Effects of Acetaminophen Exposure on Outcomes of Patients Receiving Immune Checkpoint Inhibitors for Advanced Non-Small-Cell Lung Cancer: A Propensity Score-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Benefit Analysis
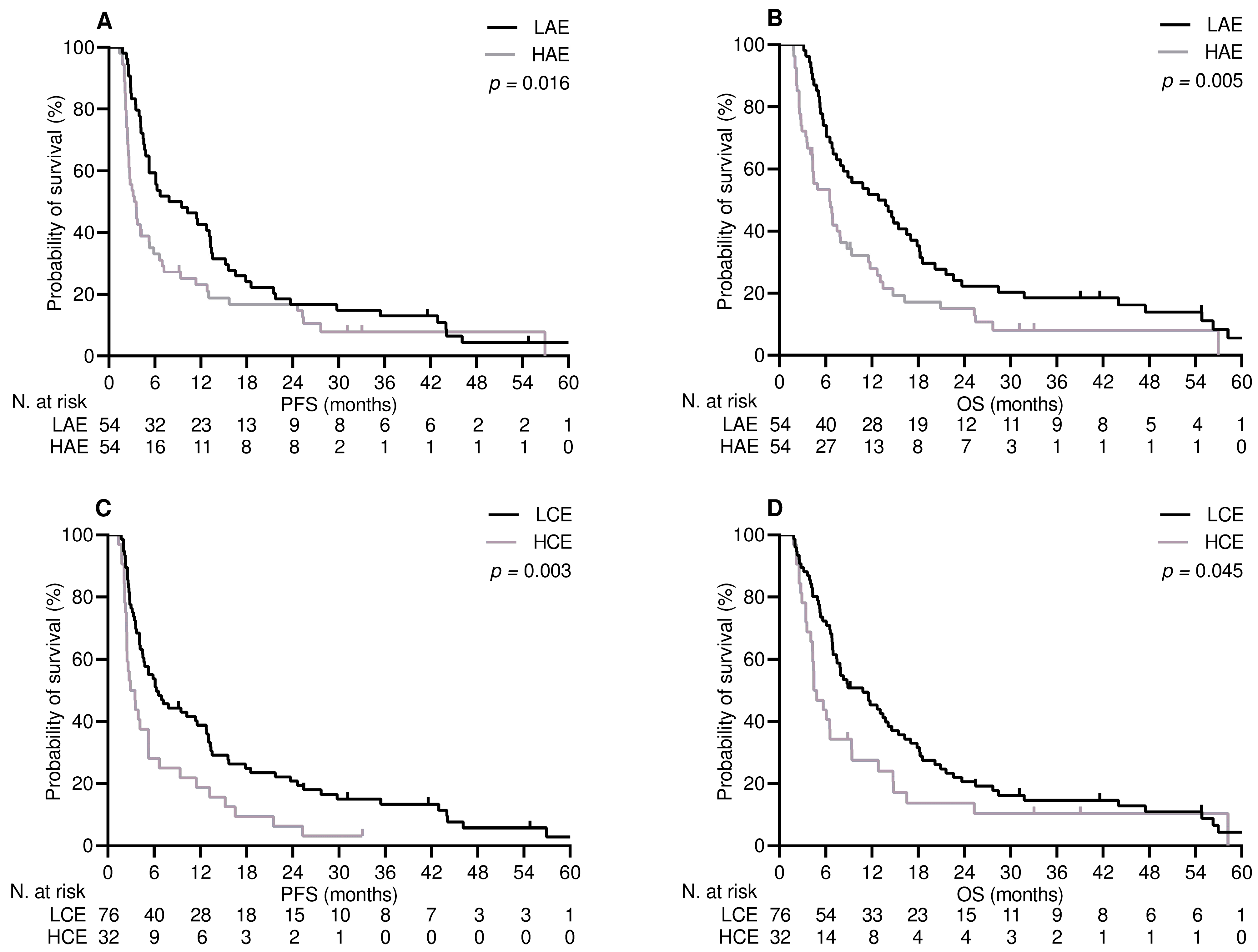
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steuer, C.E.; Ramalingam, S.S. Advances in immunotherapy and implications for current practice in non–small-cell lung cancer. JCO Oncol. Pract. 2021, 17, 662–668. [Google Scholar] [CrossRef]
- Hanna, N.H.; Robinson, A.G.; Temin, S.; Baker, S., Jr.; Brahmer, J.R.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2021, 39, 1040–1091. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.L.; John, T. Immunotherapy in Advanced NSCLC without Driver Mutations: Available Therapeutic Alternatives after Progression and Future Treatment Options. Clin. Lung Cancer 2022, 23, 643–658. [Google Scholar] [CrossRef]
- Magnuson, A.; Sattar, S.; Nightingale, G.; Saracino, R.; Skonecki, E.; Trevino, K.M. A Practical Guide to Geriatric Syndromes in Older Adults with Cancer: A Focus on Falls, Cognition, Polypharmacy, and Depression. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e96–e109. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-Induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Cruellas, M.; Yubero, A.; Zapata, M.; Galvez, E.M.; Gascón, M.; Isla, D.; Lastra, R.; Martínez-Lostao, L.; Ocariz, M.; Pardo, J.; et al. How Could Antibiotics, Probiotics, and Corticoids Modify Microbiota and Its Influence in Cancer Immune Checkpoint Inhibitors: A Review. Infect. Immun. 2021, 89, e0066520. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Badaoui, S.; Kichenadasse, G.; Karapetis, C.S.; McKinnon, R.A.; Rowland, A.; Sorich, M.J. Efficacy of Atezolizumab in Patients with Advanced NSCLC Receiving Concomitant Antibiotic or Proton Pump Inhibitor Treatment: Pooled Analysis of Five Randomized Control Trials. J. Thorac. Oncol. 2022, 17, 758–767. [Google Scholar] [CrossRef]
- Mock, J.; Wynter, E.; Young, P.; Sytov, A.; Elghawy, O.; Gentzler, R.; Novicoff, W.; Martin, L.; Hall, R. MO01.12 Association of Opioid Use with Survival in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitor Therapy. J. Thorac. Oncol. 2021, 16, S20–S21. [Google Scholar] [CrossRef]
- Cortellini, A.; D’Alessio, A.; Cleary, S.; Buti, S.; Bersanelli, M.; Bordi, P.; Tonini, G.; Vincenzi, B.; Tucci, M.; Russo, A.; et al. Type 2 diabetes mellitus and efficacy outcomes from imune checkpoint blockade in patients with cancer. Clin. Cancer Res. 2023, 29, 2714–2724. [Google Scholar] [CrossRef]
- Cantini, L.; Pecci, F.; Hurkmans, D.P.; Belderbos, R.A.; Lanese, A.; Copparoni, C.; Aerts, S.; Cornelissen, R.; Dumoulin, D.W.; Fiordoliva, I.; et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur. J. Cancer 2021, 144, 41–48. [Google Scholar] [CrossRef]
- Stokes, W.A.; Behera, M.; Jiang, R.; Gutman, D.A.; Huang, Z.; Burns, A.; Sebastian, N.T.; Sukhatme, V.; Lowe, M.C.; Ramalingam, S.S.; et al. Impact of concomitant fibrates on immunotherapy outcomes for advanced non-small cell lung cancer. Cancer Med. 2023, 12, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Guzner, A.; Wainwright, D.A.; Mohindra, N.A.; Chae, Y.K.; Behdad, A.; Villaflor, V.M. The Impact of Beta Blockers on Survival Outcomes in Patients with Non-small-cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, e57–e62. [Google Scholar] [CrossRef]
- Sebastian, N.T.; Stokes, W.A.; Behera, M.; Jiang, R.; Gutman, D.A.; Huang, Z.; Burns, A.; Sukhatme, V.; Lowe, M.C.; Ramalingam, S.S.; et al. The Association of Improved Overall Survival with NSAIDs in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. Clin. Lung Cancer 2023, 24, 287–294. [Google Scholar] [CrossRef]
- Tozuka, T.; Yanagitani, N.; Yoshida, H.; Manabe, R.; Ogusu, S.; Tsugitomi, R.; Sakamoto, H.; Amino, Y.; Ariyasu, R.; Uchibori, K.; et al. Impact of Renin-angiotensin System Inhibitors on the Efficacy of Anti-PD-1/PD-L1 Antibodies in NSCLC Patients. Anticancer Res. 2021, 41, 2093–2100. [Google Scholar] [CrossRef]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef]
- Dar, S.; Merza, N.; Qatani, A.; Rahim, M.; Varughese, T.; Mohammad, A.; Masood, F.; Reza, F.Z.; Wan, S.; Almas, T.; et al. Impact of proton-pump inhibitors on the efficacy of immune checkpoint inhibitors in non-small cell lung cancer: A systematic review and meta-analysis. Ann. Med. Surg. 2022, 78, 103752. [Google Scholar] [CrossRef]
- Chen, H.; Han, K.D.; He, Z.J.; Huang, Y.S. How to Choose a Survival Period? The Impact of Antibiotic Use on OS or PFS in NSCLC Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211033498. [Google Scholar] [CrossRef] [PubMed]
- Cani, M.; Bironzo, P.; Garetto, F.; Buffoni, L.; Cotogni, P. Immune Checkpoint Inhibitors and Opioids in Patients with Solid Tumours: Is Their Association Safe? A Systematic Literature Review. Healthcare 2022, 11, 116. [Google Scholar] [CrossRef]
- Bessede, A.; Marabelle, A.; Guégan, J.P.; Danlos, F.X.; Cousin, S.; Peyraud, F.; Chaput, N.; Spalato, M.; Roubaud, G.; Cabart, M.; et al. Impact of acetaminophen on the efficacy of immunotherapy in cancer patients. Ann. Oncol. 2022, 33, 909–915. [Google Scholar] [CrossRef]
- Acetaminophen. Available online: https://www.fda.gov/drugs/information-drug-class/acetaminophen (accessed on 9 July 2023).
- Registri Farmaci Sottoposti a Monitoraggio. Available online: https://www.aifa.gov.it/registri-farmaci-sottoposti-a-monitoraggio (accessed on 9 July 2023).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. R Website. Available online: https://www.r-project.org (accessed on 9 July 2023).
- Mushti, S.L.; Mulkey, F.; Sridhara, R. Evaluation of Overall Response Rate and Progression-Free Survival as Potential Surrogate Endpoints for Overall Survival in Immunotherapy Trials. Clin. Cancer Res. 2018, 24, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, E.; Leufkens, H.G.M.; Smyth, J.F. The use of real-world data in cancer drug development. Eur. J. Cancer 2018, 101, 69–76. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Schneeweiss, S.; Rothman, K.J.; Glynn, R.J.; Avorn, J.; Stürmer, T. Variable selection for propensity score models. Am. J. Epidemiol. 2006, 163, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V.; Whelton, P.K.; Klag, M.J. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidalantiinflammatory drugs. N. Engl. J. Med. 1994, 331, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Brooks, D.E.; Dibert, K.W.; Rivers, L.J.; Pham, N.P.T.; Ryan, M.L. 2019 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin. Toxicol. 2020, 58, 1360–1541. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Chen, Y.; Lian, Z.X.; Wei, H.; Tian, Z. Regulatory T cells ameliorate acetaminophen-induced immune-mediated liver injury. Int. Immunopharmacol. 2015, 25, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Keum, D.J.; Kwak, J.W.; Chung, H.S.; Bae, H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef]
- Thiele, K.; Solano, M.E.; Huber, S.; Flavell, R.A.; Kessler, T.; Barikbin, R.; Jung, R.; Karimi, K.; Tiegs, G.; Arck, P.C. Prenatal acetaminophen affects maternal immune and endocrine adaptation to pregnancy, induces placental damage, and impairs fetal development in mice. Am. J. Pathol. 2015, 185, 2805–2818. [Google Scholar] [CrossRef]
- Abdel Shaheed, C.; Beardsley, J.; Day, R.O.; McLachlan, A.J. Immunomodulatory effects of pharmaceutical opioids and antipyretic analgesics: Mechanisms and relevance to infection. Br. J. Clin. Pharmacol. 2022, 88, 3114–3131. [Google Scholar] [CrossRef]
- Prymula, R.; Siegrist, C.A.; Chlibek, R.; Zemlickova, H.; Vackova, M.; Smetana, J.; Lommel, P.; Kaliskova, E.; Borys, D.; Schuerman, L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: Two open-label, randomised controlled trials. Lancet 2009, 374, 1339–1350. [Google Scholar] [CrossRef]
- Saleh, E.; Moody, M.A.; Walter, E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccine Immunother. 2016, 12, 2391–2402. [Google Scholar] [CrossRef]
- WHO. Reducing pain at the time of vaccination: WHO position paper—September 2015. Vaccine 2016, 34, 3629–3630. [Google Scholar] [CrossRef]
- General Best Practice Guidelines for Immunization. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index (accessed on 9 July 2023).
- Schmiedeberg, K.; Beyer, J.; Abela, I.; Vuilleumier, N.; Schwarzmueller, M.; Pagano, S.; Von Kempis, J.; Rubbert-Roth, A. AB1335 Intake of acetaminophen suppresses antiviral humoral immune responses in patients with RA following vaccination with anti SARS-CoV-2 mRNA based vaccines. Ann. Rheum. Dis. 2023, 82, 1897–1898. [Google Scholar] [CrossRef]
- Ooi, E.E.; Dhar, A.; Petruschke, R.; Locht, C.; Buchy, P.; Low, J.G.H. Use of analgesics/antipyretics in the management of symptoms associated with COVID-19 vaccination. NPJ Vaccines 2022, 7, 31. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.; Wang, X.; Li, J.; Jiang, L. The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury. Biology 2022, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wisse, E.; Tian, Z. Liver natural killer cells: Subsets and roles in liver immunity. Cell. Mol. Immunol. 2016, 13, 328–336. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Sugiyama, S.; Horie, T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem. Biol. Interact. 2009, 179, 273–279. [Google Scholar] [CrossRef]
- Zheng, J.; He, J.; Wang, W.; Zhou, H.; Cai, S.; Zhu, L.; Qian, X.; Wang, J.; Lu, Z.; Huang, C. The impact of pain and opioids use on survival in cancer patients: Results from a population-based cohort study and a meta-analysis. Medicine 2020, 99, e19306. [Google Scholar] [CrossRef]
- Hussain, N.; Naeem, M.; Pinato, D.J. Concomitant medications and immune checkpoint inhibitor therapy for cancer: Causation or association? Hum. Vaccin. Immunother. 2021, 17, 55–61. [Google Scholar] [CrossRef]
- Yin, J.; Song, Y.; Tang, J.; Zhang, B. What is the optimal duration of immune checkpoint inhibitors in malignant tumors? Front. Immunol. 2022, 13, 983581. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Heo, M.H.; Lee, H.S.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J. Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother. Pharmacol. 2017, 80, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Derry, S.; Moore, R.A.; McNicol, E.D.; Bell, R.F.; Carr, D.B.; McIntyre, M.; Wee, B. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst. Rev. 2017, 7, CD012637. [Google Scholar]
- WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Available online: https://www.who.int/publications/i/item/9789241550390 (accessed on 9 July 2023).
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.; Lam, J.; Betts, K.A.; Yin, L.; Gao, S.; Yuan, Y.; Hartman, J.; Rao, S.; Lubinga, S.; Stenehjem, D. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer 2021, 56, 41–49. [Google Scholar] [CrossRef] [PubMed]

| Variable | All Patients, N = 80 (100%) | General Population | PSM Population | ||||
|---|---|---|---|---|---|---|---|
| LAE, N = 45 (100%) | HAE, N = 35 (100%) | p Value | LAE, N = 26 (100%) | HAE, N = 26 (100%) | p Value | ||
| Age | |||||||
| Mean (SD), years | 67.0 (8.8) | 68.0 (8.3) | 65.7 (9.4) | 0.299 | 70.1 (9.2) | 66.7 (8.7) | 0.128 |
| ≥70 years | 32 (40.0%) | 20 (44.4%) | 12 (34.3%) | 0.358 | 15 (57.7%) | 10 (38.5%) | 0.165 |
| Sex | 0.923 | 0.749 | |||||
| female | 21 (26.2%) | 12 (26.7%) | 9 (20.0%) | 6 (23.1%) | 7 (26.9%) | ||
| male | 59 (73.8%) | 33 (73.3%) | 26 (80.0%) | 20 (76.9%) | 19 (73.1%) | ||
| ECOG PS | 0.018 | 0.999 | |||||
| 0 or 1 | 68 (85.0%) | 42 (93.3%) | 26 (74.3%) | 23 (88.5%) | 23 (88.5%) | ||
| 2 | 19 (15.0%) | 3 (6.7%) | 9 (25.7%) | 3 (11.5%) | 3 (11.5%) | ||
| Histology | 0.670 | 0.442 | |||||
| Squamous | 10 (12.5%) | 5 (11.1%) | 5 (14.3%) | 3 (11.5%) | 5 (19.2%) | ||
| Nonsquamous | 70 (87.5%) | 40 (88.9%) | 30 (85.7%) | 23 (88.5%) | 21 (80.8%) | ||
| No. of metastatic sites | 0.413 | 0.158 | |||||
| ≤2 | 43 (53.7%) | 26 (57.8%) | 19 (54.3%) | 18 (69.2%) | 13 (50.0%) | ||
| >2 | 37 (46.3%) | 17 (42.3%) | 18 (45.7%) | 8 (30.8%) | 13 (50.0%) | ||
| Bone metastases | 0.757 | 0.999 | |||||
| Not present | 63 (78.8%) | 36 (80.0%) | 27 (77.2%) | 22 (84.6%) | 22 (84.6%) | ||
| Any | 17 (21.2%) | 9 (20.0%) | 8 (22.8%) | 4 (15.4%) | 4 (15.4%) | ||
| CNS metastases | 0.923 | 0.337 | |||||
| Not present | 59 73.8%) | 33 (73.4%) | 26 (74.3%) | 21 (80.8%) | 18 (69.2%) | ||
| Any | 21 (26.2%) | 12 (26.6%) | 9 (25.7%) | 5 (19.2%) | 8 (30.8%) | ||
| Liver metastases | 0.060 | 0.124 | |||||
| Not present | 72 (90.0%) | 43 (95.6%) | 29 (82.9%) | 24 (92.3%) | 20 (76.9%) | ||
| Any | 8 (10.0%) | 2 (4.4%) | 6 (17.1%) | 2 (7.7%) | 6 (23.1%) | ||
| PD-L1 TPS | 0.363 | 0.798 | |||||
| <1% | 32 (40.0%) | 21 (46.7%) | 11 (31.4%) | 5 (19.2) | 7 (26.9%) | ||
| ≥1% and ≤49% | 11 (13.7%) | 6 (13.3%) | 5 (14.3%) | 3 (11.5%) | 3 (11.5%) | ||
| ≥50% | 37 (46.2%) | 18 (40.0%) | 19 (54.3%) | 18 (69.2%) | 16 (61.5%) | ||
| BMI | |||||||
| Mean (SD), (kg/m2) | 25.6 (5.2) | 25.9 (5.1) | 25.2 (5.4) | 0.485 | 26.4 (5.5) | 25.4 (5.5) | 0.577 |
| ≥25 | 41 (51.2%) | 26 (57.8%) | 15 (42.8) | 0.185 | 14 (53.8%) | 11 (42.3%) | 0.405 |
| Smoking habit | 0.141 | 0.638 | |||||
| Never | 9 (11.2%) | 3 (6.7%) | 6 (17.1%) | 2 (7.7%) | 3 (11.5%) | ||
| Ever | 71 (88.8%) | 42 (93.3%) | 29 (82.9%) | 24 (93.3%) | 23 (88.5%) | ||
| Type of treatment | 0.265 | 0.351 | |||||
| Pembrolizumab | 42 (52.5%) | 21 (46.7%) | 21 (60.0%) | 18 (69.2%) | 17 (65.4%) | ||
| Pemetrexed-based | 33 (41.2%) | 22 (48.9%) | 11 (31.4%) | 8 (30.8%) | 7 (26.9%) | ||
| Paclitaxel-based | 5 (6.3%) | 2 (4.4) | 3 (8.6%) | - | 2 (7.7%) | ||
| Reasons for APAP intake | 0.377 | 0.704 | |||||
| Cancer-related | 37 (46.2%) | 18 (40.0%) | 19 (54.3%) | 11 (42.3%) | 14 (53.8%) | ||
| Treatment-related | 24 (30.0%) | 16 (35.6%) | 8 (22.8%) | 9 (34.6%) | 7 (26.9%) | ||
| Others | 19 (23.8%) | 11 (24.4%) | 8 (22.8%) | 6 (23.1%) | 5 (19.2%) | ||
| Corticosteroids (yes) | 24 (30.0%) | 8 (17.8%) | 16 (45.7%) | 0.007 | 8 (30.8%) | 10 (38.5%) | 0.560 |
| Systemic antibiotics (yes) | 13 (16.2%) | 9 (20.0%) | 4 (11.4%) | 0.303 | 6 (23.1%) | 3 (11.5%) | 0.271 |
| PPI (yes) | 39 (48.7%) | 22 (48.9% | 17 (48.5%) | 0.978 | 15 (57.7%) | 9 (34.6%) | 0.095 |
| Statins (yes) | 20 25.0%) | 11 (24.4%) | 9 (25.7%) | 0.896 | 7 (26.9%) | 7 (26.9%) | 0.999 |
| Fibrates (yes) | 8 (10.0%) | 3 (6.7%) | 5 (14.3%) | 0.260 | 2 (7.7%) | 4 (15.4%) | 0.385 |
| NSAIDs or ASA (yes) | 28 (35.0%) | 15 (33.3%) | 13 (28.9%) | 0.723 | 9 (34.6%) | 11 (42.3%) | 0.569 |
| Beta-blockers (yes) | 24 (30.0%) | 13 (28.9%) | 11 (31.4%) | 0.806 | 8 (30.8%) | 10 (38.5%) | 0.560 |
| ACEi or ARBs (yes) | 35 (43.7%) | 16 (35.6%) | 19 (54.3%) | 0.094 | 9 (34.6%) | 12 (46.2%) | 0.397 |
| Metformin (yes) | 10 (12.5%) | 5 (11.1%) | 5 (14.3%) | 0.670 | 2 (7.7%) | 4 (15.4%) | 0.385 |
| Oral or transdermal opioids (yes) | 32 (40.0%) | 16 (35.6%) | 16 (45.7%) | 0.358 | 10 (38.5%) | 12 (46.2%) | 0.575 |
| Variable | All Patients, N = 145 (100%) | General Population | PSM Population | ||||
|---|---|---|---|---|---|---|---|
| LAE, N = 70 (100%) | HAE, N = 75 (100%) | p Value | LAE, N = 54 (100%) | HAE, N = 54 (100%) | p Value | ||
| Age | |||||||
| Mean (SD), years | 70.1 (8.9) | 69.2 (9.9) | 70.9 (8.0) | 0.375 | 67.5 (10.9) | 70.3 (8.2) | 0.209 |
| ≥70 years | 89 (61.4%) | 43 (61.4%) | 46 (61.3%) | 0.991 | 29 (53.7%) | 31 (57.4%) | 0.698 |
| Sex | 0.058 | 0.380 | |||||
| Female | 44 (30.3%) | 16 (22.9%) | 28 (37.3%) | 12 (22.2%) | 16 (29.6%) | ||
| Male | 101 (69.7%) | 54 (77.1%) | 47 (69.7%) | 42 (77.8%) | 38 (70.4%) | ||
| ECOG PS | 0.497 | 0.421 | |||||
| 0 or 1 | 116 (80.6%) | 58 (82.9%) | 58 (78.4%) | 46 (85.2%) | 42 (77.8%) | ||
| 2 | 28 (19.4%) | 12 (17.1%) | 16 (21.6%) | 8 (14.8%) | 12 (22.2%) | ||
| Histology | 0.549 | 0.683 | |||||
| Squamous | 47 (32.4%) | 21 (30.0%) | 26 (34.7%) | 17 (31.5%) | 19 (35.2%) | ||
| Nonsquamous | 98 (67.6%) | 49 (70.0%) | 49 (65.3%) | 37 (68.5%) | 35 (64.8%) | ||
| No. of metastatic sites | 0.325 | 0.245 | |||||
| ≤2 | 83 (57.2%) | 43 (61.4%) | 40 (53.3%) | 33 (61.1%) | 27 (50.0%) | ||
| >2 | 62 (42.8%) | 27 (38.6%) | 35 (46.7%) | 21 (38.9%) | 27 (50.0%) | ||
| Bone metastases | 0.562 | 0.814 | |||||
| Not present | 113 (79.1%) | 56 (80.0%) | 57 (76.0%) | 43 (79.6%) | 42 (77.8%) | ||
| Any | 32 (22.1%) | 14 (20.0%) | 18 (24.0%) | 11 (20.4%) | 12 (22.2%) | ||
| SNC metastases | 0.659 | 0.643 | |||||
| Not present | 118 (81.4%) | 58 (82.9%) | 55 (80.0%) | 43 (79.6%) | 41 (75.9%) | ||
| Any | 27 (18.6%) | 12 (17.1%) | 15 (20.0%) | 11 (20.4%) | 13 (24.1%) | ||
| Liver metastases | 0.884 | 0.767 | |||||
| Not present | 129 (89.9%) | 62 (88.6%) | 67 (89.3%) | 48 (88.9%) | 47 (87.0%) | ||
| Any | 16 (11.0%) | 8 (11.4%) | 8 (10.7%) | 6 (11.1%) | 7 (13.0%) | ||
| PD-L1 TPS | 0.221 | 0.051 | |||||
| <1% | 60 (41.4%) | 28 (40.0%) | 32 (42.7%) | 24 (44.4%) | 21 (38.9%) | ||
| ≥1% and ≤49% | 71 (49.0%) | 38 (54.3%) | 33 (44.0%) | 29 (53.7%) | 25 (46.3%) | ||
| ≥50% | 14 (9.7%) | 4 (5.7%) | 10 (13.3%) | 1 (1.8%) | 8 (14.8%) | ||
| BMI | |||||||
| Mean (SD), kg/m2 | 25.8 (5.1) | 26.6 (5.2) | 25.1 (5.0) | 0.109 | 26.2 (4.6) | 25.7 (5.1) | 0.511 |
| ≥25 | 76 (52.4%) | 37 (52.8%) | 39 (52.0%) | 0.916 | 27 (50.0%) | 27 (50.0%) | 0.999 |
| Smoking habit | 0.166 | 0.999 | |||||
| Never smoker | 18 (12.5%) | 6 (8.6%) | 12 (16.2%) | 6 (11.1%) | 6 (11.1%) | ||
| Ever | 126 (87.5%) | 64 (91.4%) | 62 (83.8%) | 48 (88.9%) | 48 (88.9%) | ||
| Type of treatment | 0.404 | 0.054 | |||||
| Nivolumab | 88 (60.7%) | 46 (65.7%) | 42 (56.0%) | 38 (70.3%) | 30 (55.5%) | ||
| Atezolizumab | 12 (8.3%) | 6 (8.6%) | 6 (8.0%) | 6 (11.1%) | 3 (5.5%) | ||
| Pembrolizumab | 45 (31.0%) | 18 (25.7%) | 27 (36.0%) | 10 (18.5%) | 21 (38.9%) | ||
| Reasons for APAP intake | 0.461 | 0.624 | |||||
| Cancer-related | 74 (51.0%) | 32 (45.7%) | 42 (56.0%) | 25 (46.3%) | 30 (55.5%) | ||
| Treatment-related | 50 (34.5%) | 27 (38.6%) | 23 (30.6%) | 21 (38.9%) | 17 (31.5%) | ||
| Others | 21 (14.5%) | 11 (15.7%) | 10 (12.3%) | 8 (14.8%) | 7 (13.0%) | ||
| Corticosteroids (yes) | 43 (29.7%) | 11 (15.7%) | 32 (42.7%) | <0.001 | 14 (25.9%) | 18 (33.3%) | 0.399 |
| Systemic antibiotics (yes) | 21 (14.5%) | 10 (14.3%) | 11 (14.7%) | 0.948 | 9 (16.6%) | 9 (16.6%) | 0.999 |
| PPI (yes) | 66 (45.5% | 32 (45.7%) | 34 (45.3%) | 0.963 | 25 (46.3%) | 27 (50.0%) | 0.700 |
| Statins (yes) | 25 (17.2%) | 14 (20.0%) | 11 (14.7%) | 0.396 | 9 (16.6%) | 6 (11.1%) | 0.404 |
| Fibrates (yes) | 12 (8.3%) | 6 (8.6%) | 6 (8%) | 0.901 | 4 (7.4%) | 3 (5.5%) | 0.696 |
| NSAIDs or ASA (yes) | 51 (35.2%) | 24 (34.3%) | 27 (36.0%) | 0.829 | 20 (37.0%) | 20 (37.0%) | 0.999 |
| Beta-blockers (yes) | 18 (12.4%) | 11 (15.7%) | 7 (9.3%) | 0.244 | 8 (14.8%) | 5 (9.2%) | 0.375 |
| ACEi/ARBs (yes) | 42 (29.0%) | 22 (31.4%) | 20 (26.7%) | 0.528 | 18 (33.3%) | 14 (25.9%) | 0.399 |
| Metformin (yes) | 12 (8.3%) | 7 (10.%) | 5 (6.7%) | 0.467 | 5 (9.2%) | 4 (7.4%) | 0.728 |
| Oral or transdermal opioids (yes) | 52 (35.9%) | 32 (45.7%) | 20 (26.7%) | 0.017 | 20 (37.0%) | 19 (35.1%) | 0.841 |
| Covariate | First-Line PSM Population | Second-Line PSM Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||
| NCB N = 26 (100%) | DCB N = 26 (100%) | p Value | OR (95% CI) | p Value | NCB N = 62 (100%) | DCB N = 46 (100%) | p Value | OR (95% CI) | p Value | |
| Age | 0.999 | - | - | 0.999 | - | |||||
| ≤70 years | 14 (53.8) | 13 (50.0%) | 28 (45.2%) | 20 (43.5%) | ||||||
| >70 years | 12 (45.2%) | 13 (50.0%) | 34 (54.8%) | 26 (56.5%) | ||||||
| Sex | 0.999 | - | - | 0.828 | - | |||||
| Female | 7 (26.9%) | 6 (23.1%) | 16 (25.8%) | 12 (26.1%) | ||||||
| Male | 19 (73.1%) | 20 (76.9%) | 46 (74.2%) | 34 (73.9%) | ||||||
| ECOG PS | 0.668 | - | - | 0.617 | - | |||||
| 0 or 1 | 22 (84.6%) | 24 (92.3%) | 49 (79.1%) | 39 (84.8%) | ||||||
| 2 | 4 (15.4%) | 2 (7.7%) | 13 (20.9%) | 7 (15.2%) | ||||||
| Histology | 0.703 | - | - | 0.838 | - | |||||
| Squamous | 3 (11.5%) | 5 (19.2%) | 20 (32.3%) | 16 (34.8%) | ||||||
| Nonsquamous | 23 (88.5%) | 21 (80.8%) | 42 (67.7%) | 30 (65.2%) | ||||||
| No. of metastatic sites | 0.572 | - | - | 0.018 | 0.209 | |||||
| ≤2 | 14 (53.8%) | 17 (65.4%) | 28 (45.2%) | 32 (69.6%) | 1.00 | |||||
| >2 | 12 (46.2%) | 9 (34.6%) | 34 (54.8%) | 14 (30.4%) | 0.52 (0.18–1.44) | |||||
| Bone metastases | 0.703 | - | - | 0.032 | 0.171 | |||||
| Not present | 21 (80.3%) | 23 (88.5%) | 44 (71%) | 41 (89.1%) | 1.00 | |||||
| Any | 5 (19.2%) | 3 (11.5%) | 18 (29.0%) | 5 (10.9%) | 0.38 (0.10–1.50) | |||||
| SNC metastases | 0.523 | - | - | 0.354 | - | - | ||||
| Not present | 18 (69.2%) | 21 (80.8%) | 46 (74.2%) | 38 (82.6%) | ||||||
| Any | 8 (30.8%) | 5 (19.2%) | 16 (25.8%) | 8 (17.4%) | ||||||
| Liver metastases | 0.703 | - | - | 0.999 | - | - | ||||
| Not present | 2 (80.%) | 23 (80.8%) | 54 (87.1%) | 41 (89.1%) | ||||||
| Any | 5 (19.2%) | 3 (11.5%) | 8 (12.9%) | 5 (10.9%) | ||||||
| PD-L1 TPS | 0.210 | - | - | 0.119 | - | - | ||||
| <1% | 7 (26.9%) | 5 (19.2%) | 31 (50.0%) | 14 (30.4%) | ||||||
| ≥1% and ≤49% | 1 (3.8%) | 5 (19.2%) | 27 (43.5%) | 27 (58.7%) | ||||||
| ≥50% | 18 (69.2%) | 16 (61.5%) | 4 (6.5%) | 5 (10.9%) | ||||||
| BMI | 0.781 | - | - | 0.560 | - | - | ||||
| ≥25 kg/m2 | 12 (46.2%) | 13 (50.0%) | 29 (47.5%) | 25 (53.2%) | ||||||
| Smoking habit | 0.999 | - | - | 0.758 | - | - | ||||
| Never smoker | 2 (7.7%) | 3 (11.5%) | 8 (12.9%) | 4 (8.7%) | ||||||
| Ever | 24 (92.3%) | 23 (88.5%) | 54 (87.1%) | 42 (91.3%) | ||||||
| Type of treatment | 0.313 | - | - | - | - | - | - | - | ||
| Pembrolizumab | 19 (73.1%) | 16 (61.5%) | ||||||||
| Pemetrexed-based | 7 (26.9%) | 8 (30.8%) | ||||||||
| Paclitaxel-based | - | 2 (7.7%) | ||||||||
| Type of treatment | - | - | - | - | - | 0.991 | - | - | ||
| Nivolumab | 39 (62.9%) | 29 (63.0%) | ||||||||
| Atezolizumab | 5 (8.1%) | 4 (8.7%) | ||||||||
| Pembrolizumab | 18 (29.0%) | 13 (28.3%) | ||||||||
| APAP exposure | 0.012 | 0.008 | <0.001 | <0.001 | ||||||
| Low | 8 (30.8%) | 18 (69.2%) | 1.00 | 20 (32.2%) | 34 (73.9%) | 1.00 | ||||
| High | 18 (69.2%) | 8 (30.8%) | 0.18 (0.05–0.64) | 42 (67.8%) | 12 (26.1%) | 0.17 (0.07–0.43) | ||||
| Reasons for APAP intake | 0.165 | - | - | 0.182 | - | - | ||||
| Cancer-related | 15 (57.7%) | 10 (38.5%) | 35 (56.4%) | 20 (43.5%) | ||||||
| Cancer-unrelated | 11 (42.3%) | 16 (61.5%) | 27 (43.6%) | 26 (56.5%) | ||||||
| Corticosteroids | 0.040 | 0.027 | 0.019 | 0.033 | ||||||
| No | 13 (50.0%) | 21 (80.8%) | 1.00 | 38 (61.3%) | 38 (82.6%) | 1.00 | ||||
| Yes | 13 (50.0%) | 5 (19.2%) | 0.21 (0.05–0.84) | 24 (39.3%) | 8 (17.4%) | 0.33 (0.12–0.91) | ||||
| Systemic antibiotics (yes) | 6 (23.1%) | 3 (11.5%) | 0.465 | - | - | 9 (14.8%) | 9 (19.1%) | 0.608 | - | - |
| PPI (yes) | 13 (50.0%) | 11 (42.3%) | 0.781 | - | - | 27 (44.3%) | 25 (53.2%) | 0.438 | - | - |
| Statins (yes) | 7 (26.9%) | 7 (26.9%) | 0.999 | - | - | 9 (14.8%) | 6 (12.8%) | 0.999 | - | - |
| Fibrates (yes) | 3 (11.5%) | 3 (11.5%) | 0.999 | - | - | 3 (4.9%) | 4 (8.5%) | 0.466 | - | - |
| NSAIDs or ASA (yes) | 10 (38.5%) | 10 (38.5%) | 0.999 | - | - | 19 (31.1%) | 21 (44.7%) | 0.165 | - | - |
| Beta-blockers (yes) | 12 (46.2%) | 6 (23.1%) | 0.144 | - | - | 8 (13.1%) | 5 (10.6%) | 0.773 | - | - |
| ACEi/ARBs (yes) | 11 (42.3%) | 10 (38.5%) | 0.999 | - | - | 16 (26.2%) | 16 (34.0%) | 0.402 | - | - |
| Metformin (yes) | 4 (15.4%) | 2 (7.7%) | 0.668 | - | - | 5 (8.2%) | 4 (8.5%) | 0.999 | - | - |
| Oral or transdermal opioids (yes) | 12 (46.2%) | 10 (38.5%) | 0.779 | - | - | 23 (37.7%) | 16 (34%) | 0.840 | - | - |
| Covariate | Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Median (95% CI), Months | p Value | HR (95% CI) | p Value | Median (95% CI), Months | p Value | HR (95% CI) | p Value | |
| APAP exposure | 0.002 | 0.001 | 0.002 | 0.003 | ||||
| Low | 16.5 (9.5–23.4) | 1.00 | 20.5 (14.2–26.7) | 1.00 | ||||
| High | 5.2 (3.3–7.0) | 0.34 (0.18–0.66) | 9.3 (7.8–10.8) | 0.36 (0.18–0.71) | ||||
| Corticosteroids | 0.002 | 0.002 | 0.003 | 0.002 | ||||
| No | 11.5 (7.3–15.7) | 1.00 | 17.9 (15.4–20.4) | 1.00 | ||||
| Yes | 5.7 (3.8–7.5) | 0.33 (0.17–0.66) | 9.8 (9.3–10.3) | 0.33 (0.16–0.67) | ||||
| Covariate | Progression-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Median (95% CI), Months | p Value | HR (95% CI) | p Value | Median (95% CI), Months | p Value | HR (95% CI) | p Value | |
| APAP exposure | 0.016 | 0.016 | 0.005 | 0.005 | ||||
| Low | 7.8 (1.4–14.2) | 1.00 | 12.8 (6.6–19.0) | 1.00 | ||||
| High | 3.3 (2.3–4.3) | 0.61 (0.40–0.91) | 6.5 (3.6–9.4) | 0.54 (0.35–0.83) | ||||
| Corticosteroids | 0.003 | 0.002 | 0.045 | 0.012 | ||||
| No | 6.3 (3.5–9.0) | 1.00 | 10.8 (6.8–14.8) | 1.00 | ||||
| Yes | 2.9 (1.6–4.2) | 0.50 (0.32–0.78) | 4.4 (2.7–6.1) | 0.56 (0.35–0.88) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelli, F.; Virtuoso, A.; Giannarelli, D.; Fabbri, A.; Giron Berrios, J.R.; Marrucci, E.; Fiore, C.; Ruggeri, E.M. Effects of Acetaminophen Exposure on Outcomes of Patients Receiving Immune Checkpoint Inhibitors for Advanced Non-Small-Cell Lung Cancer: A Propensity Score-Matched Analysis. Curr. Oncol. 2023, 30, 8117-8133. https://doi.org/10.3390/curroncol30090589
Nelli F, Virtuoso A, Giannarelli D, Fabbri A, Giron Berrios JR, Marrucci E, Fiore C, Ruggeri EM. Effects of Acetaminophen Exposure on Outcomes of Patients Receiving Immune Checkpoint Inhibitors for Advanced Non-Small-Cell Lung Cancer: A Propensity Score-Matched Analysis. Current Oncology. 2023; 30(9):8117-8133. https://doi.org/10.3390/curroncol30090589
Chicago/Turabian StyleNelli, Fabrizio, Antonella Virtuoso, Diana Giannarelli, Agnese Fabbri, Julio Rodrigo Giron Berrios, Eleonora Marrucci, Cristina Fiore, and Enzo Maria Ruggeri. 2023. "Effects of Acetaminophen Exposure on Outcomes of Patients Receiving Immune Checkpoint Inhibitors for Advanced Non-Small-Cell Lung Cancer: A Propensity Score-Matched Analysis" Current Oncology 30, no. 9: 8117-8133. https://doi.org/10.3390/curroncol30090589
APA StyleNelli, F., Virtuoso, A., Giannarelli, D., Fabbri, A., Giron Berrios, J. R., Marrucci, E., Fiore, C., & Ruggeri, E. M. (2023). Effects of Acetaminophen Exposure on Outcomes of Patients Receiving Immune Checkpoint Inhibitors for Advanced Non-Small-Cell Lung Cancer: A Propensity Score-Matched Analysis. Current Oncology, 30(9), 8117-8133. https://doi.org/10.3390/curroncol30090589





