Reduced Risk of All-Cause, Cancer-, and Cardiovascular Disease-Related Mortality among Patients with Primary Malignant Cardiac Tumors Receiving Chemotherapy in the United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of Study Variables
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burke, A. Primary malignant cardiac tumors. Semin. Diagn. Pathol. 2008, 25, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Kumar, S.; Noshirwani, A.; Harky, A. The Current Management of Cardiac Tumours: A Comprehensive Literature Review. Braz. J. Cardiovasc. Surg. 2020, 35, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.H.; Al-Kindi, S.G.; Hoimes, C.; Park, S.J. Characteristics and Survival of Malignant Cardiac Tumors: A 40-Year Analysis of >500 Patients. Circulation 2015, 132, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Meghrajani, V.; Desai, R.; Gupta, N. Primary Malignant Cardiac Tumors: A Rare Disease with an Adventurous Journey. J. Am. Heart Assoc. 2020, 9, e016032. [Google Scholar] [CrossRef]
- Aloysius, M.M.; Shrivastava, S.; Rojulpote, C.; Naseer, R.; Hanif, H.; Babic, M.; Gentilezza, K.; Boruah, P.K.; Pancholy, S. Racial and ethnic characteristics and cancer-specific survival in Primary Malignant Cardiac Tumors. Front. Cardiovasc. Med. 2022, 9, 961160. [Google Scholar] [CrossRef]
- Rahouma, M.; Arisha, M.J.; Elmously, A.; El-Sayed Ahmed, M.M.; Spadaccio, C.; Mehta, K.; Baudo, M.; Kamel, M.; Mansor, E.; Ruan, Y.; et al. Cardiac tumors prevalence and mortality: A systematic review and meta-analysis. Int. J. Surg. 2020, 76, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Chiavarelli, M.; Glauber, M.; Tanganelli, P.; Scalese, M.; Cesareo, F.; Guerrini, F.; Capati, E.; Focardi, M.; Severi, S. Incidence rate of primary cardiac tumors: A 14-year population study. J. Cardiovasc. Med. 2016, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.Y.; Del Nido, P.J.; Alexander, M.E.; Cecchin, F.; Berul, C.I.; Triedman, J.K.; Geva, T.; Walsh, E.P. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J. Am. Coll. Cardiol. 2011, 58, 1903–1909. [Google Scholar] [CrossRef]
- Guan, T.; Li, Y.; Qiu, Z.; Zhang, Y.; Lin, W.; Lai, Y.; Wang, K.; Shen, Y.; Du, L.; Liu, C. Nomograms and risk classification systems predicting overall and cancer-specific survival in primary malignant cardiac tumor. J. Card. Surg. 2019, 34, 1540–1549. [Google Scholar] [CrossRef]
- Barreiro, M.; Renilla, A.; Jimenez, J.M.; Martin, M.; Al Musa, T.; Garcia, L.; Barriales, V. Primary cardiac tumors: 32 years of experience from a Spanish tertiary surgical center. Cardiovasc. Pathol. 2013, 22, 424–427. [Google Scholar] [CrossRef]
- Awad, A.K.; Elgenidy, A.; Afifi, A.M.; Sa, M.P.; Ramlawi, B. Specific causes of death among patients with cardiac sarcoma in the United States-An analysis of The Surveillance, Epidemiology, and End Results (SEER) Program. J. Card. Surg. 2022, 37, 3961–3963. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 293–311. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, H.; Cao, Y.; Nian, F.; Xu, Y.; Chen, W.; Jiang, B.; Auchoybur, M.L.; Tao, Z.; Tang, S.; et al. Risk factors for early death in primary malignant cardiac tumors: An analysis of over 40 years and 500 patients. Int. J. Cardiol. 2018, 270, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Bianco, V.; Habertheuer, A.; Kilic, A.; Gleason, T.G.; Aranda-Michel, E.; Harinstein, M.E.; Martinez-Meehan, D.; Arnaoutakis, G.; Okusanya, O. Long-Term Outcomes of Primary Cardiac Malignancies: Multi-Institutional Results From the National Cancer Database. J. Am. Coll. Cardiol. 2020, 75, 2338–2347. [Google Scholar] [CrossRef]
- Antwi-Amoabeng, D.; Meghji, Z.; Thakkar, S.; Ulanja, M.B.; Taha, M.; Adalja, D.; Al-Khafaji, J.; Gullapalli, N.; Beutler, B.D.; Boampong-Konam, K.; et al. Survival Differences in Men and Women With Primary Malignant Cardiac Tumor: An Analysis Using the Surveillance, Epidemiology and End Results (SEER) Database From 1973 to 2015. J. Am. Heart Assoc. 2020, 9, e014846. [Google Scholar] [CrossRef]
- Bui, Q.; Ngo, T.N.M.; Mazur, J.; Pham, V.; Palmer, C.; Truong, B.Q.; Chung, E.S.; Vuong, H.G.; Truong, V.T. Long-term outcomes of primary cardiac malignant tumors: Difference between African American and Caucasian population. Cancer Med. 2021, 10, 8838–8845. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Luo, R.; Wei, Y.; Wang, F.; Zhang, Y.; Karlson, K.J.; Zhang, Z.; Reardon, M.J.; Dobrilovic, N. Survival outcomes in patients with primary cardiac sarcoma in the United States. J. Thorac. Cardiovasc. Surg. 2021, 162, 107–115. [Google Scholar] [CrossRef]
- Randhawa, J.S.; Budd, G.T.; Randhawa, M.; Ahluwalia, M.; Jia, X.; Daw, H.; Spiro, T.; Haddad, A. Primary Cardiac Sarcoma: 25-Year Cleveland Clinic Experience. Am. J. Clin. Oncol. 2016, 39, 593–599. [Google Scholar] [CrossRef]
- Brisson, R.J.; Quinn, T.J.; Deraniyagala, R.L. The role of chemotherapy in the management of olfactory neuroblastoma: A 40-year surveillance, epidemiology, and end results registry study. Health Sci. Rep. 2021, 4, e257. [Google Scholar] [CrossRef]
- Rahouma, M.; Khairallah, S.; Dabsha, A.; Baudo, M.; El-Sayed Ahmed, M.M.; Gambardella, I.; Lau, C.; Esmail, Y.M.; Mohamed, A.; Girardi, L.; et al. Geographic variation in malignant cardiac tumors and their outcomes: SEER database analysis. Front. Oncol. 2023, 13, 1071770. [Google Scholar] [CrossRef]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.J.; Xu, A.; Lythgoe, M.P.; Prasad, V. Cancer Drug Approvals That Displaced Existing Standard-of-Care Therapies, 2016–2021. JAMA Netw. Open 2022, 5, e222265. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Overview of the Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 12 September 2020).
- Noone, A.M.; Lund, J.L.; Mariotto, A.; Cronin, K.; McNeel, T.; Deapen, D.; Warren, J.L. Comparison of SEER Treatment Data with Medicare Claims. Med. Care 2016, 54, e55–e64. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xing, Y.; Cormier, J.N.; Chang, G.J. The validity of cause of death coding within the Surveillance, Epidemiology, and End Results (SEER) Registry. J. Clin. Oncol. 2009, 27, 6544. [Google Scholar] [CrossRef]
- O’Connor, E.S.; Greenblatt, D.Y.; LoConte, N.K.; Gangnon, R.E.; Liou, J.I.; Heise, C.P.; Smith, M.A. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3381–3388. [Google Scholar] [CrossRef]
- Murphy, C.C.; Harlan, L.C.; Warren, J.L.; Geiger, A.M. Race and Insurance Differences in the Receipt of Adjuvant Chemotherapy Among Patients with Stage III Colon Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2530–2536. [Google Scholar] [CrossRef]
- Neuner, J.M.; Kong, A.; Blaes, A.; Riley, D.; Chrischilles, E.; Smallwood, A.; Lizarraga, I.; Schroeder, M. The association of socioeconomic status with receipt of neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2019, 173, 179–188. [Google Scholar] [CrossRef]
- Sanford, N.N.; Aguilera, T.A.; Folkert, M.R.; Ahn, C.; Mahal, B.A.; Zeh, H.; Beg, M.S.; Mansour, J.; Sher, D.J. Sociodemographic Disparities in the Receipt of Adjuvant Chemotherapy Among Patients With Resected Stage I-III Pancreatic Adenocarcinoma. J. Natl. Compr. Canc Netw. 2019, 17, 1292–1300. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Lumley, T.; Forestplot: Advanced Forest Plot Using ‘grid’ Graphics. R package version 1.9. 2019. Available online: https://CRAN.R-project.org/package=forestplot (accessed on 26 August 2023).
- Ho, D.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef]
- Greifer, N. Cobalt: Covariate Balance Tables and Plots. Available online: https://CRAN.R-project.org/package=cobalt (accessed on 24 June 2023).
- Greifer, N. WeightIt: Weighting for Covariate Balance in Observational Studies. Available online: https://CRAN.R-project.org/package=WeightIt (accessed on 24 June 2023).
- Taguchi, S. Comprehensive review of the epidemiology and treatments for malignant adult cardiac tumors. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 257–262. [Google Scholar] [CrossRef]
- Simpson, L.; Kumar, S.K.; Okuno, S.H.; Schaff, H.V.; Porrata, L.F.; Buckner, J.C.; Moynihan, T.J. Malignant primary cardiac tumors: Review of a single institution experience. Cancer 2008, 112, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Elbardissi, A.W.; Dearani, J.A.; Daly, R.C.; Mullany, C.J.; Orszulak, T.A.; Puga, F.J.; Schaff, H.V. Survival after resection of primary cardiac tumors: A 48-year experience. Circulation 2008, 118, S7–S15. [Google Scholar] [CrossRef]
- Hamidi, M.; Moody, J.S.; Weigel, T.L.; Kozak, K.R. Primary cardiac sarcoma. Ann. Thorac. Surg. 2010, 90, 176–181. [Google Scholar] [CrossRef]
- Hasan, S.M.; Witten, J.; Collier, P.; Tong, M.Z.; Pettersson, G.B.; Smedira, N.G.; Toth, A.; Shepard, D.; Blackstone, E.H.; Roselli, E.E. Outcomes after resection of primary cardiac sarcoma. JTCVS Open 2021, 8, 384–390. [Google Scholar] [CrossRef]
- Yu, L.; Gu, T.; Shi, E.; Xiu, Z.; Fang, Q.; Wang, C.; Wang, X.; Cheng, Y. Primary malignant cardiac tumors. J. Cancer Res. Clin. Oncol. 2014, 140, 1047–1055. [Google Scholar] [CrossRef]
- Yin, L.; He, D.; Shen, H.; Ling, X.; Li, W.; Xue, Q.; Wang, Z. Surgical treatment of cardiac tumors: A 5-year experience from a single cardiac center. J. Thorac. Dis. 2016, 8, 911–919. [Google Scholar] [CrossRef]
- Burazor, I.; Aviel-Ronen, S.; Imazio, M.; Markel, G.; Grossman, Y.; Yosepovich, A.; Adler, Y. Primary malignancies of the heart and pericardium. Clin. Cardiol. 2014, 37, 582–588. [Google Scholar] [CrossRef]
- Siontis, B.L.; Zhao, L.; Leja, M.; McHugh, J.B.; Shango, M.M.; Baker, L.H.; Schuetze, S.M.; Chugh, R. Primary Cardiac Sarcoma: A Rare, Aggressive Malignancy with a High Propensity for Brain Metastases. Sarcoma 2019, 2019, 1960593. [Google Scholar] [CrossRef] [PubMed]
- Abu Saleh, W.K.; Ramlawi, B.; Shapira, O.M.; Al Jabbari, O.; Ravi, V.; Benjamin, R.; Durand, J.B.; Leja, M.J.; Blackmon, S.H.; Bruckner, B.A.; et al. Improved Outcomes With the Evolution of a Neoadjuvant Chemotherapy Approach to Right Heart Sarcoma. Ann. Thorac. Surg. 2017, 104, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Llombart-Cussac, A.; Pivot, X.; Contesso, G.; Rhor-Alvarado, A.; Delord, J.P.; Spielmann, M.; Tursz, T.; Le Cesne, A. Adjuvant chemotherapy for primary cardiac sarcomas: The IGR experience. Br. J. Cancer 1998, 78, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Aleman, B.M.; Moser, E.C.; Nuver, J.; Suter, T.M.; Maraldo, M.V.; Specht, L.; Vrieling, C.; Darby, S.C. Cardiovascular disease after cancer therapy. EJC Suppl. 2014, 12, 18–28. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, A.; Sandhu, N.P.; Villarraga, H.R.; Mulvagh, S.L.; Kohli, M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef]
- Healy, M.A.; Morris, A.M.; Abrahamse, P.; Ward, K.C.; Kato, I.; Veenstra, C.M. The accuracy of chemotherapy ascertainment among colorectal cancer patients in the surveillance, epidemiology, and end results registry program. BMC Cancer 2018, 18, 481. [Google Scholar] [CrossRef]




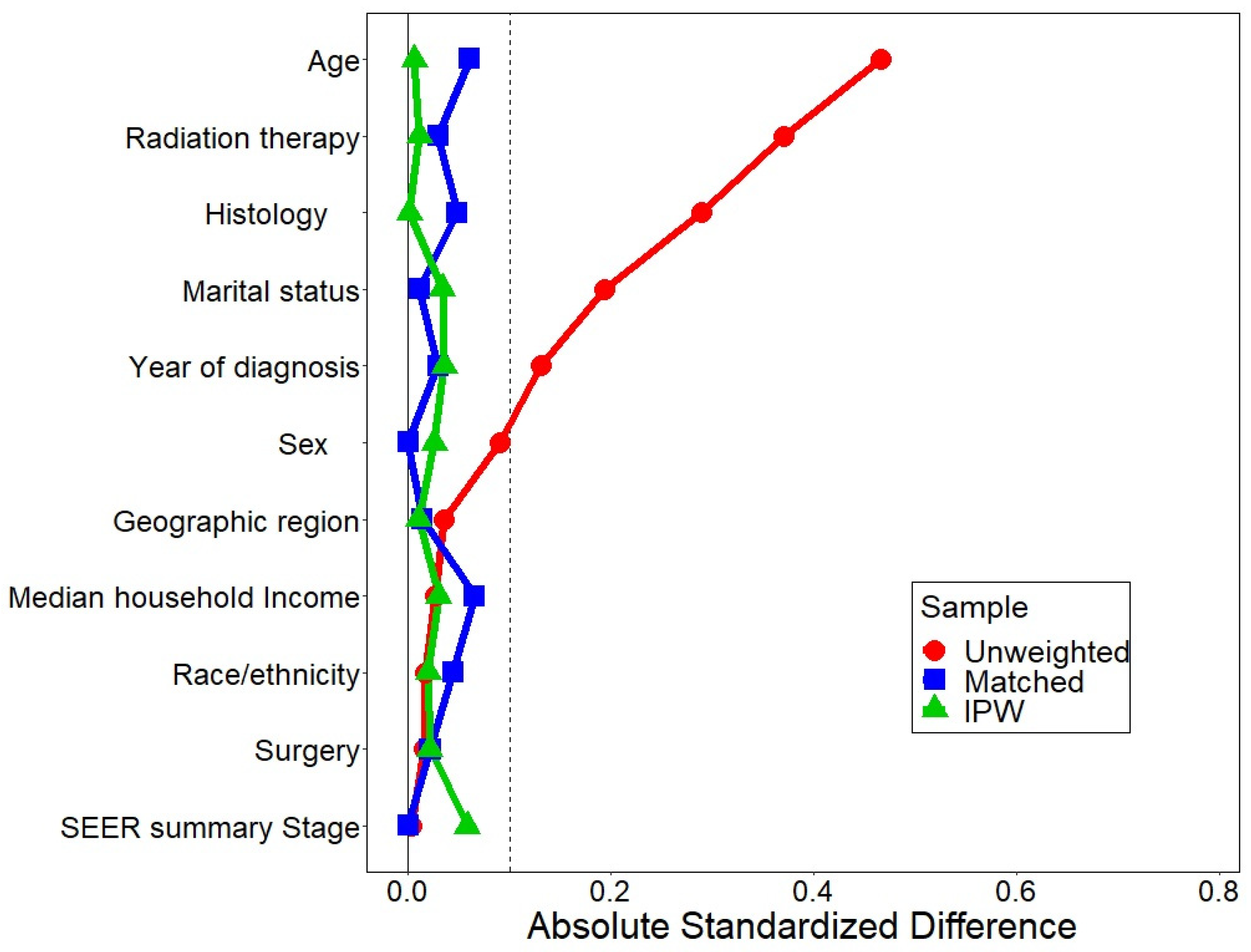
| Characteristics | Chemotherapy | p Value | |
|---|---|---|---|
| No (n = 263) | Yes (n = 300) | ||
| Age, years | <0.001 | ||
| 20–44 | 57 (21.7) | 104 (34.7) | |
| 45–64 | 89 (33.8) | 122 (40.7) | |
| ≥65 | 117 (44.5) | 74 (24.7) | |
| Sex | 0.353 | ||
| Male | 136 (51.7) | 167 (55.7) | |
| Female | 127 (48.3) | 133 (44.3) | |
| Year of diagnosis | 0.336 | ||
| 2000–2004 | 59 (22.4) | 49 (16.3) | |
| 2005–2009 | 57 (21.7) | 70 (23.3) | |
| 2010–2014 | 67 (25.5) | 81 (27.0) | |
| 2015–2020 | 80 (30.4) | 100 (33.3) | |
| Race and ethnicity | 0.565 | ||
| Non-Hispanic White | 155 (58.9) | 174 (58.0) | |
| Non-Hispanic Black | 22 (8.4) | 34 (11.3) | |
| Hispanic | 56 (21.3) | 52 (17.3) | |
| Other | 30 (11.4) | 40 (13.3) | |
| Region | 0.967 | ||
| Midwest | 13 (4.9) | 16 (5.3) | |
| Northeast | 43 (16.3) | 53 (17.7) | |
| South | 47 (17.9) | 51 (17.0) | |
| West | 160 (60.8) | 180 (60.0) | |
| Marital status, married | 127 (48.3) | 173 (57.7) | 0.026 |
| Median household income | 0.794 | ||
| <$75,000 | 140 (53.2) | 163 (54.3) | |
| ≥$75,000 | 123 (46.8) | 137 (45.7) | |
| Location, rural | 22 (8.4) | 33 (11.0) | 0.293 |
| SEER summary stage | <0.001 | ||
| Localized | 86 (32.7) | 92 (30.7) | |
| Regional | 71 (27.0) | 79 (26.3) | |
| Distant | 74 (28.1) | 119 (39.7) | |
| Unknown/unstaged | 32 (12.2) | 10 (3.3) | |
| Histology | <0.001 | ||
| Sarcoma | 203 (77.2) | 192 (64.0) | |
| Lymphoma | 52 (19.8) | 103 (34.3) | |
| Mesothelioma and others | 8 (3.0) | 5 (1.7) | |
| Surgery | 0.822 | ||
| Yes | 129 (49.0) | 150 (50.0) | |
| No | 134 (51.0) | 150 (50.0) | |
| Radiation therapy | <0.001 | ||
| Yes | 26 (10.0) | 70 (23.6) | |
| No | 235 (90.0) | 227 (76.4) | |
| All-Cause Mortality | Cancer Mortality | CVD Mortality | |
|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |
| Before PS matching—unadjusted | 0.52 (0.43–0.62) | 0.59 (0.48–0.72) | 0.23 (0.12–0.46) |
| Before PS matching—adjusted | 0.56 (0.45–0.69) | 0.63 (0.50–0.80) | 0.27 (0.12–0.58) |
| After PS matching | 0.65 (0.52–0.81) | 0.71 (0.56–0.89) | 0.30 (0.13–0.69) |
| IPTW | 0.63 (0.52–0.76) | 0.67 (0.55–0.82) | 0.36 (0.19–0.68) |
| Characteristics | Chemotherapy | ASD | p Value | |
|---|---|---|---|---|
| No (n = 185) | Yes (n = 185) | |||
| Age, years | 0.056 | 0.894 | ||
| 20–44 | 53 (28.6) | 57 (30.8) | ||
| 45–64 | 67 (36.2) | 66 (35.7) | ||
| ≥65 | 65 (35.1) | 62 (33.5) | ||
| Sex | 0.033 | 0.835 | ||
| Male | 98 (53.0) | 101 (54.6) | ||
| Female | 87 (47.0) | 84 (45.4) | ||
| Year of diagnosis | 0.025 | 0.901 | ||
| 2000–2004 | 33 (17.8) | 34 (18.4) | ||
| 2005–2009 | 40 (21.6) | 44 (23.8) | ||
| 2010–2014 | 52 (28.1) | 46 (24.9) | ||
| 2015–2020 | 60 (32.4) | 61 (33.0) | ||
| Race and ethnicity | 0.044 | 0.161 | ||
| Non-Hispanic White | 112 (60.5) | 108 (58.4) | ||
| Non-Hispanic Black | 17 (9.2) | 23 (12.4) | ||
| Hispanic | 13 (7.0) | 24 (13.0) | ||
| Other | 40 (21.6) | 28 (15.1) | ||
| Region | 3 (1.6) | 2 (1.1) | 0.037 | 0.929 |
| Midwest | 11 (5.9) | 9 (4.9) | ||
| Northeast | 32 (17.3) | 36 (19.5) | ||
| South | 33 (17.8) | 33 (17.8) | ||
| West | 109 (58.9) | 107 (57.8) | ||
| Marital status, married | 91 (49.2) | 98 (53.0) | 0.076 | 0.533 |
| Median household income | 0.000 | 1.000 | ||
| <$75,000 | 101 (54.6) | 101 (54.6) | ||
| ≥$75,000 | 84 (45.4) | 84 (45.4) | ||
| Location, rural | 15 (8.1) | 23 (12.4) | 0.142 | 0.230 |
| SEER summary stage | 0.000 | 1.000 | ||
| Localized | 60 (32.4) | 60 (32.4) | ||
| Regional | 55 (29.7) | 55 (29.7) | ||
| Distant | 63 (34.1) | 63 (34.1) | ||
| Unknown/unstaged | 7 (3.8) | 7 (3.8) | ||
| Histology | 0.012 | 0.910 | ||
| Sarcoma | 129 (69.7) | 128 (69.2) | ||
| Lymphoma | 48 (25.9) | 56 (30.3) | ||
| Mesothelioma and others | 8 (4.3) | 1 (0.5) | ||
| Surgery | 0.000 | 1.000 | ||
| Yes | 91 (49.2) | 91 (49.2) | ||
| No | 94 (50.8) | 94 (50.8) | ||
| Radiation therapy, yes | 0.000 | 1.000 | ||
| Yes | 159 (85.9) | 159 (85.9) | ||
| No | 26 (14.1) | 26 (14.1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, D.; Goodart, C.R.; Kothari, G.K.; Ebong, I.A.; Nwabuo, C.C. Reduced Risk of All-Cause, Cancer-, and Cardiovascular Disease-Related Mortality among Patients with Primary Malignant Cardiac Tumors Receiving Chemotherapy in the United States. Curr. Oncol. 2023, 30, 8488-8500. https://doi.org/10.3390/curroncol30090618
Appiah D, Goodart CR, Kothari GK, Ebong IA, Nwabuo CC. Reduced Risk of All-Cause, Cancer-, and Cardiovascular Disease-Related Mortality among Patients with Primary Malignant Cardiac Tumors Receiving Chemotherapy in the United States. Current Oncology. 2023; 30(9):8488-8500. https://doi.org/10.3390/curroncol30090618
Chicago/Turabian StyleAppiah, Duke, Carina R. Goodart, Grishma K. Kothari, Imo A. Ebong, and Chike C. Nwabuo. 2023. "Reduced Risk of All-Cause, Cancer-, and Cardiovascular Disease-Related Mortality among Patients with Primary Malignant Cardiac Tumors Receiving Chemotherapy in the United States" Current Oncology 30, no. 9: 8488-8500. https://doi.org/10.3390/curroncol30090618
APA StyleAppiah, D., Goodart, C. R., Kothari, G. K., Ebong, I. A., & Nwabuo, C. C. (2023). Reduced Risk of All-Cause, Cancer-, and Cardiovascular Disease-Related Mortality among Patients with Primary Malignant Cardiac Tumors Receiving Chemotherapy in the United States. Current Oncology, 30(9), 8488-8500. https://doi.org/10.3390/curroncol30090618





