arm Exploratory Study of Neoadjuvant Therapy | Recruiting | Non-small-cell lung cancer | N/A | NCT05527808 |
| 25 | Drug: Durvalumab; drug: Tremelimumab; drug: Cisplatin-based neoadjuvant chemotherapy | Non-IPP | Twelve weeks before surgery | Durvalumab (MEDI4736) and TREmelimumab in NEOadjuvant Bladder Cancer Patients (DUTRENEO) | Completed | Bladder cancer | 2 | NCT03472274 |
| 26 | Drug: Axitinib (VEGF-TKI); procedure: cytoreductive nephrectomy (CN); procedure: metastasectomy (MET); biological: Pembrolizumab | Non-IPP | 5–6 weeks before surgery and 3–6 weeks after | Pembrolizumab With or Without Axitinib for Treatment of Locally Advanced or Metastatic Clear Cell Kidney Cancer in Patients Undergoing Surgery | Recruiting | Metastatic/recurrent clear-cell renal cell carcinoma; renal cell cancer | 2 | NCT04370509 |
| 27 | Drug: Nivolumab 10 MG/ML; drug: Ipilimumab 200 MG in a 40 ML injection | Non-IPP | Seven weeks before surgery and four weeks after | Peri-operative Association of Immunotherapy (Pre-operative Association of Nivolumab and Ipilimumab, Post-operative Nivolumab Alone) in Localized Microsatellite Instability (MSI) and/or Deficient Mismatch Repair (dMMR) Oeso-gastric Adenocarcinoma | Recruiting | Localized esogastric adenocarcinoma; MSI and or dMMR | 2 | NCT04006262 |
| 28 | Drug: Nivolumab; drug: carboplatin; drug: nab-paclitaxel | Non-IPP | Eight weeks before and two weeks after surgery | A Two-arm (Phase 2) Exploratory Study of Nivolumab Monotherapy or in Combination With Nab-paclitaxel and Carboplatin in Early Stage NSCLC in China | Recruiting | Non Small Cell Lung Cancer | 2 | NCT04015778 |
| 29 | Drug: neoadjuvant radiation plus SOX and PD-1 antibody | Non-IPP | 5 weeks before surgery and 3–8 weeks after | Neoadjuvant Immunotherapy and Chemoradiotherapy for Locally Advanced Esophagogastric Junction Adenocarcinoma | Not yet recruiting | Adenocarcinoma of esophagogastric junction | 2 | NCT05505461 |
| 30 | Drug: Tirelizumab; drug: Paclitaxel; drug: Carboplatin; radiation: neoadjuvant radiotherapy | Non-IPP | 7–9 weeks before surgery | Selected Chemotherapy Combined Immunotherapy Treated High Risk Patient After NCRT in Resected Locally Advanced ESCC | Recruiting | Esophageal squamous cell carcinoma | 2 | NCT05189730 |
| 31 | Drug: Toripalimab; radiation: stereotactic body radiation therapy (SBRT) | Non-IPP | 6–9 weeks before surgery (after unknown) | Neoadjuvant Radiotherapy Combined With Toripalimab for Locally Advanced Head and Neck Squamous Cell Carcinoma | Not yet recruiting | Locally advanced head and neck squamous cell carcinoma | 2 | NCT05861557 |
| 32 | Drug: camrelizumab; drug: Paclitaxel for injection (albumin-bound); drug: Cisplatin | Non-IPP | Nine weeks before surgery | Efficacy of Neoadjuvant PD-1 Blockade Plus Chemotherapy for Esophageal Squamous Cell Carcinoma | Completed | Esophageal squamous cell Carcinoma | 2 | NCT04225364 |
| 33 | Drug: Carillizumab; procedure: esophagectomy; other: samples | Non-IPP | 4–6 weeks before surgery | Carrelizumab, Chemotherapy and Apatinib in the Neoadjuvant Treatment of Resectable Esophageal Squamous Cell Carcinoma | Active, not recruiting | Esophageal squamous cell carcinoma | 2 | NCT04666090 |
| 34 | Drug: Nivolumab; drug: relatlimab; drug: Oxaliplatin; drug: Docetaxel; drug: 5-Fluorouracil (5-FU); drug: folic acid (FA) | Not described | Perioperative Immunotherapy vs. Chemo-immunotherapy in Patients With Advanced GC and AEG | Active, not recruiting | Gastric cancer esophagogastric junction adenocarcinoma | 2 | NCT04062656 | |
| 35 | Combination product: Toripalimab combined with cisplatin and paclitaxel; combination product: placebo combined with cisplatin and paclitaxel | Not described | Perioperative Toripalimab (JS001) Combined With Neoadjuvant Chemotherapy in Patients With Resectable Locally Advanced Thoracic Esophageal Squamous Cell Carcinoma | Recruiting | Resectable locally advanced thoracic esophageal squamous cell carcinoma | 3 | NCT04848753 | |
| 36 | Drug: neoadjuvant immunotherapy; drug: neoadjuvant chemotherapy | Not described | Effect of Neoadjuvant Anti-PD-1 Immunotherapy on Perioperative Analgesia and Postoperative Delirium | Recruiting | NSCLC | N/A | NCT05273827 | |
| 37 | Drug: Atezolizumab; drug: Capecitabine; drug: Docetaxel; drug: Fluorouracil; drug: Leucovorin Calcium; drug: Oxaliplatin; procedure: positron emission tomography; procedure: surgical procedure | Not described | Testing Immunotherapy (Atezolizumab) With or Without Chemotherapy in Locoregional MSI-H/dMMR Gastric and Gastroesophageal Junction (GEJ) Cancer | Not yet recruiting | Stage I-II-III-IVA—localized gastric cancer and gastroesophageal junction adenocarcinoma AJCC v8 | 2 | NCT05836584 | |
| 38 | Procedure: surgery | Not described | Safety and Feasibility of Surgery After Conversion Therapy for Locally Advanced and Advanced NSCLC | Recruiting | Pulmonary Neoplasm|Advanced Cancer|Locally Advanced Cancer | N/A | NCT04945928 | |
| 39 | Drug: Oxaliplatin; drug: Tegafur–Gimeracil–Oteracil; drug: Sintilimab; radiation: concurrent chemoradiation; procedure: D2/R0 gastrectomy | Not described | Perioperative Chemoimmunotherapy With/Without Preoperative Chemoradiation for Locally Advanced Gastric Cancer | Recruiting | Stomach neoplasms; esophagogastric junction disorder | 2 | NCT05161572 | |
| 40 | Drug: Oxaliplatin; drug: SOX neoadjuvant; drug: Sintilimab neoadjuvant; procedure: gastrectomy plus D2 lymph node dissection; drug: SOX adjuvant; drug: Sintilimab adjuvant | Not described | Transarterial Neoadjuvant Chemotherapy vs. Traditional Intravenous Chemotherapy For Locally Advanced Gastric Cancer With SOX + PD-1 | Recruiting | Locally advanced gastric carcinoma | 3 | NCT05593458 | |
| 41 | Drug: Toripalimab; drug: Docetaxel; drug: Fluorouracil; drug: Leucovorin; drug: Oxaliplatin | Not described | Toripalimab Combined With FLOT —Neoadjuvant Chemotherapy in Patients With Resectable Gastric Cancer | Recruiting | Gastric cancer | Phase 2 | NCT04354662 | |
| 42 | Drug: Anti-PD-1 Monoclonal Antibody JS001; drug: chemotherapy | Not described | The Efficacy of JS001 Combined With Chemotherapy in Patients With Locally Advanced Colon Cancer | Recruiting | Colonic neoplasms | 1|2 | NCT03985891 | |
| 43 | Procedure: Hepatectomy Drug: Pembrolizumab Drug: Vactosertib | Not described | Preoperative Immunotherapy (Pembrolizumab) for Patients With Colorectal Cancer and Resectable Hepatic Metastases | Recruiting | Metastatic malignant neoplasm in the liver; stage IV colorectal cancer | 2 | NCT03844750 | |
| 44 | Drug: Serplilumab; drug: Capecitabine; drug: Oxaliplatin; drug: Celecoxib | Not described | Serplulimab Combined With CAPEOX + Celecoxib as Neoadjuvant Treatment for Locally Advanced Rectal Cancer | Recruiting | pMMR|MSS|MSI-L|locally advanced rectal carcinoma | 2 | NCT05731726 | |
| 45 | Drug: Nivolumab; drug: Ipilimumab | Not described | A Study of Immunotherapy Drugs Nivolumab and Ipilimumab in Patients w/Resectable Malignant Peritoneal Mesothelioma | Active, not recruiting | Mesothelioma|peritoneal mesothelioma | 2 | NCT05041062 | |
| 46 | Drug: Tislelizumab; drug: gemcitabine and cisplatin; radiation: modified hypofractionation | Not described | Risk-stratification Based Bladder-sparing Modalities for Muscle-invasive Bladder Cancer | Recruiting | Bladder cancer | 2 | NCT05531123 | |
| 47 | Drug: neoadjuvant PD-1 inhibitor plus COX inhibitor; drug: neoadjuvant PD-1 inhibitor | Not described | Toripalimab With or Without Celecoxib as Neoadjuvant Therapy in Resectable dMMR/MSI-H Colorectal Cancer | Recruiting | Colorectal cancer, dMMR, and MSI-H | 1|2 | NCT03926338 | |
| 48 | Drug: Durvalumab Drug: Tremelimumab Drug: Enfortumab Vedotin Procedure: Radical Cystectomy | Not described | Treatment Combination of Durvalumab, Tremelimumab and Enfortumab Vedotin or Durvalumab and Enfortumab Vedotin in Patients With Muscle Invasive Bladder Cancer Ineligible to Cisplatin or Who Refuse Cisplatin | Recruiting | Muscle-invasive bladder cancer | 3 | NCT04960709 | |
| 49 | Drug: Nivolumab | Not described | Pre-operative Immunotherapy in Stage II-III Urothelial Cancer | Active, not recruiting | Urothelial carcinoma | 1 | NCT04871594 | |
| 50 | Drug: camrelizumab; drug: albumin paclitaxel; drug: cisplatin | Not described | A Study of Perioperative Camrelizumab Combined With Chemotherapy in Patients With Resectable ESCC | Recruiting | Esophageal cancer | 2 | NCT05182944 | |
| 51 | Drug: Tislelizumab | Not described | Neoadjuvant PD-1 Monoclonal Antibody in Locally Advanced Upper Tract Urothelial Carcinoma | Unknown status | Locally advanced upper urinary tract urothelial carcinoma | 2 | NCT04672330 | |
| 52 | Drug: Carboplatin; drug: Ipilimumab; drug: Nivolumab; drug: Paclitaxel; procedure: positron emission tomography and radiation therapy | Not described | Nivolumab and Ipilimumab in Treating Patients With Esophageal and Gastroesophageal Junction Adenocarcinoma Undergoing Surgery | Suspended | Esophageal/gastroesophageal junction adenocarcinoma AJCC v8 stage II-III-IVA | 2|3 | NCT03604991 | |
| 53 | Drug: PD-1 antibody combined with FOLFIRINOX regimen; drug: PD-1 antibody combined with SOX program | Not described | Immune Checkpoint Inhibitor PD-1 Antibody Combined With Chemotherapy in the Perioperative Treatment of Locally Advanced Resectable Gastric or Gastroesophageal Junction Adenocarcinoma | Recruiting | Locally advanced gastric adenocarcinoma | 2 | NCT04908566 | |
| 54 | Drug: sintilimab; drug: Trastuzumab; drug: S-1 plus oxaliplatin | Not described | SOX Combined With Sintilimab and Trastuzumab Versus SOX Regimen in the Perioperative Treatment of HER2-positive Locally Advanced Gastric Adenocarcinoma | Not yet recruiting | HER2-positive gastric or gastroesophageal junction adenocarcinoma | 2 | NCT05218148 | |
| 55 | Drug: Serplulimab, Albumin paclitaxel, and carboplatin AUC = 5; procedure: esophagectomy | Not described | Serplulimab Combined With Chemotherapy in Patients With Resectable Esophageal Squamous Cell Carcinoma | Recruiting | Esophageal squamous cell carcinoma | 2 | NCT05659251 | |
| 56 | Drug: PD-1 antibody; drug: Capecitabine; drug: Oxaliplatin radiotherapy | Not described | The Combination of Hypofractionated Radiotherapy and Immunotherapy in Locally Recurrent Rectal Cancer | Not yet recruiting | Recurrent rectal cancer | 2 | NCT05628038 | |
| 57 | Drug: neoadjuvant PD-L1 inhibitor | Not described | Envafolimab as Neoadjuvant Immuntherapy in Resectable Local Advanced dMMR/MSI-H Colorectal Cancer | Recruiting | Colorectal cancer, dMMR, and MSI-H | 2 | NCT05371197 |
| Phase | Cancer Type | Patient N | Treatment | Timing | Citation |
|---|---|---|---|---|---|
| I | Glioblastoma | 21 | ICI + GT | IPP | [62] |
| III | Melanoma | 676 | ICI | Non-IPP | [108] |
| III | Melanoma | 418 | ICI + CT | Non-IPP | [109] |
| I/II | Melanoma, NSCLC | 1260 | ICI | Non-IPP | [110] |
| Ib | Metastatic melanoma | 655 | ICI | Non-IPP | [111] |
| III | Bladder | 317 | CT | Non-IPP | [114] |
| III | Esophageal | 368 | CT + RT | Non-IPP | [115] |
| III | NSCLC | 505 | ICI + CT | Non-IPP | [117] |
| II | NSCLC | 44 | ICI | Non-IPP | [118] |
| I | Melanoma | 27 | ICI | IPP | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandbank, E.; Eckerling, A.; Margalit, A.; Sorski, L.; Ben-Eliyahu, S. Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease. Curr. Oncol. 2023, 30, 7450-7477. https://doi.org/10.3390/curroncol30080540
Sandbank E, Eckerling A, Margalit A, Sorski L, Ben-Eliyahu S. Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease. Current Oncology. 2023; 30(8):7450-7477. https://doi.org/10.3390/curroncol30080540
Chicago/Turabian StyleSandbank, Elad, Anabel Eckerling, Adam Margalit, Liat Sorski, and Shamgar Ben-Eliyahu. 2023. "Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease" Current Oncology 30, no. 8: 7450-7477. https://doi.org/10.3390/curroncol30080540
APA StyleSandbank, E., Eckerling, A., Margalit, A., Sorski, L., & Ben-Eliyahu, S. (2023). Immunotherapy during the Immediate Perioperative Period: A Promising Approach against Metastatic Disease. Current Oncology, 30(8), 7450-7477. https://doi.org/10.3390/curroncol30080540





