Novel Cellular and Immunotherapy: Toxicities and Perioperative Implications
Abstract
:1. Introduction—A New Paradigm of Perioperative Cancer Care
2. Immune-Checkpoint Inhibitors
2.1. Specific Toxicities and Perioperative Implications
2.1.1. Gastrointestinal
2.1.2. Pulmonary
2.1.3. Cardiac
2.1.4. Endocrine
3. Chimeric Antigen-Receptor T Cells (CAR-T)
4. Bispecific T-Cell Engager (BiTE) Therapy
5. Other Cellular Therapies
6. Toxicity of Cellular Therapy and Perioperative Considerations
6.1. Cytokine-Release Syndrome (CRS)
6.2. Neurotoxicity
6.3. Perioperative Considerations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Mays, A.C.; Yarlagadda, B.; Achim, V.; Jackson, R.; Pipkorn, P.; Huang, A.T.; Rajasekaran, K.; Sridharan, S.; Rosko, A.J.; Orosco, R.K.; et al. Examining the relationship of immunotherapy and wound complications following flap reconstruction in patients with head and neck cancer. Head Neck 2021, 43, 1509–1520. [Google Scholar] [CrossRef]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356, Erratum in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef]
- Ikeuchi, K.; Okuma, Y.; Tabata, T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer 2016, 99, 148–150. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef]
- Bonaca Marc, P.; Olenchock, B.A.; Salem, J.-E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the setting of cancer therapeutics. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Z.; Zhang, Q.; Zhang, X. Immune checkpoint inhibitors and adrenal insufficiency: A large-sample case series study. Ann. Transl. Med. 2022, 10, 251. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723, Erratum in N. Engl. J. Med. 2010, 363, 1290. [Google Scholar] [CrossRef]
- Goswami S, Aparicio A, Subudhi SK. Immune Checkpoint Therapies in Prostate Cancer. Cancer J. 2016, 22, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Data Presented at AACR Support Potential of Peregrine’s PS-Targeting Immunotherapy Bavituximab to Enhance Anti-Tumor and Immune-Stimulating Effects of Anti-CTLA-4 and Anti-PD-1 Treatments in Models of Melanoma and Colon Cancer. Reuters. Archived from the Original on 2014-05-21. Available online: https://www.biospace.com/article/releases/data-presented-at-american-association-for-cancer-research-support-potential-of-peregrine-pharmaceuticals-inc-s-ps-targeting-immunotherapy-bavituxim/ (accessed on 1 August 2023).
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Opdivo-Nivolumab Injection. DailyMed. 17 December 2019. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394 (accessed on 11 March 2020).
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423. [Google Scholar] [CrossRef]
- Elias, A.W.; Kasi, P.M.; Stauffer, J.A.; Thiel, D.D.; Colibaseanu, D.T.; Mody, K.; Joseph, R.W.; Bagaria, S.P. The Feasibility and Safety of Surgery in Patients Receiving Immune Checkpoint Inhibitors: A Retrospective Study. Front. Oncol. 2017, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337, Erratum in Cell 2022, 185, 576. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126, Erratum in J. Clin. Oncol. 2022, 40, 315. [Google Scholar] [CrossRef]
- Tunio, N.A.; Desai, A.; Dalal, S.; Kurin, M.; Waghray, N. S218 Prevalence and Outcomes of Immune Checkpoint Inhibitor (ICI) Associated Colitis: A Population-Based Cohort Study. Am. J. Gastroenterol. 2022, 117, e157. [Google Scholar] [CrossRef]
- Omori, G.; Takada, K.; Murase, K.; Hayasaka, N.; Nakamura, H.; Iyama, S.; Ohnuma, H.; Miyanishi, K.; Fukuta, F.; Tanaka, T.; et al. Successful mycophenolate mofetil treatment of a patient with severe steroid-refractory hepatitis evoked by nivolumab plus ipilimumab treatment for relapsed bladder cancer. Clin. Case Rep. 2020, 9, 654–659. [Google Scholar] [CrossRef]
- Mahmud, N.; Fricker, Z.; Hubbard, R.A.; Ioannou, G.N.; Lewis, J.D.; Taddei, T.H.; Rothstein, K.D.; Serper, M.; Goldberg, D.S.; Kaplan, D.E. Risk Prediction Models for Post-Operative Mortality in Patients With Cirrhosis. Hepatology 2021, 73, 204–218. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.S.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology 2019, 19, 587–594. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Yoo, J.W.; Joshi, N.; Farrell, J.J. Mo1236—Incidence of Acute Pancreatitis with the use of Immune Checkpoint Inhibitors (ICI) in Solid Tumors: A Systematic Review and Meta-Analysis. Gastroenterology 2018, 154 (Suppl. S1), S-714. [Google Scholar] [CrossRef]
- Waller, A.; Long, B.; Koyfman, A.; Gottlieb, M. Acute Pancreatitis: Updates for Emergency Clinicians. J. Emerg. Med. 2018, 55, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Clotman, K.; Janssens, K.; Specenier, P.; Weets, I.; De Block, C.E.M. Programmed Cell Death-1 Inhibitor–Induced Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 3144–3154. [Google Scholar] [CrossRef]
- Banavasi, H.; Kim, S.; Alkassis, S.; Daoud, A.; Laktineh, A.; Nagasaka, M.; Sukari, A.; Soubani, A.O. Immune checkpoint inhibitor-induced pneumonitis: Incidence, clinical characteristics, and outcomes. Hematol. Oncol. Stem Cell Ther. 2021, 16, 144–150. [Google Scholar] [CrossRef]
- Poulose, V. Incidence of Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer. Chest 2022, 161, e196–e197. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Naidoo, J.; Cottrell, T.R.; Lipson, E.J.; Forde, P.M.; Illei, P.B.; Yarmus, L.B.; Voong, K.R.; Feller-Kopman, D.; Lee, H.; Riemer, J.; et al. Chronic immune checkpoint inhibitor pneumonitis. J. Immunother. Cancer 2020, 8, e000840, Erratum in J. Immunother Cancer 2020, 8, e000840corr1. [Google Scholar] [CrossRef]
- Barrón, F.; Sánchez, R.; Arroyo-Hernández, M.; Blanco, C.; Zatarain-Barrón, Z.L.; Catalán, R.; Ramos-Ramírez, M.; Cardona, A.F.; Flores-Estrada, D.; Arrieta, O. Risk of Developing Checkpoint Immune Pneumonitis and Its Effect on Overall Survival in Non-small Cell Lung Cancer Patients Previously Treated with Radiotherapy. Front. Oncol. 2020, 10, 570233. [Google Scholar] [CrossRef] [PubMed]
- Tiu, B.C.; Zubiri, L.; Iheke, J.; Pahalyants, V.; Theodosakis, N.; Ugwu-Dike, P.; Seo, J.; Tang, K.; Sise, E.M.; Sullivan, R.; et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: A multi-institutional cohort study. J. Immunother. Cancer 2022, 10, e004670. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Cai, K.; Chen, C.; Chen, J.; Chen, K.-N.; Chen, Q.-X.; Cheng, C.; Dai, T.-Y.; Fan, J.; Fan, Z.; et al. Expert consensus on perioperative immunotherapy for local advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 3713–3736. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.-P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41, Erratum in Eur. Respir. J. 2009, 34, 782. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Ful-minant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Norwood, T.G.; Westbrook, B.C.; Johnson, D.B.; Litovsky, S.H.; Terry, N.L.; McKee, S.B.; Gertler, A.S.; Moslehi, J.J.; Conry, R.M. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 2017, 5, 91. [Google Scholar] [CrossRef]
- Vartanov, A.; Kalotra, A.; Varughese, J.; Gautam, S.; Kandel, S.; Hosmer, W. Immunotherapy-associated complete heart block in a patient with NSCLC: A case report and literature review. Respir. Med. Case Rep. 2021, 33, 101390. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef]
- Pelletier, K.; Škrtić, M.; Kitchlu, A. Cancer therapy-induced hyponatremia: A case-illustrated review. J. Onco-Nephrol. 2021, 5, 70–78. [Google Scholar] [CrossRef]
- Özdemir, B.C. Immune checkpoint inhibitor-related hypogonadism and infertility: A neglected issue in immuno-oncology. J. Immunother. Cancer 2021, 9, e002220. [Google Scholar] [CrossRef]
- Jessel, S.; Weiss, S.A.; Austin, M.; Mahajan, A.; Etts, K.; Zhang, L.; Aizenbud, L.; Perdigoto, A.L.; Hurwitz, M.; Sznol, M.; et al. Immune Checkpoint Inhibitor-Induced Hypophysitis and Patterns of Loss of Pituitary Function. Front. Oncol. 2022, 12, 836859. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; Newschaffer, C.; Olivi, A.; Pomper, M.G.; Burger, P.C.; Rose, N.R. Autoimmune hypophysitis. Endocr. Rev. 2005, 26, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- El Sabbagh, R.; Azar, N.S.; Eid, A.A.; Azar, S.T. Thyroid Dysfunctions Due to Immune Checkpoint Inhibitors: A Review. Int. J. Gen. Med. 2020, 13, 1003–1009. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, P.; Huang, H. Engineering better chimeric antigen receptor T cells. Exp. Hematol. Oncol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.-A.N.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.-A.; Yang, J.; Zhang, G.; Li, J.; Song, L.; Su, Y.; Shi, Y.; Zhang, M.; He, J.; et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020, 4, 2325–2338. [Google Scholar] [CrossRef]
- Kyte, J.A. Strategies for Improving the Efficacy of CAR T Cells in Solid Cancers. Cancers 2022, 14, 571. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Helwick, C. Novel BiTE antibody mediates contact between T cells and cancer cells. Oncol. NEWS Int. 2008, 17. [Google Scholar]
- Dombret, H.; Topp, M.S.; Schuh, A.C.; Wei, A.H.; Durrant, S.; Bacon, C.L.; Tran, Q.; Zimmerman, Z.; Kantarjian, H. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk. Lymphoma 2019, 60, 2214–2222. [Google Scholar] [CrossRef]
- Diefenbach, C.; Assouline, S.; Bosch, F.; Cheah, C.Y.; Kim, W.S.; Matasar, M.J.; Panizo, C.; Yoon, D.H.; Bender, B.; Hernandez, G.; et al. An individualized risk mitigation approach for safety: Experience from the mo-sunetuzumab (CD20/CD3 bispecific antibody) development program in relation to neurotoxicity risk [abstract]. Blood 2019, 134 (Suppl. S1), 4728. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Teachey, D.T. Spotlight on Tocilizumab in the Treatment of CAR-T-Cell-Induced Cytokine Release Syndrome: Clinical Ev-idence to Date. Ther. Clin. Risk Manag. 2020, 16, 705–714. [Google Scholar]
- Patel, S.; Cenin, D.; Corrigan, D.; Hamilton, B.K.; Kalaycio, M.; Sobecks, R.M.; Anwer, F.; Khouri, J.; Dean, R.M.; Winter, A.; et al. Siltuximab for First-Line Treatment of Cytokine Release Syndrome: A Response to the National Shortage of Tocilizumab. Blood 2022, 140 (Suppl. S1), 5073–5074. [Google Scholar] [CrossRef]
- Wehrli, M.; Gallagher, K.; Chen, Y.-B.; Leick, M.B.; McAfee, S.L.; El-Jawahri, A.R.; DeFilipp, Z.; Horick, N.; O’Donnell, P.; Spitzer, T.; et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J. Immunother. Cancer 2022, 10, e003847. [Google Scholar] [CrossRef]
- Daver, N.; McClain, K.; Allen, C.E.; Parikh, S.A.; Otrock, Z.; Rojas-Hernandez, C.; Blechacz, B.; Wang, S.; Minkov, M.; Jordan, M.B.; et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017, 123, 3229–3240. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Ziaja, M. Septic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2013, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Parekh, S.; Santomasso, B.D.; Pérez-Larraya, J.G.; van de Donk, N.W.C.J.; Arnulf, B.; Mateos, M.-V.; Lendvai, N.; Jackson, C.C.; De Braganca, K.C.; et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022, 12, 32. [Google Scholar] [CrossRef]
- Shah, N.N.; Highfill, S.L.; Shalabi, H.; Yates, B.; Jin, J.; Wolters, P.L.; Ombrello, A.; Steinbers, S.M.; Martin, S.; Delbrook, C.; et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 Car T-Cell Trial. J. Clin. Oncol. 2020, 38, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tang, D.; Han, Y.; Shen, E.; Ahmed, O.A.; Guo, C.; Shen, H.; Zeng, S. A comprehensive analysis of the fatal toxic effects associated with CD19 CAR-T cell therapy. Aging 2020, 12, 18741–18753. [Google Scholar] [CrossRef] [PubMed]
- Gutgarts, V.; Jain, T.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Pennisi, M.; Jaimes, E.A.; Perales, M.-A.; Sathick, J. Acute Kidney Injury after CAR-T Cell Therapy: Low Incidence and Rapid Recovery. Biol. Blood Marrow Transplant. 2020, 26, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
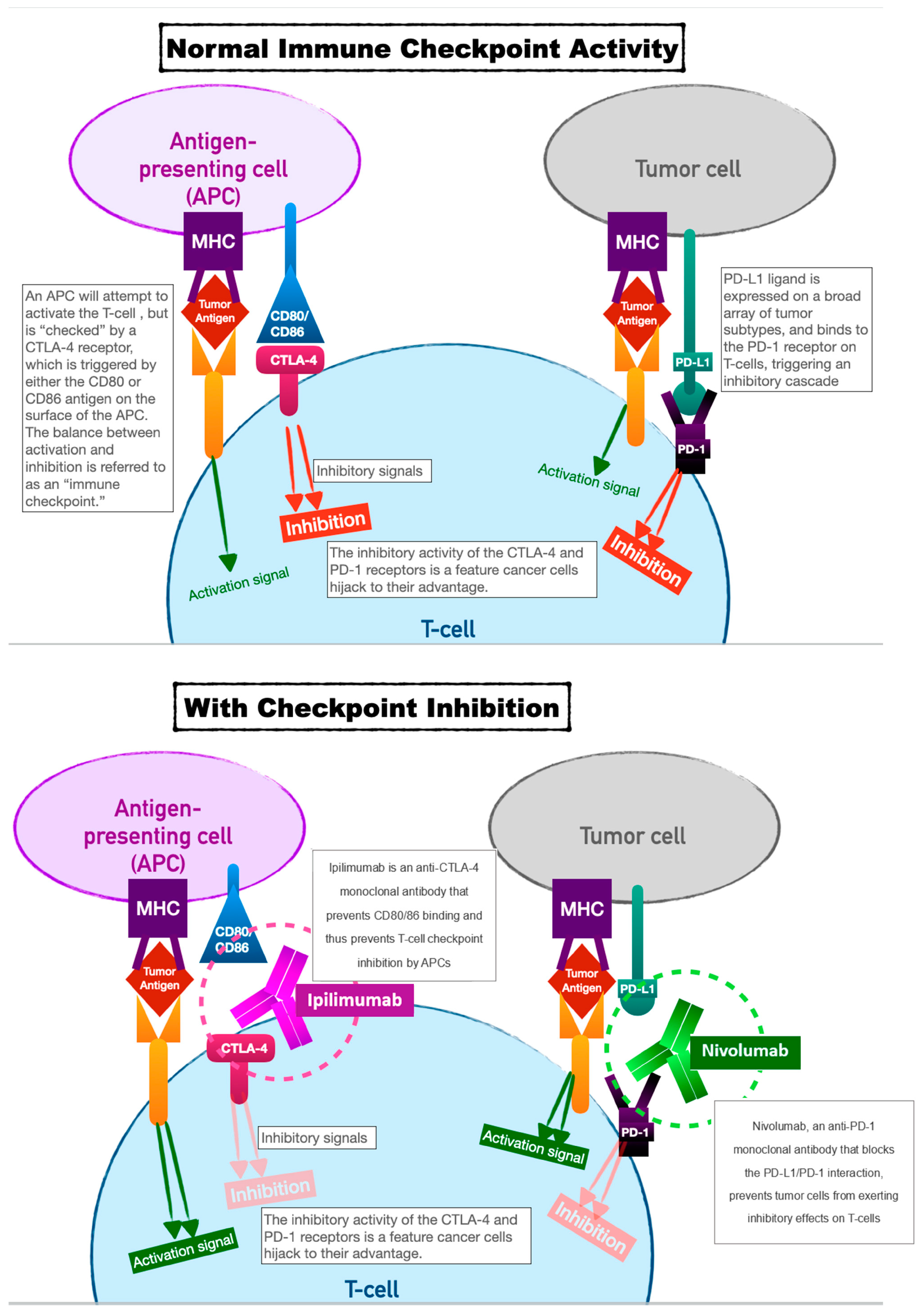

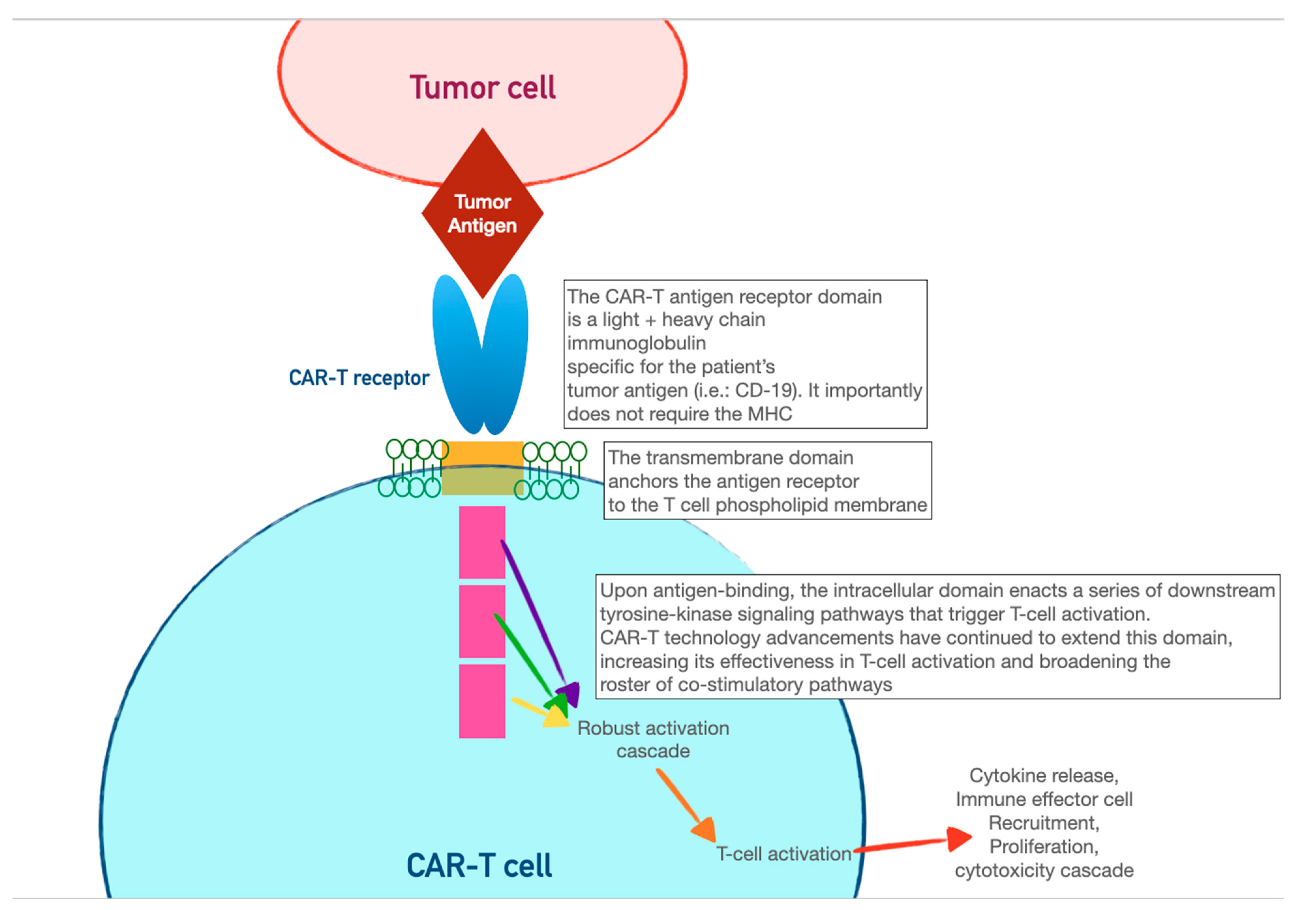
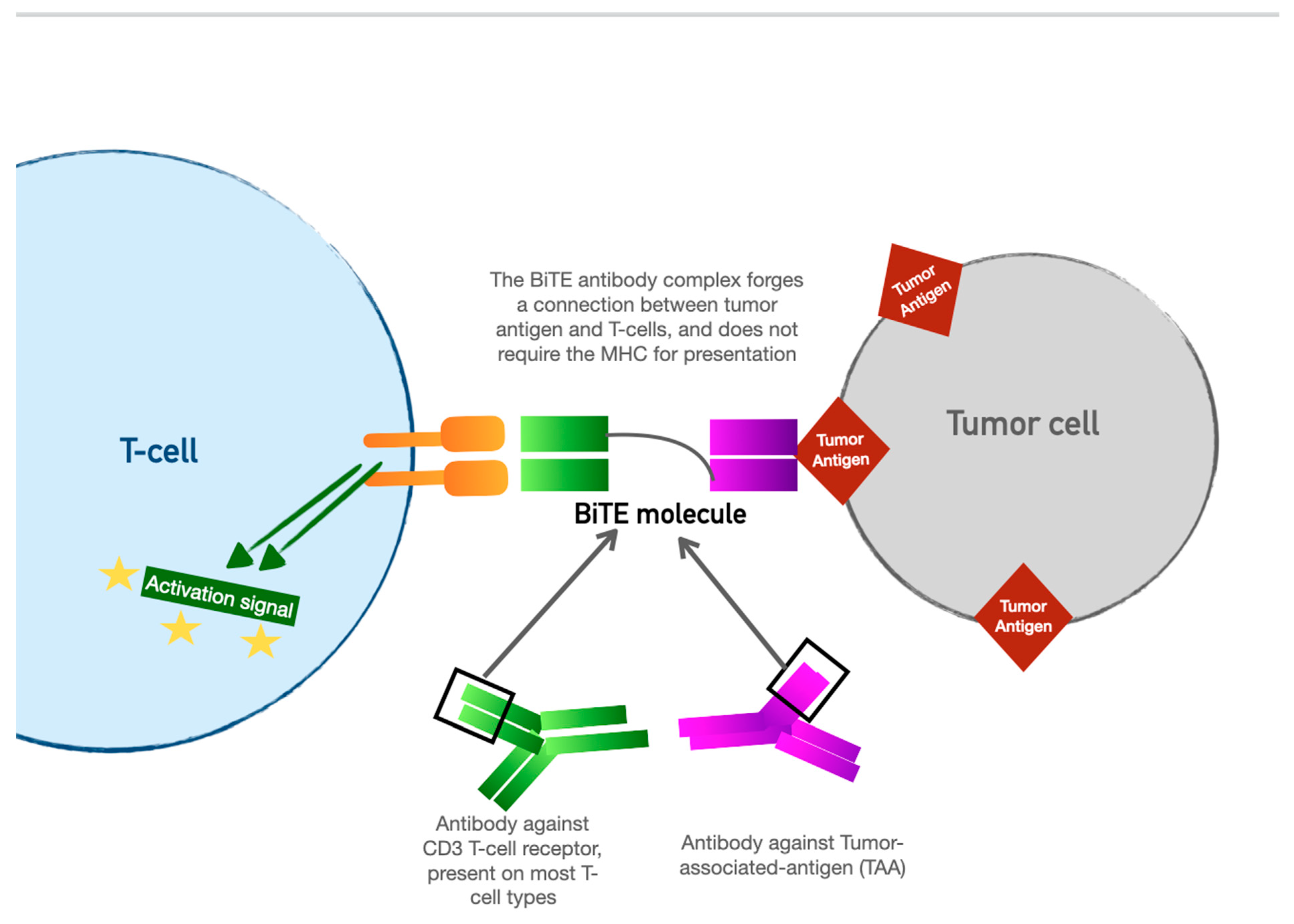
| Immune-Checkpoint Inhibitor Toxicity Category | Manifestations | Treatment | Perioperative Considerations |
|---|---|---|---|
| Central hypothyroidism | Fatigue, headache, low T4, low/normal TSH | Thyroid hormone replacement | Thyroid function tests within 3 months for all patients on ICI therapy |
| Hypogonadism | Hot flashes, decreased libido, decreased FSH and LH | Hormone replacement therapy | Screening for other signs of pituitary dysfunction |
| Pulmonary | Hypersensitivity pneumonitis, pulmonary fibrosis, organizing pneumonia, ARDS | Corticosteroids and supportive care | Screening for respiratory symptoms, baseline CXR, pulmonary risk stratification with baseline spirometry and cardio-pulmonary exercise testing |
| Enterocolitis | Diarrhea, gastritis, hepatitis (manifests as occult rise in AST/ALT over first few months of therapy) | Low-dose corticosteroids | Screening for reflux, gastritis symptoms; recent LFTs if therapy was started within the last few months |
| Pancreatic dysfunction | New-onset type 1 diabetes and ICI pancreatitis | Corticosteroids, resuscitative fluids, insulin therapy | Amylase and lipase levels for patients reporting abdominal pain, ruling out other causes of abdominal pain or pancreatitis; screening with preoperative blood glucose |
| Cardiac | Myocarditis, pericarditis, conduction system disorders | Corticosteroids and standard AHA/ACC heart failure guidelines | Screening for symptoms of heart failure, and baseline EKGs for patients on ICI therapy, cardiac biomarkers, and cardiac MRI for patients with symptoms of myocarditis |
| Toxicity | Incidence | Symptoms | Mechanism | Evidence-Based Treatments | Investigational Treatments |
|---|---|---|---|---|---|
| Cytokine Release Syndrome | Up to 50% | Vasodilation, confusion, fever, headache | Systemic inflammation induced by mass T-lymphocyte activation | Corticosteroids, Tocilizumab, time | Siltuximab, Anakinra |
| Neurotoxicity | 3–5% | Encephalopathy, hypoactive delirium, lethargy, tremors, seizures (see ICANS scoring) | Central nervous system infiltration of lymphocytes | Steroids, Tocilizumab, anti-epileptic drugs, time | Siltuximab, Anakinra |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Camilli, A.; Fischer, G. Novel Cellular and Immunotherapy: Toxicities and Perioperative Implications. Curr. Oncol. 2023, 30, 7638-7653. https://doi.org/10.3390/curroncol30080554
De Camilli A, Fischer G. Novel Cellular and Immunotherapy: Toxicities and Perioperative Implications. Current Oncology. 2023; 30(8):7638-7653. https://doi.org/10.3390/curroncol30080554
Chicago/Turabian StyleDe Camilli, Alessandro, and Gregory Fischer. 2023. "Novel Cellular and Immunotherapy: Toxicities and Perioperative Implications" Current Oncology 30, no. 8: 7638-7653. https://doi.org/10.3390/curroncol30080554
APA StyleDe Camilli, A., & Fischer, G. (2023). Novel Cellular and Immunotherapy: Toxicities and Perioperative Implications. Current Oncology, 30(8), 7638-7653. https://doi.org/10.3390/curroncol30080554




