Addressing Inequity in Spatial Access to Lung Cancer Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Geocoding
2.3. Travel Time
2.4. Statistical Analysis
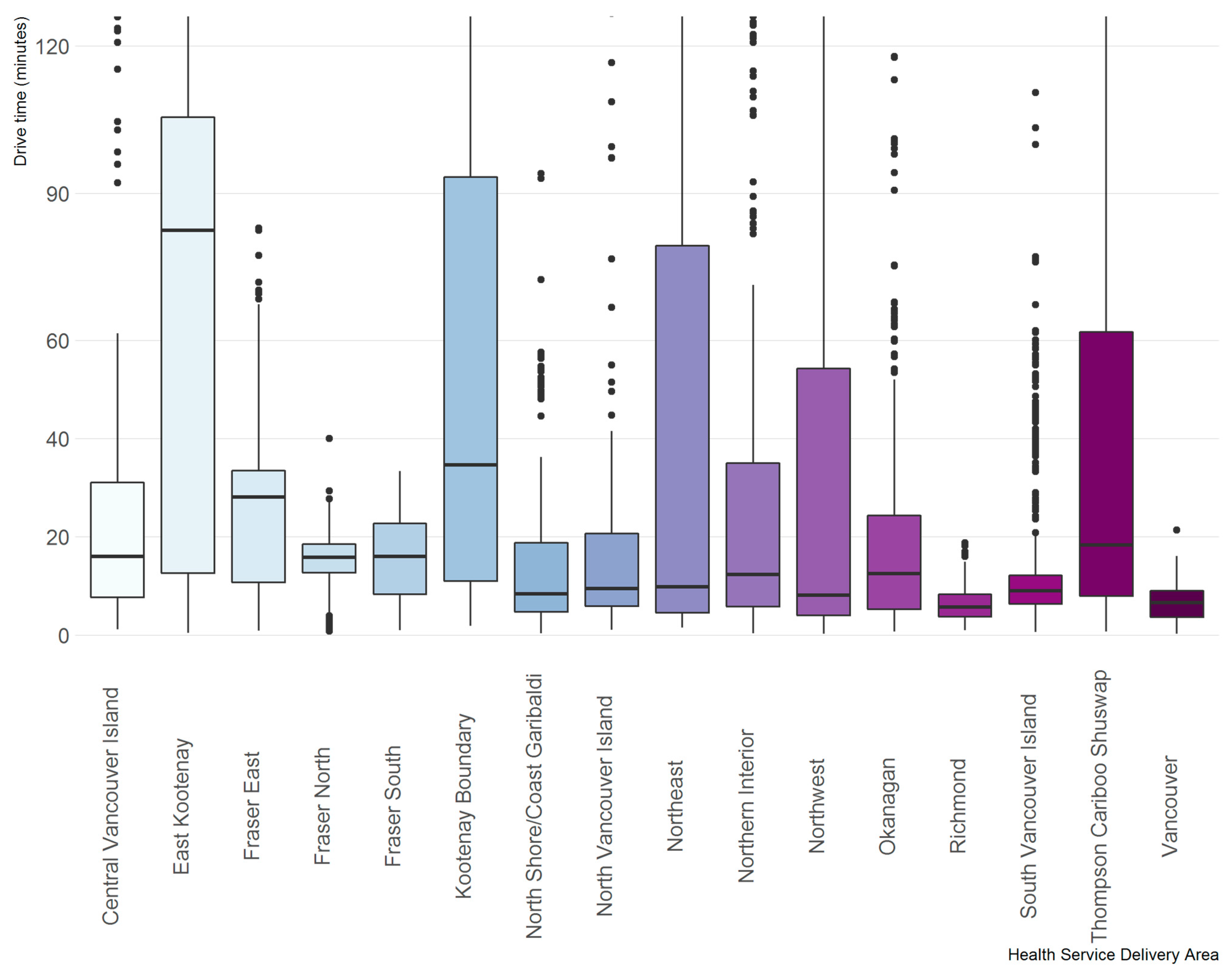
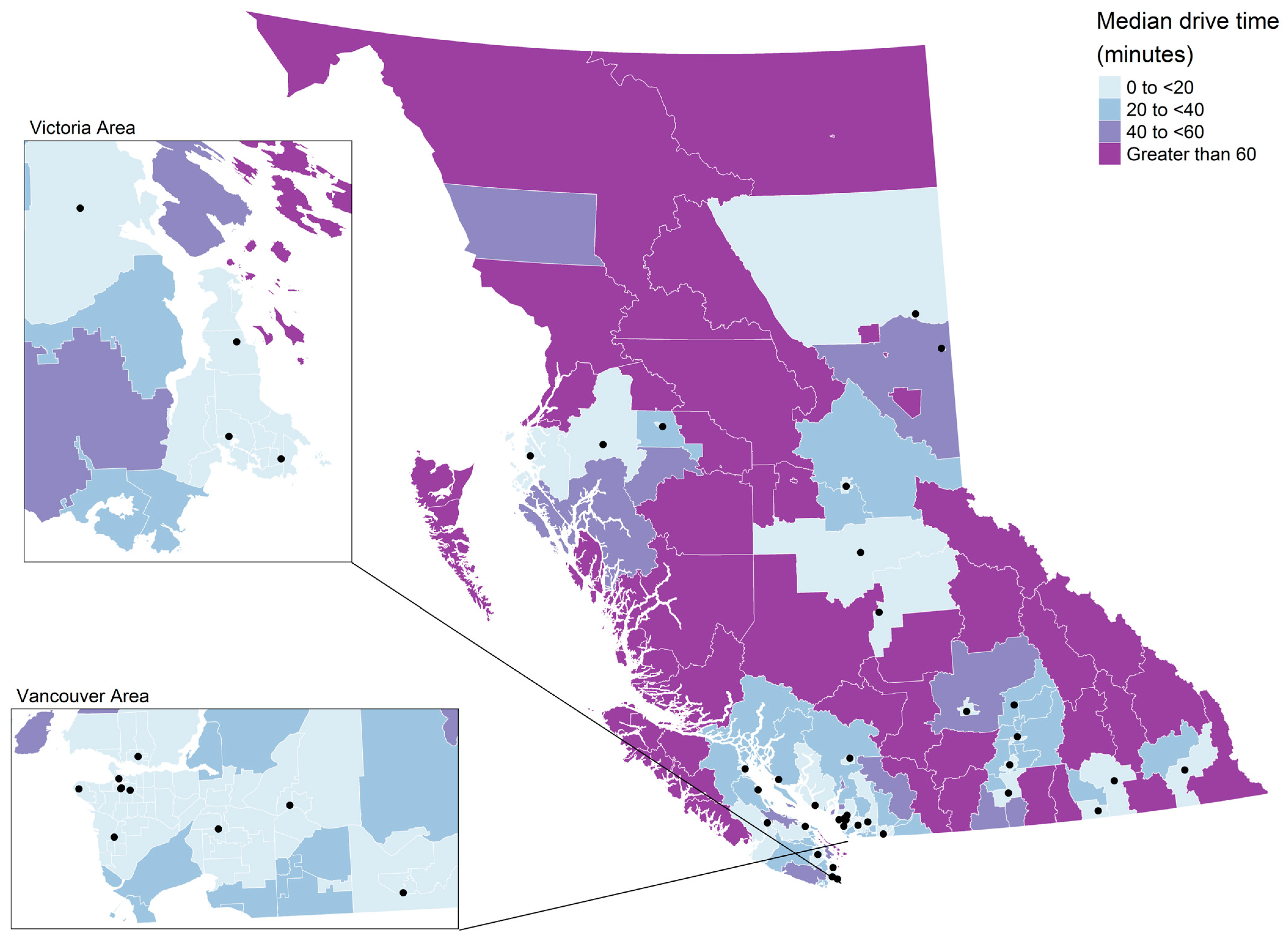
3. Results
4. Discussion
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society and Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2021; Canadian Cancer Society: Toronto, ON, Canada, 2021. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchianò, A. Prolonged Lung Cancer Screening Reduced 10-Year Mortality in the MILD Trial: New Confirmation of Lung Cancer Screening Efficacy. Ann. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, S.A.; Kazerooni, E.A.; Studts, J.L.; Smith, R.A.; Bandi, P.; Sauer, A.G.; Cotter, M.; Sineshaw, H.M.; Jemal, A.; Silvestri, G.A. State Variation in Low-Dose Computed Tomography Scanning for Lung Cancer Screening in the United States. JNCI J. Natl. Cancer Inst. 2021, 113, 1044–1052. [Google Scholar] [CrossRef]
- Mitra, D.; Shaw, A.; Tjepkema, M.; Peters, P. Social Determinants of Lung Cancer Incidence in Canada: A 13-Year Prospective Study. Health Rep. 2015, 26, 12–20. [Google Scholar]
- Singh, G.K.; Jemal, A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J. Environ. Public Health 2017, 2017, e2819372. [Google Scholar] [CrossRef]
- Simkin, J.; Dummer, T.J.B.; Erickson, A.C.; Otterstatter, M.C.; Woods, R.R.; Ogilvie, G. Small Area Disease Mapping of Cancer Incidence in British Columbia Using Bayesian Spatial Models and the Smallareamapp R Package. Front. Oncol. 2022, 12, 833265. [Google Scholar] [CrossRef]
- Withrow, D.R.; Pole, J.D.; Nishri, E.D.; Tjepkema, M.; Marrett, L.D. Cancer Survival Disparities Between First Nation and Non-Aboriginal Adults in Canada: Follow-up of the 1991 Census Mortality Cohort. Cancer Epidemiol. Biomark. Prev. 2017, 26, 145–151. [Google Scholar] [CrossRef]
- Islami, F.; Guerra, C.E.; Minihan, A.; Yabroff, K.R.; Fedewa, S.A.; Sloan, K.; Wiedt, T.L.; Thomson, B.; Siegel, R.L.; Nargis, N.; et al. American Cancer Society’s Report on the Status of Cancer Disparities in the United States, 2021. CA Cancer J. Clin. 2022, 72, 112–143. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Society: Toronto, ON, Canada, 2020. [Google Scholar]
- Tailor, T.D.; Choudhury, K.R.; Tong, B.C.; Christensen, J.D.; Sosa, J.A.; Rubin, G.D. Geographic Access to CT for Lung Cancer Screening: A Census Tract-Level Analysis of Cigarette Smoking in the United States and Driving Distance to a CT Facility. J. Am. Coll. Radiol. 2019, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sahar, L.; Douangchai Wills, V.L.; Liu, K.K.; Kazerooni, E.A.; Dyer, D.S.; Smith, R.A. Using Geospatial Analysis to Evaluate Access to Lung Cancer Screening in the United States. Chest 2021, 159, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Sahar, L.; Douangchai Wills, V.L.; Liu, K.K.; Fedewa, S.A.; Rosenthal, L.; Kazerooni, E.A.; Dyer, D.S.; Smith, R.A. Geographic Access to Lung Cancer Screening among Eligible Adults Living in Rural and Urban Environments in the United States. Cancer 2022, 128, 1584–1594. [Google Scholar] [CrossRef]
- St-Jacques, S.; Philibert, M.D.; Langlois, A.; Daigle, J.-M.; Pelletier, É.; Major, D.; Brisson, J. Geographic Access to Mammography Screening Centre and Participation of Women in the Quebec Breast Cancer Screening Programme. J. Epidemiol. Community Health 2013, 67, 861–867. [Google Scholar] [CrossRef]
- McDonald, J.T.; Wang, Y.; Liu, Z. Participation and Retention in the Breast Cancer Screening Program in New Brunswick Canada. Prev. Med. Rep. 2017, 6, 214–220. [Google Scholar] [CrossRef]
- Canadian Partnership Against Cancer. Examining Disparities in Cancer Control System Performance Special Focus Report; Canadian Partnership against Cancer: Toronto, ON, Canada, 2014. [Google Scholar]
- Honein-AbouHaidar, G.N.; Baxter, N.N.; Moineddin, R.; Urbach, D.R.; Rabeneck, L.; Bierman, A.S. Trends and Inequities in Colorectal Cancer Screening Participation in Ontario, Canada, 2005–2011. Cancer Epidemiol. 2013, 37, 946–956. [Google Scholar] [CrossRef]
- Kerner, J.; Liu, J.; Wang, K.; Fung, S.; Landry, C.; Lockwood, G.; Zitzelsberger, L.; Mai, V. Canadian Cancer Screening Disparities: A Recent Historical Perspective. Curr. Oncol. 2015, 22, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Simkin, J.; Ogilvie, G.; Hanley, B.; Elliott, C. Differences in Colorectal Cancer Screening Rates across Income Strata by Levels of Urbanization: Results from the Canadian Community Health Survey (2013/2014). Can. J. Public Health. 2019, 110, 62–71. [Google Scholar] [CrossRef]
- Statistics Canada. Table 17-10-0005-01 Population Estimates on July 1st, by Age and Sex. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 (accessed on 2 September 2021).
- Ministry of Health Community Health Service Areas-CHSA-Datasets-Data Catalogue. Available online: https://catalogue.data.gov.bc.ca/dataset/community-health-service-areas-chsa (accessed on 3 January 2020).
- Tammemagi, M.C.; Schmidt, H.; Martel, S.; McWilliams, A.; Goffin, J.R.; Johnston, M.R.; Nicholas, G.; Tremblay, A.; Bhatia, R.; Liu, G.; et al. Participant Selection for Lung Cancer Screening by Risk Modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] Study): A Single-Arm, Prospective Study. Lancet Oncol. 2017, 18, 1523–1531. [Google Scholar] [CrossRef]
- Ministry of Health BC Health Care Facilities (Hospital)-Datasets-Data Catalogue. Available online: https://catalogue.data.gov.bc.ca/dataset/bc-health-care-facilities-hospital (accessed on 25 May 2021).
- Statistics Canada the Canadian Index of Multiple Deprivation. Available online: https://www150.statcan.gc.ca/n1/pub/45-20-0001/452000012019002-eng.htm (accessed on 9 February 2022).
- Project OSRM. Available online: http://project-osrm.org/ (accessed on 25 May 2021).
- Riatelab/Osrm: Shortest Paths and Travel Time from OpenStreetMap with R. Available online: https://github.com/riatelab/osrm (accessed on 25 May 2021).
- Liu, E.; Santibáñez, P.; Puterman, M.L.; Weber, L.; Ma, X.; Sauré, A.; Olivotto, I.A.; Halperin, R.; French, J.; Tyldesley, S. A Quantitative Analysis of the Relationship Between Radiation Therapy Use and Travel Time. Int. J. Radiat. Oncol. 2015, 93, 710–718. [Google Scholar] [CrossRef]
- Niranjan, S.J.; Opoku-Agyeman, W.; Carroll, N.W.; Dorsey, A.; Tipre, M.; Baskin, M.L.; Dransfield, M.T. Distribution and Geographic Accessibility of Lung Cancer Screening Centers in the United States. Ann. Am. Thorac. Soc. 2021, 18, 1577–1580. [Google Scholar] [CrossRef]
- Bivand, R.S.; Wong, D.W.S. Comparing Implementations of Global and Local Indicators of Spatial Association. TEST 2018, 27, 716–748. [Google Scholar] [CrossRef]
- Lavergne, M.R.; Johnston, G.M.; Gao, J.; Dummer, T.J.B.; Rheaume, D.E. Variation in the Use of Palliative Radiotherapy at End of Life: Examining Demographic, Clinical, Health Service, and Geographic Factors in a Population-Based Study. Palliat. Med. 2011, 25, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Tyldesley, S.; McGahan, C. Utilisation of Radiotherapy in Rural and Urban Areas in British Columbia Compared with Evidence-Based Estimates of Radiotherapy Needs for Patients with Breast, Prostate and Lung Cancer. Clin. Oncol. 2010, 22, 526–532. [Google Scholar] [CrossRef]
- Huang, J.; Wai, E.S.; Lau, F.; Blood, P.A. Palliative Radiotherapy Utilization for Cancer Patients at End of Life in British Columbia: Retrospective Cohort Study. BMC Palliat. Care 2014, 13, 49. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hystad, P.; Carpiano, R.M.; Demers, P.A.; Johnson, K.C.; Brauer, M. Neighbourhood Socioeconomic Status and Individual Lung Cancer Risk: Evaluating Long-Term Exposure Measures and Mediating Mechanisms. Soc. Sci. Med. 2013, 97, 95–103. [Google Scholar] [CrossRef]
- Drope, J.; Liber, A.C.; Cahn, Z.; Stoklosa, M.; Kennedy, R.; Douglas, C.E.; Henson, R.; Drope, J. Who’s Still Smoking? Disparities in Adult Cigarette Smoking Prevalence in the United States. CA Cancer J. Clin. 2018, 68, 106–115. [Google Scholar] [CrossRef] [PubMed]
- BC Cancer Agency. British Columbia 2011 Regional Cancer Report; BC Cancer Agency: Vancouver, BC, Canda, 2011.
- Simkin, J.; Woods, R.; Elliott, C. Cancer Mortality in Yukon 1999–2013: Elevated Mortality Rates and a Unique Cancer Profile. Int. J. Circumpolar Health 2017, 76, 1324231. [Google Scholar] [CrossRef]
- Atkins, G.T.; Kim, T.; Munson, J. Residence in Rural Areas of the United States and Lung Cancer Mortality: Disease Incidence, Treatment Disparities, and Stage-Specific Survival. Ann. Am. Thorac. Soc. 2017, 14, 403–411. [Google Scholar] [CrossRef]
- Jemal, A.; Fedewa, S.A. Lung Cancer Screening with Low-Dose Computed Tomography in the United States—2010 to 2015. JAMA Oncol. 2017, 3, 1278. [Google Scholar] [CrossRef]
- Zahnd, W.E.; Eberth, J.M. Lung Cancer Screening Utilization: A Behavioral Risk Factor Surveillance System Analysis. Am. J. Prev. Med. 2019, 57, 250–255. [Google Scholar] [CrossRef]
- Rankin, N.M.; McWilliams, A.; Marshall, H.M. Lung Cancer Screening Implementation: Complexities and Priorities. Respirology 2020, 25, 5–23. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Bergamo, C.; Lin, J.J.; Lurslurchachai, L.; Diefenbach, M.; Smith, C.; Nelson, J.E.; Wisnivesky, J.P. Beliefs and Attitudes about Lung Cancer Screening among Smokers. Lung Cancer 2012, 77, 526–531. [Google Scholar] [CrossRef]
- Richmond, J.; Mbah, O.M.; Dard, S.Z.; Jordan, L.C.; Cools, K.S.; Samuel, C.A.; Khan, J.M.; Manning, M.A. Evaluating Potential Racial Inequities in Low-Dose Computed Tomography Screening for Lung Cancer. J. Natl. Med. Assoc. 2020, 112, 209–214. [Google Scholar] [CrossRef]
- Ersek, J.L.; Eberth, J.M.; McDonnell, K.K.; Strayer, S.M.; Sercy, E.; Cartmell, K.B.; Friedman, D.B. Knowledge of, Attitudes toward, and Use of Low-Dose Computed Tomography for Lung Cancer Screening among Family Physicians. Cancer 2016, 122, 2324–2331. [Google Scholar] [CrossRef]
- Jeon, J.; Holford, T.R.; Levy, D.T.; Feuer, E.J.; Cao, P.; Tam, J.; Clarke, L.; Clarke, J.; Kong, C.Y.; Meza, R. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065. Ann. Intern. Med. 2018, 169, 684. [Google Scholar] [CrossRef]
- Gould, M.K.; Huang, B.Z.; Tammemagi, M.C.; Kinar, Y.; Shiff, R. Machine Learning for Early Lung Cancer Identification Using Routine Clinical and Laboratory Data. Am. J. Respir. Crit. Care Med. 2021, 204, 445–453. [Google Scholar] [CrossRef]
- Khan, S.; Pinault, L.; Tjepkema, M.; Wilkins, R. Positional Accuracy of Geocoding from Residential Postal Codes versus Full Street Addresses. Health Rep. 2018, 29, 3–9. [Google Scholar]
- Pinault, L.; Khan, S.; Tjepkema, M. Accuracy of Matching Residential Postal Codes to Census Geography. Health Rep. 2020, 31, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Postal CodeOM Conversion File Plus (PCCF+), Reference Guide; Statistics Canada: Ottawa, ON, Canada, 2021.
- BC Cancer Get Screened. Available online: http://www.bccancer.bc.ca/screening/lung/get-screened (accessed on 25 August 2023).
- Magarinos, J.; Lutzow, L.; Dass, C.; Ma, G.X.; Erkmen, C.P. Feasibility of Single-Encounter Telemedicine Lung Cancer Screening: A Retrospective Cohort Study in an Underserved Population. Cancer Control 2023, 30, 10732748221121392. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (N) N = 12,688 | Proportion (%) |
|---|---|---|
| Age (years) | ||
| Median [IQR] | 70 (64–75) | |
| Age groups (years) | ||
| 55–59 | 1211 | 9.5 |
| 60–64 | 2096 | 16.5 |
| 65–69 | 2722 | 21.5 |
| 70–74 | 3168 | 25 |
| 75–80 | 3491 | 27.5 |
| Sex | ||
| Females | 6553 | 51.6 |
| Males | 6135 | 48.4 |
| Year of diagnosis | ||
| 2015 | 2472 | 19.5 |
| 2016 | 2400 | 18.9 |
| 2017 | 2528 | 19.9 |
| 2018 | 2567 | 20.2 |
| 2019 | 2721 | 21.4 |
| Histology type | ||
| Non-small cell lung cancer | 10,194 | 80.3 |
| Small cell lung cancer | 1226 | 9.7 |
| Sarcomas and other specified malignant neoplasms | 15 | 0.1 |
| Unspecified | 1253 | 9.9 |
| Stage 1 | ||
| I | 2602 | 20.5 |
| II | 979 | 7.7 |
| III | 2587 | 20.4 |
| IV | 5539 | 43.7 |
| Unknown | 961 | 7.6 |
| Occult | 20 | 0.2 |
| CHSA urban-rural classifications | ||
| Metropolitan | 4573 | 36 |
| Large Urban | 1675 | 13.2 |
| Medium Urban | 2332 | 18.4 |
| Small Urban | 1223 | 9.6 |
| Rural Hub | 896 | 7.1 |
| Rural | 1884 | 14.8 |
| Remote | 105 | 0.8 |
| CIMD variables | ||
| Ethnocultural composition 1 | ||
| Q1 | 2687 | 21.2 |
| Q2 | 3114 | 24.5 |
| Q3 | 2781 | 21.9 |
| Q4 | 2218 | 17.5 |
| Q5 | 1869 | 14.7 |
| Missing | 19 | 0.1 |
| Situational vulnerability 2 | ||
| Q1 | 2250 | 17.7 |
| Q2 | 2479 | 19.5 |
| Q3 | 2444 | 19.3 |
| Q4 | 2629 | 20.7 |
| Q5 | 2867 | 22.6 |
| Missing | 19 | 0.1 |
| Residential instability 2 | ||
| Q1 | 1935 | 15.3 |
| Q2 | 2350 | 18.5 |
| Q3 | 2482 | 19.6 |
| Q4 | 2663 | 21 |
| Q5 | 3239 | 25.5 |
| Missing | 19 | 0.1 |
| Economic dependency 2 | ||
| Q1 | 1858 | 14.6 |
| Q2 | 2200 | 17.3 |
| Q3 | 2375 | 18.7 |
| Q4 | 2506 | 19.8 |
| Q5 | 3730 | 29.4 |
| Missing | 19 | 0.1 |
| Variable | Total (N) N = 12,688 | Proportion (%) |
|---|---|---|
| Travel time (minutes) | ||
| Median [IQR] | 11.7 [6.2–23.2] | |
| Travel time categories (minutes) | ||
| <20 | 8856 | 69.8 |
| 20 to 40 | 2246 | 17.7 |
| 40 to 60 | 572 | 4.5 |
| 60+ | 1014 | 8.0 |
| Drive Time Categories | p Value | ||||
|---|---|---|---|---|---|
| Variable | <20 | 20–40 | 40–60 | 60+ | |
| Age | 0.37 | ||||
| 55–59 | 852 (9.6) | 208 (9.3) | 55 (9.6) | 96 (9.5) | |
| 60–64 | 1464 (16.5) | 361 (16.1) | 86 (15.0) | 185 (18.2) | |
| 65–69 | 1894 (21.4) | 457 (20.3) | 137 (24.0) | 234 (23.1) | |
| 70–74 | 2198 (24.8) | 585 (26.0) | 134 (23.4) | 251 (24.8) | |
| 75+ | 2448 (27.6) | 635 (28.3) | 160 (28.0) | 248 (24.5) | |
| Sex | 0.018 | ||||
| Females | 4555 (51.4) | 1218 (54.2) | 283 (49.5) | 497 (49) | |
| Males | 4301 (48.6) | 1028 (45.8) | 289 (50.5) | 517 (51) | |
| Stage | 0.28 | ||||
| I | 1847 (22.6) | 454 (21.8) | 95 (18.1) | 206 (22) | |
| II | 677 (8.3) | 189 (9.1) | 45 (8.6) | 68 (7.3) | |
| III | 1792 (22) | 466 (22.3) | 112 (21.3) | 217 (23.2) | |
| IV | 3843 (47.1) | 978 (46.9) | 273 (52) | 445 (47.5) | |
| Level of urbanization | <0.001 | ||||
| Metropolitan | 4204 (47.5) | 368 (16.4) | <5 (0.9) | <5 (0.5) | |
| Large Urban | 1616 (18.2) | <60 (2.7) | <5 (0.9) | <5 (0.5) | |
| Medium Urban | 1704 (19.2) | 600 (26.7) | <30 (5.2) | <5 (0.5) | |
| Small Urban | 757 (8.5) | 367 (16.3) | <100 (17.5) | <5 (0.5) | |
| Rural Hub | 124 (1.4) | 297 (13.2) | 171 (29.9) | 304 (30) | |
| Rural | 445 (5) | 551 (24.5) | 276 (48.3) | 612 (60.4) | |
| Remote | 6 (0.1) | <5 (0.2) | <5(0.9) | <5 (0.5) | |
| CIMD variables | |||||
| Ethnocultural composition * | |||||
| Q1 | 1289 (14.6) | 693 (30.9) | 200 (35.4) | 505 (50.0) | <0.001 |
| Q2 | 1796 (20.3) | 712 (31.8) | 242 (42.8) | 364 (36.0) | |
| Q3 | 2011 (22.7) | 553 (24.7) | 100 (17.7) | 117 (11.6) | |
| Q4 | 1965 (22.2) | 205 (9.1) | 23 (4.1) | 25 (2.5) | |
| Q5 | 1791 (20.2) | 78 (3.5) | 0 (0) | 0 (0) | |
| Situational vulnerability * | <0.001 | ||||
| Q1 | 1601 (18.1) | 525 (23.4) | 76 (13.5) | 48 (4.7) | |
| Q2 | 1755 (19.8) | 491 (21.9) | 117 (20.7) | 116 (11.5) | |
| Q3 | 1714 (19.4) | 445 (19.9) | 124 (21.9) | 161 (15.9) | |
| Q4 | 1836 (20.7) | 423 (18.9) | 131 (23.2) | 239 (23.6) | |
| Q5 | 1946 (22.0) | 357 (15.9) | 117 (20.7) | 447 (44.2) | |
| Residential instability * | <0.001 | ||||
| Q1 | 1231 (13.9) | 460 (20.5) | 76 (13.5) | 168 (16.6) | |
| Q2 | 1385 (15.6) | 551 (24.6) | 156 (27.6) | 258 (25.5) | |
| Q3 | 1509 (17.0) | 467 (20.8) | 214 (37.9) | 292 (28.9) | |
| Q4 | 1902 (21.5) | 427 (19.1) | 95 (16.8) | 239 (23.6) | |
| Q5 | 2825 (31.9) | 336 (15) | 24 (4.2) | 54 (5.3) | |
| Economic dependency * | <0.001 | ||||
| Q1 | 1477 (16.7) | 225 (10) | 63 (11.2) | 93 (9.2) | |
| Q2 | 1763 (19.9) | 320 (14.3) | 37 (6.5) | 80 (7.9) | |
| Q3 | 1818 (20.5) | 372 (16.6) | 74 (13.1) | 111 (11) | |
| Q4 | 1834 (20.7) | 417 (18.6) | 91 (16.1) | 164 (16.2) | |
| Q5 | 1960 (22.1) | 907 (40.5) | 300 (53.1) | 563 (55.7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simkin, J.; Khoo, E.; Darvishian, M.; Sam, J.; Bhatti, P.; Lam, S.; Woods, R.R. Addressing Inequity in Spatial Access to Lung Cancer Screening. Curr. Oncol. 2023, 30, 8078-8091. https://doi.org/10.3390/curroncol30090586
Simkin J, Khoo E, Darvishian M, Sam J, Bhatti P, Lam S, Woods RR. Addressing Inequity in Spatial Access to Lung Cancer Screening. Current Oncology. 2023; 30(9):8078-8091. https://doi.org/10.3390/curroncol30090586
Chicago/Turabian StyleSimkin, Jonathan, Edwin Khoo, Maryam Darvishian, Janette Sam, Parveen Bhatti, Stephen Lam, and Ryan R. Woods. 2023. "Addressing Inequity in Spatial Access to Lung Cancer Screening" Current Oncology 30, no. 9: 8078-8091. https://doi.org/10.3390/curroncol30090586
APA StyleSimkin, J., Khoo, E., Darvishian, M., Sam, J., Bhatti, P., Lam, S., & Woods, R. R. (2023). Addressing Inequity in Spatial Access to Lung Cancer Screening. Current Oncology, 30(9), 8078-8091. https://doi.org/10.3390/curroncol30090586





