Concordance of Abundance for Mutational EGFR and Co-Mutational TP53 with Efficacy of EGFR-TKI Treatment in Metastatic Patients with Non-Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Abundance of EGFR Mutations
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Included Patients
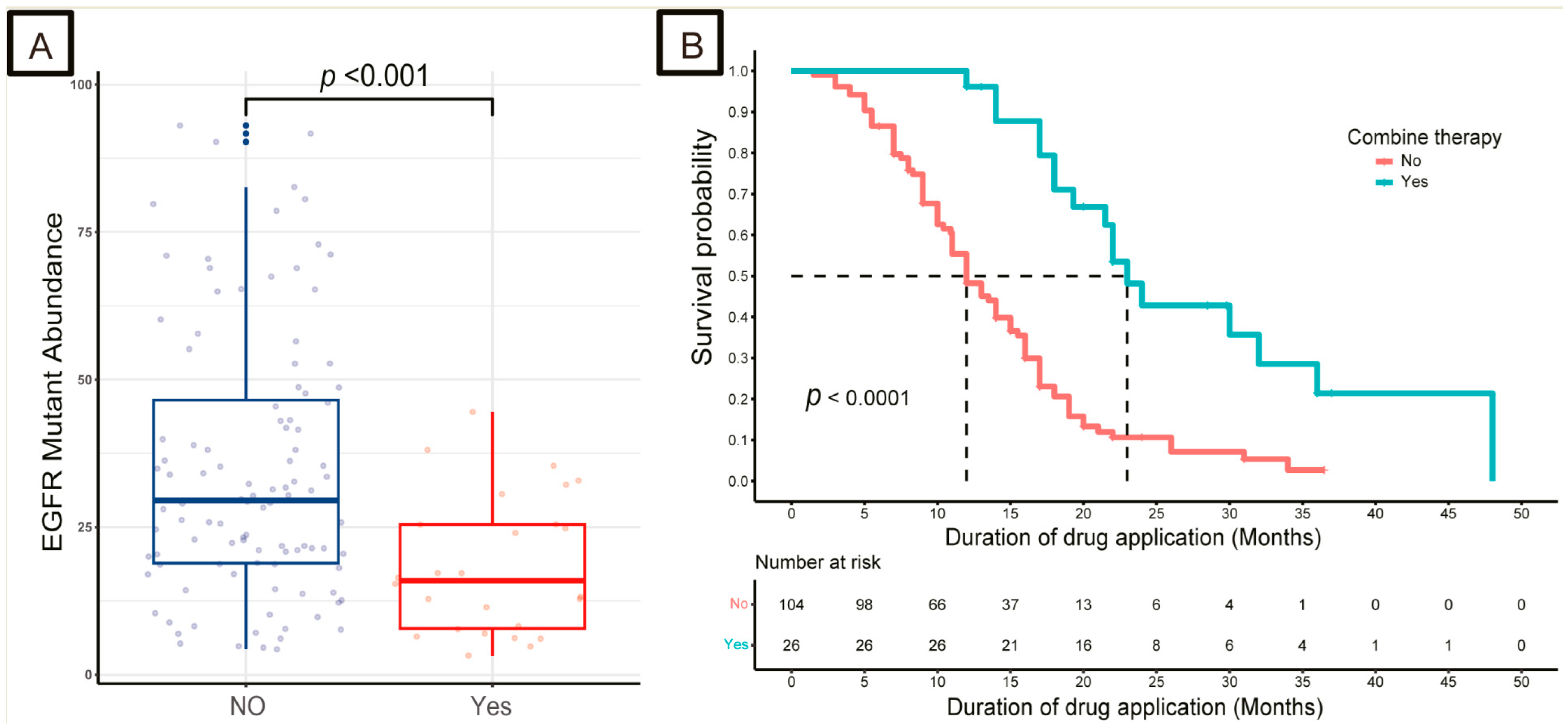
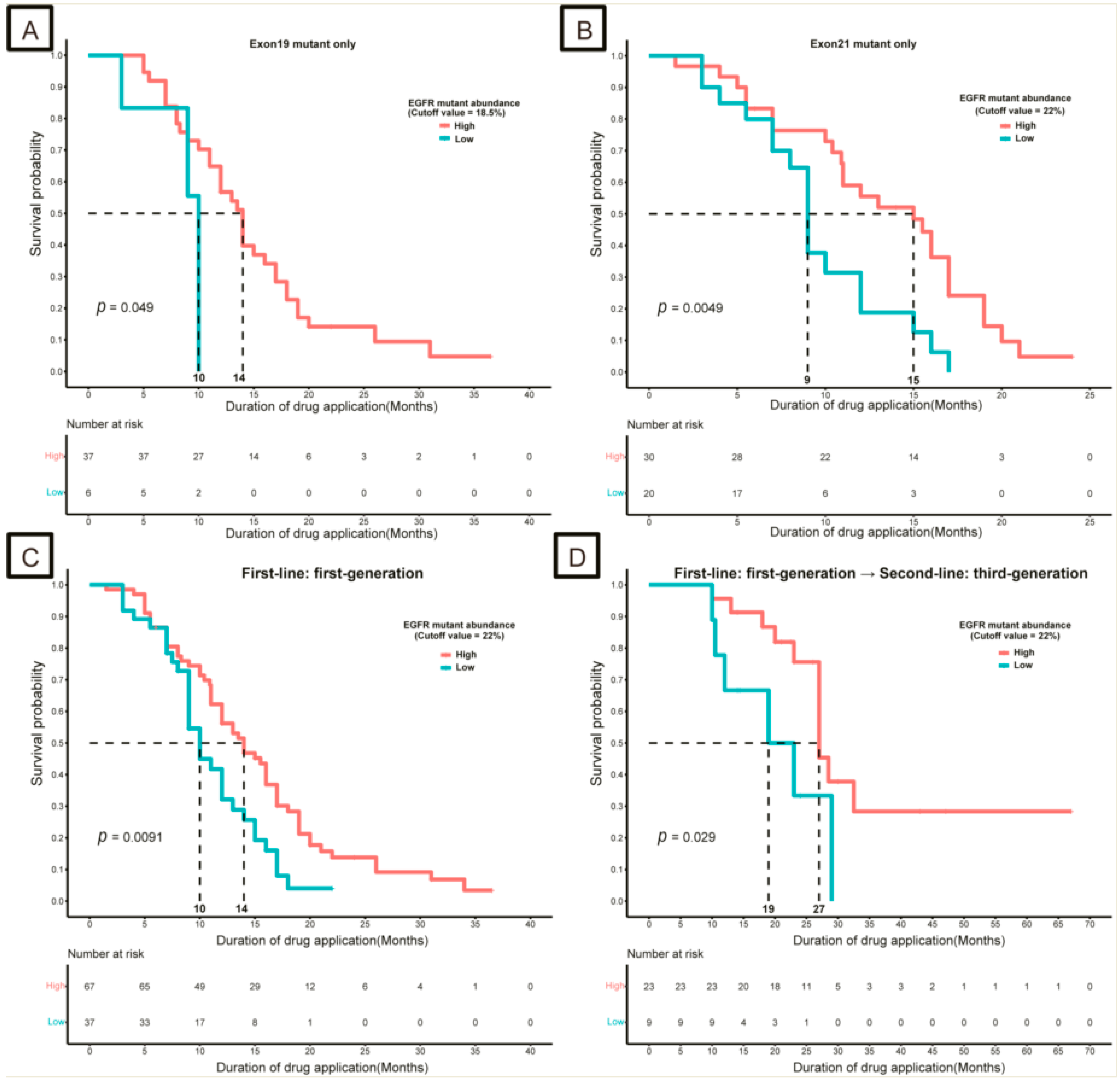
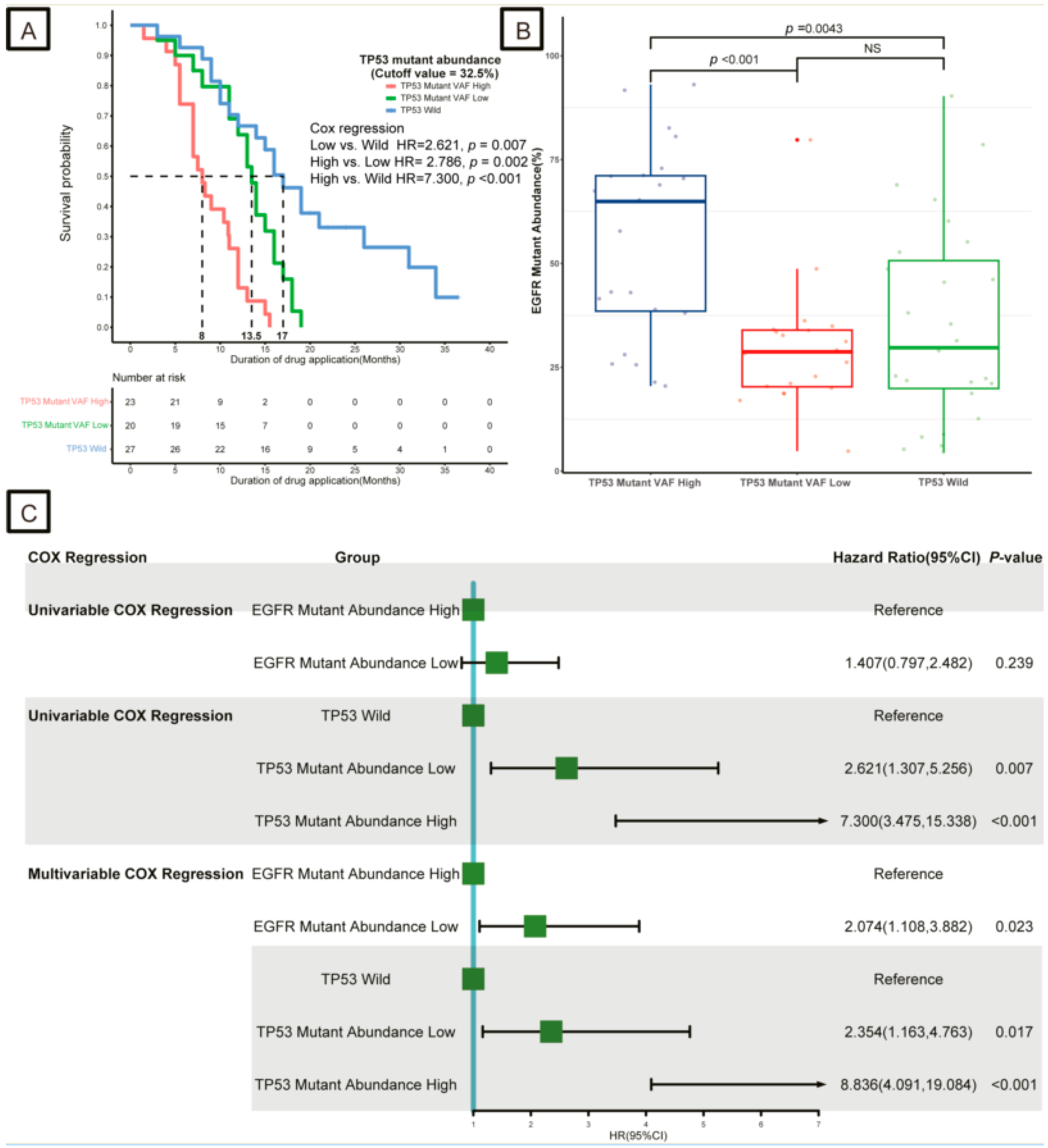
3.2. Impact of EGFR and TP53 Mutation Abundance on Response to TKIs
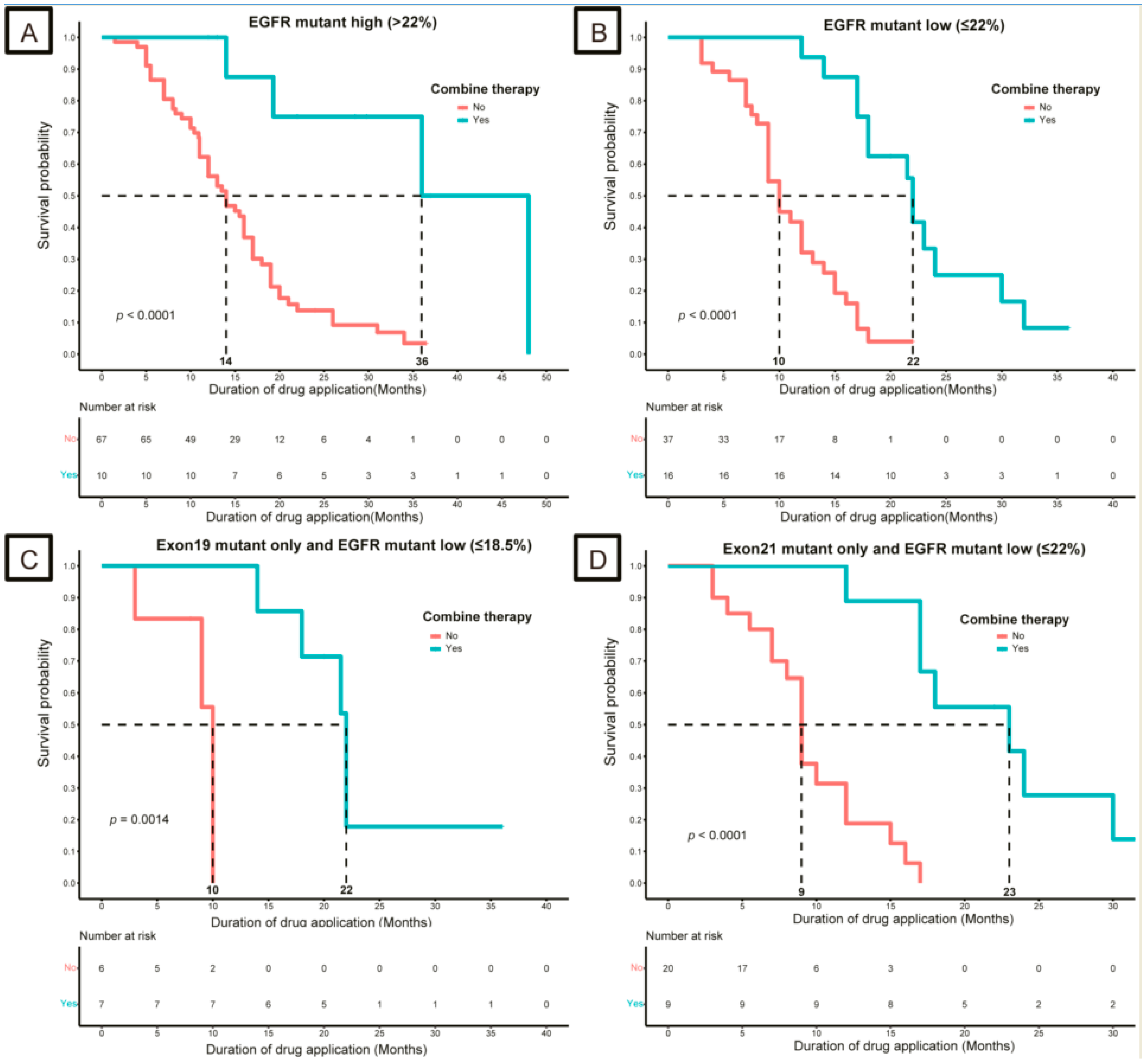
3.3. Strategies for Patients with Low-Abundance Mutations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, E248. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol. Res. 2019, 139, 395–411. [Google Scholar] [CrossRef]
- Castellanos, E.; Feld, E.; Horn, L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR-Mutated Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 612–623. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Sun, X.; Xu, S.; Yang, Z.; Zheng, P.; Zhu, W. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: A patent review (2014–present). Expert Opin. Ther. Pat. 2021, 31, 223–238. [Google Scholar] [CrossRef]
- Tan, C.-S.; Kumarakulasinghe, N.B.; Huang, Y.-Q.; Ang, Y.L.E.; Choo, J.R.-E.; Goh, B.-C.; Soo, R.A. Third generation EGFR TKIs: Current data and future directions. Mol. Cancer 2018, 17, 29. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Yang, G.; Su, C.; Ren, S.; Zhao, C.; Hu, R.; Chen, X.; Gao, G.; Guo, Z.; et al. Comprehensive Analysis of EGFR-Mutant Abundance and Its Effect on Efficacy of EGFR TKIs in Advanced NSCLC with EGFR Mutations. J. Thorac. Oncol. 2017, 12, 1388–1397. [Google Scholar] [CrossRef]
- Blakely, C.M.; Watkins, T.B.K.; Wu, W.; Gini, B.; Chabon, J.J.; McCoach, C.E.; McGranahan, N.; A Wilson, G.; Birkbak, N.J.; Olivas, V.R.; et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat. Genet. 2017, 49, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Barnet, M.B.; O’Toole, S.; Horvath, L.G.; Selinger, C.; Yu, B.; Ng, C.C.; Boyer, M.; Cooper, W.A.; Kao, S. EGFR-Co-Mutated Advanced NSCLC and Response to EGFR Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2017, 12, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Gao, F.; Fu, S.; Wang, Y.; Fang, W.; Huang, Y.; Zhang, L. Concomitant Genetic Alterations with Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, B.; Shim, J.H.; Lee, S.-H.; Park, W.-Y.; Choi, Y.-L.; Sun, J.-M.; Ahn, J.-S.; Ahn, M.-J.; Park, M. Concurrent Genetic Alterations Predict the Progression to Target Therapy in EGFR-Mutated Advanced NSCLC. J. Thorac. Oncol. 2019, 14, 193–202. [Google Scholar] [CrossRef]
- Nahar, R.; Zhai, W.; Zhang, T.; Takano, A.; Khng, A.J.; Lee, Y.Y.; Liu, X.; Lim, C.H.; Koh, T.P.T.; Aung, Z.W.; et al. Elucidating the genomic architecture of Asian EGFR-mutant lung adenocarcinoma through multi-region exome sequencing. Nat. Commun. 2018, 9, 216. [Google Scholar] [CrossRef]
- Helena, A.Y.; Suzawa, K.; Jordan, E.J.; Zehir, A.; Ni, A.; Kim, H.R.; Kris, M.G.; Hellmann, M.D.; Li, B.T.; Somwar, R.; et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin. Cancer Res. 2018, 24, 3108–3118. [Google Scholar] [CrossRef]
- Cai, W.; Lin, D.; Wu, C.; Li, X.; Zhao, C.; Zheng, L.; Chuai, S.; Fei, K.; Zhou, C.; Hirsch, F.R. Intratumoral Heterogeneity of ALK-Rearranged and ALK/EGFR Coaltered Lung Adenocarcinoma. J. Clin. Oncol. 2015, 33, 3701–3709. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, X.-C.; Chen, Z.-H.; Yin, X.-L.; Yang, J.-J.; Xu, C.-R.; Yan, H.-H.; Chen, H.-J.; Su, J.; Zhong, W.-Z.; et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 3316–3321. [Google Scholar] [CrossRef]
- Schrock, A.B.; Frampton, G.M.; Herndon, D.; Greenbowe, J.R.; Wang, K.; Lipson, D.; Yelensky, R.; Chalmers, Z.R.; Chmielecki, J.; Elvin, J.A.; et al. Comprehensive Genomic Profiling Identifies Frequent Drug-Sensitive EGFR Exon 19 Deletions in NSCLC not Identified by Prior Molecular Testing. Clin. Cancer Res. 2016, 22, 3281–3285. [Google Scholar] [CrossRef]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker Testing for Patients with Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef]
- Rozenblum, A.B.; Ilouze, M.; Dudnik, E.; Dvir, A.; Soussan-Gutman, L.; Geva, S.; Peled, N. Clinical Impact of Hybrid Capture-Based Next-Generation Sequencing on Changes in Treatment Decisions in Lung Cancer. J. Thorac. Oncol. 2017, 12, 258–268. [Google Scholar] [CrossRef]
- Drilon, A.; Wang, L.; Arcila, M.E.; Balasubramanian, S.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.; Lipson, D.; Miller, V.A.; Kris, M.G.; et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin. Cancer Res. 2015, 21, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Kim, E.Y.; Kim, H.R.; Ali, S.M.; Greenbowe, J.R.; Shim, H.S.; Chang, H.; Lim, S.; Paik, S.; Cho, B.C. Genomic profiling of lung adenocarcinoma patients reveals therapeutic targets and confers clinical benefit when standard molecular testing is negative. Oncotarget 2016, 7, 24172–24178. [Google Scholar] [CrossRef] [PubMed]
- Chinese Society of Clinical Oncology, Expert Committee on Non-Small Cell Lung Cancer. Chinese Expert Consensus on Next Generation Sequencing Diagnosis for Non-small Cell Lung Cancer (2020 Edition). Zhongguo Fei Ai Za Zhi 2020, 23, 741–761. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Han, B.; Gu, A.; Zhang, Y.; Jiao, S.C.; Wang, C.-L.; He, J.; Jia, X.; Zhang, L.; Peng, J.; et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer 2014, 86, 207–212. [Google Scholar] [CrossRef]
- Swanton, C. Intratumor heterogeneity: Evolution through space and time. Cancer Res. 2012, 72, 4875–4882. [Google Scholar] [CrossRef]
- Turtoi, A.; Blomme, A.; Castronovo, V. Intratumoral heterogeneity and consequences for targeted therapies. Bull. Cancer 2015, 102, 17–23. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Tang, W.; Ma, J.; Wei, B.; Niu, Y.; Zhang, G.; Li, P.; Yan, X.; Ma, Z. Mutation abundance affects the therapeutic efficacy of EGFR-TKI in patients with advanced lung adenocarcinoma: A retrospective analysis. Cancer Biol. Ther. 2018, 19, 687–694. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, J.; Chen, H.; Bai, H.; An, T.; Zhao, J.; Wang, Z.; Zhuo, M.; Wang, S.; Wang, J. Analysis of EGFR mutation status in tissue and plasma for predicting response to EGFR-TKIs in advanced non-small-cell lung cancer. Oncol. Lett. 2017, 13, 2425–2431. [Google Scholar] [CrossRef]
- Remon, J.; Steuer, C.E.; Ramalingam, S.S.; Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018, 29, i20–i27. [Google Scholar] [CrossRef]
- Labbé, C.; Cabanero, M.; Korpanty, G.J.; Tomasini, P.; Doherty, M.K.; Mascaux, C.; Jao, K.; Pitcher, B.; Wang, R.; Pintilie, M.; et al. Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer 2017, 111, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Aisner, D.L.; Sholl, L.M.; Berry, L.D.; Rossi, M.R.; Chen, H.; Fujimoto, J.; Moreira, A.L.; Ramalingam, S.S.; Villaruz, L.C.; Otterson, G.A.; et al. The Impact of Smoking and TP53 Mutations in Lung Adenocarcinoma Patients with Targetable Mutations-The Lung Cancer Mutation Consortium (LCMC2). Clin. Cancer Res. 2018, 24, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Vokes, N.I.; Chambers, E.; Nguyen, T.; Coolidge, A.; Lydon, C.A.; Le, X.; Sholl, L.; Heymach, J.V.; Nishino, M.; Van Allen, E.M.; et al. Concurrent TP53 Mutations Facilitate Resistance Evolution in EGFR-Mutant Lung Adenocarcinoma. J. Thorac. Oncol. 2022, 17, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Hou, H.; Liang, Y.; Zhang, X. Prognostic value of TP53 concurrent mutations for EGFR-TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: A meta-analysis. BMC Cancer 2020, 20, 328. [Google Scholar] [CrossRef]
- Xu, Y.; Tong, X.; Yan, J.; Wu, X.; Shao, Y.W.; Fan, Y. Short-Term Responders of Non-Small Cell Lung Cancer Patients to EGFR Tyrosine Kinase Inhibitors Display High Prevalence of TP53 Mutations and Primary Resistance Mechanisms. Transl. Oncol. 2018, 11, 1364–1369. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, L.; Liu, Y.; Zhu, J.; Xin, Y.; Liu, X.; Wang, Y.; Zhang, T.; Yang, C.; Wang, S.; et al. Comprehensive characterization and clinical impact of concomitant genomic alterations in EGFR-mutant NSCLCs treated with EGFR kinase inhibitors. Lung Cancer 2020, 145, 63–70. [Google Scholar] [CrossRef]
- Li, X.-M.; Li, W.-F.; Lin, J.-T.; Yan, H.-H.; Tu, H.-Y.; Chen, H.-J.; Wang, B.-C.; Wang, Z.; Zhou, Q.; Zhang, X.-C.; et al. Predictive and Prognostic Potential of TP53 in Patients with Advanced Non-Small-Cell Lung Cancer Treated with EGFR-TKI: Analysis of a Phase III Randomized Clinical Trial (CTONG 0901). Clin. Lung Cancer 2021, 22, 100–109. [Google Scholar] [CrossRef]
- Canale, M.; Petracci, E.; Delmonte, A.; Bronte, G.; Chiadini, E.; Ludovini, V.; Dubini, A.; Papi, M.; Baglivo, S.; De Luigi, N.; et al. Concomitant TP53 Mutation Confers Worse Prognosis in EGFR-Mutated Non-Small Cell Lung Cancer Patients Treated with TKIs. J. Clin. Med. 2020, 9, 1047. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, F.; Wang, Y.; Wu, Q.; Wang, B.; Yao, Y.; Zhang, Y.; Han-Zhang, H.; Ye, J.; Zhang, L.; et al. Mutations in exon 8 of TP53 are associated with shorter survival in patients with advanced lung cancer. Oncol. Lett. 2019, 18, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, Y.; Morita, S.; Sugawara, S.; Kato, T.; Fukuhara, T.; Gemma, A.; Takahashi, K.; Fujita, Y.; Harada, T.; Minato, K.; et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer with Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J. Clin. Oncol. 2020, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, H.; Li, P.; Zhang, G.; Zhang, M.; Yang, J.; Zhang, X.; Zheng, X.; Ma, Z. Efficacy of first-line treatment with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) alone or in combination with chemotherapy for advanced non-small cell lung cancer (NSCLC) with low-abundance mutation. Lung Cancer 2019, 128, 6–12. [Google Scholar] [CrossRef]
- Saito, R.; Sugawara, S.; Ko, R.; Azuma, K.; Morita, R.; Maemondo, M.; Oizumi, S.; Takahashi, K.; Kagamu, H.; Tsubata, Y.; et al. Phase 2 study of osimertinib in combination with platinum and pemetrexed in patients with previously untreated EGFR-mutated advanced non-squamous non-small cell lung cancer: The OPAL Study. Eur. J. Cancer 2023, 185, 83–93. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | High-EGFR Group (N = 86) | Low-EGFR Group (N = 44) |
|---|---|---|
| Age (years, range) | 63 (56, 69) | 67 (56, 73) |
| Gender | ||
| Male | 39 (45%) | 18 (41%) |
| Female | 47 (55%) | 26 (59%) |
| First-line therapy | ||
| Icotinib | 60 (70%) | 27 (61%) |
| Gefitinib | 26 (30%) | 16 (36%) |
| Erlotinib | 0 (0%) | 1 (2.3%) |
| Combination therapy (first-line) | ||
| No | 76 (88%) | 28 (64%) |
| Chemotherapy | 4 (4.7%) | 8 (18%) |
| Target therapy | 5 (5.8%) | 7 (16%) |
| Chemotherapy plus target therapy | 1 (1.2%) | 1 (2.3%) |
| Second-line therapy | ||
| Third-generation TKIs | 30 (35%) | 13 (30%) |
| Osimertinib | 27 (31%) | 10 (23%) |
| Almonertinib | 2 (2.3%) | 1 (2.3%) |
| Furmonertinib | 1 (1.2%) | 2 (4.5%) |
| Other or unknown | 56 (65%) | 31 (70%) |
| Best therapy response (first-line) | ||
| CR/PR | 66 (77%) | 34 (77%) |
| SD | 19 (22%) | 7 (16%) |
| PD | 1 (1.2%) | 3 (6.8%) |
| EGFR mutant number | ||
| 1 | 74 (86%) | 41 (93%) |
| ≥2 | 12 (14%) | 3 (6.8%) |
| EGFR mutant type | ||
| E19 only | 39 (45%) | 16 (36%) |
| E21 only | 38 (44%) | 25 (57%) |
| Others | 9 (10%) | 3 (6.8%) |
| TP53 mutant | ||
| Yes | 44 (51%) | 11 (25%) |
| No | 24 (28%) | 8 (18%) |
| Undetected or Unknown | 18 (21%) | 25 (57%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, H.; Yu, N.; Xiang, X. Concordance of Abundance for Mutational EGFR and Co-Mutational TP53 with Efficacy of EGFR-TKI Treatment in Metastatic Patients with Non-Small-Cell Lung Cancer. Curr. Oncol. 2023, 30, 8464-8476. https://doi.org/10.3390/curroncol30090616
Wang Y, Liu H, Yu N, Xiang X. Concordance of Abundance for Mutational EGFR and Co-Mutational TP53 with Efficacy of EGFR-TKI Treatment in Metastatic Patients with Non-Small-Cell Lung Cancer. Current Oncology. 2023; 30(9):8464-8476. https://doi.org/10.3390/curroncol30090616
Chicago/Turabian StyleWang, Youping, Hong Liu, Ningjuan Yu, and Xueping Xiang. 2023. "Concordance of Abundance for Mutational EGFR and Co-Mutational TP53 with Efficacy of EGFR-TKI Treatment in Metastatic Patients with Non-Small-Cell Lung Cancer" Current Oncology 30, no. 9: 8464-8476. https://doi.org/10.3390/curroncol30090616
APA StyleWang, Y., Liu, H., Yu, N., & Xiang, X. (2023). Concordance of Abundance for Mutational EGFR and Co-Mutational TP53 with Efficacy of EGFR-TKI Treatment in Metastatic Patients with Non-Small-Cell Lung Cancer. Current Oncology, 30(9), 8464-8476. https://doi.org/10.3390/curroncol30090616





