Pediatric-Inspired Regimens in the Treatment of Acute Lymphoblastic Leukemia in Adolescents and Young Adults: A Systematic Review
Abstract
:1. Introduction
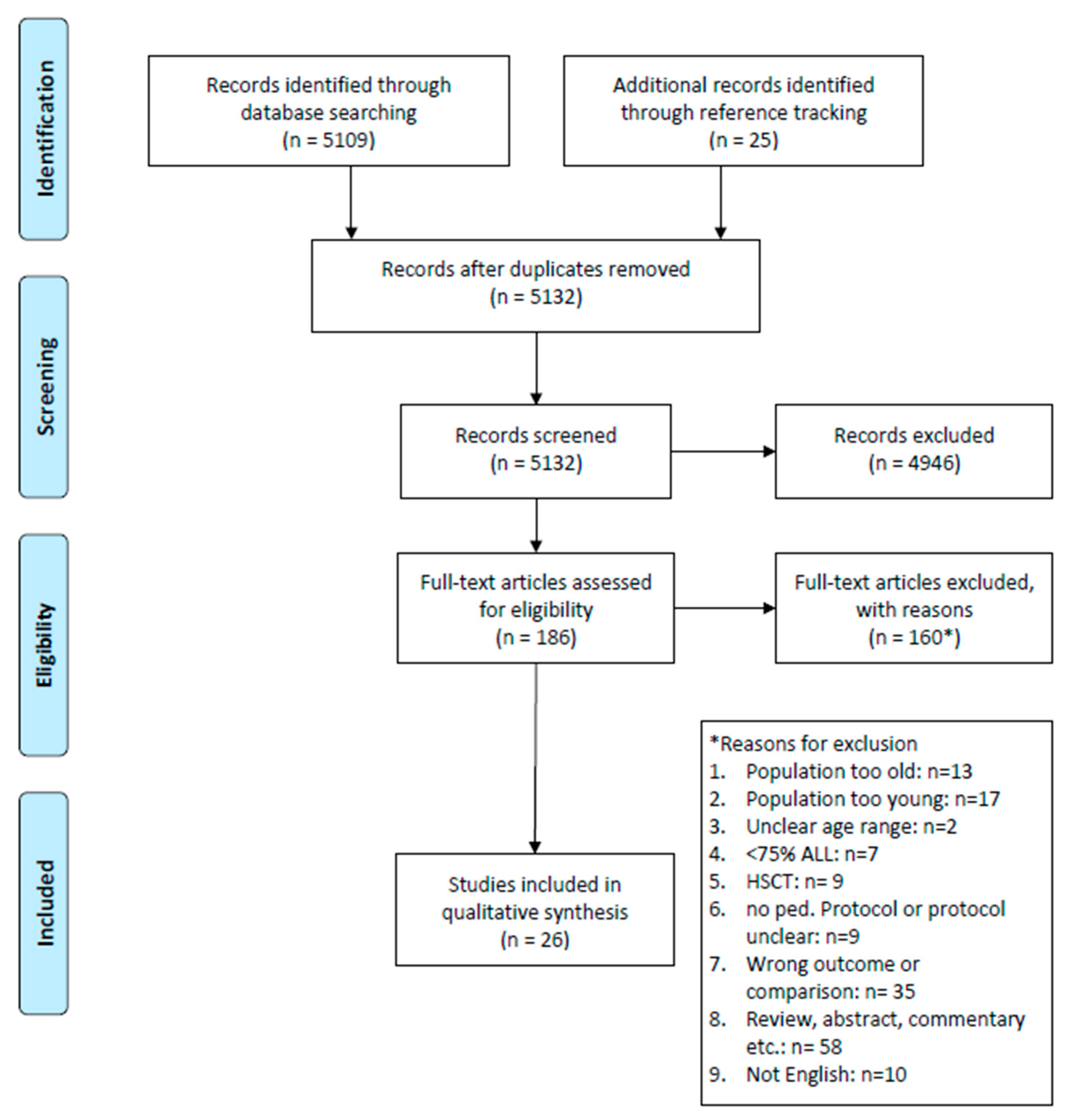
2. Materials and Methods
3. Results
3.1. Description of Studies and Regimens
3.2. Treatment Outcomes and Toxicity in AYA Patients When Treated with PIR versus Conventional Adult Regimens
3.3. Age-Stratified Analysis of Outcomes and Toxicities in AYA Patients Treated with PIR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferrari, A.; Stark, D.; Peccatori, F.A.; Fern, L.; Laurence, V.; Gaspar, N.; Bozovic-Spasojevic, I.; Smith, O.; De Munter, J.; Derwich, K.; et al. Adolescents and young adults (AYA) with cancer: A position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 2021, 6, 100096. [Google Scholar] [CrossRef]
- Trama, A.; Botta, L.; Foschi, R.; Ferrari, A.; Stiller, C.; Desandes, E.; Maule, M.M.; Merletti, F.; Gatta, G. Survival of European adolescents and young adults diagnosed with cancer in 2000-07: Population-based data from EUROCARE-5. Lancet Oncol. 2016, 17, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.R.; Advani, A.S. Treatment of Acute Lymphoblastic Leukemia in Adolescents and Young Adults. J. Adolesc. Young Adult Oncol. 2011, 1, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Wolach, O.; Vidal, L.; Gafter-Gvili, A.; Shpilberg, O.; Raanani, P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: Systematic review and meta-analysis. Am. J. Hematol. 2012, 87, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Boissel, N.A.-O.; Baruchel, A.A.-O. Acute lymphoblastic leukemia in adolescent and young adults: Treat as adults or as children? Blood 2018, 132, 351–361. [Google Scholar] [CrossRef]
- Rizzari, C.; Putti, M.C.; Colombini, A.; Casagranda, S.; Ferrari, G.M.; Papayannidis, C.; Iacobucci, I.; Abbenante, M.C.; Sartor, C.; Martinelli, G. Rationale for a pediatric-inspired approach in the adolescent and young adult population with acute lymphoblastic leukemia, with a focus on asparaginase treatment. Hematol. Rep. 2014, 6, 5554. [Google Scholar] [CrossRef]
- Schafer, E.S.; Hunger, S.P. Optimal therapy for acute lymphoblastic leukemia in adolescents and young adults. Nat. Rev. Clin. Oncol. 2011, 8, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Advani, A.S.; Larsen, E.; Laumann, K.; Luger, S.M.; Liedtke, M.; Devidas, M.; Chen, Z.; Yin, J.; Foster, M.C.; Claxton, D.; et al. Comparison of CALGB 10403 (Alliance) and COG AALL0232 toxicity results in young adults with acute lymphoblastic leukemia. Blood Adv. 2021, 5, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Alacacioglu, I.; Medeni, S.S.; Ozsan, G.H.; Payzin, B.; Sevindik, O.G.; Acar, C.; Katgi, A.; Ozdemirkan, F.; Piskin, O.; Ozcan, M.A.; et al. Is the BFM Regimen Feasible for the Treatment of Adult Acute Lymphoblastic Leukemia? A Retrospective Analysis of the Outcomes of BFM and Hyper-CVAD Chemotherapy in Two Centers. Chemotherapy 2014, 60, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Al-Khabori, M.; Minden, M.D.; Yee, K.W.L.; Gupta, V.; Schimmer, A.D.; Schuh, A.C.; Xu, W.; Brandwein, J.M. Improved survival using an intensive, pediatric-based chemotherapy regimen in adults with T-cell acute lymphoblastic leukemia. Leuk. Lymphoma 2010, 21, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Huante, E.; Espinosa-Bautista, K.; Rangel-Patiño, J.; Demichelis-Gómez, R. Comparison of Two Pediatric-Inspired Regimens to Hyper-CVAD in Hispanic Adolescents and Young Adults With Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, 55–62. [Google Scholar] [CrossRef]
- Brandwein, J.M.; Atenafu, E.G.; Schuh, A.C.; Yee, K.W.; Schimmer, A.D.; Gupta, V.; Minden, M.D. Predictors of outcome in adults with BCR-ABL negative acute lymphoblastic leukemia treated with a pediatric-based regimen. Leuk. Res. 2014, 38, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.A.-O.; Devidas, M.; Chen, Z.; Salzer, W.L.; Raetz, E.A.; Rabin, K.R.; Heerema, N.A.; Carroll, A.A.-O.X.; Gastier-Foster, J.M.; Borowitz, M.J.; et al. Outcomes in adolescent and young adult patients (16 to 30 years) compared to younger patients treated for high-risk B-lymphoblastic leukemia: Report from Children’s Oncology Group Study AALL0232. Leukemia 2022, 36, 648–655. [Google Scholar] [CrossRef]
- Cheng, C.N.; Li, S.S.; Hsu, Y.T.; Chen, Y.P.; Chen, T.Y.; Chen, J.S. Outcome of young adult patients with very-high-risk acute lymphoblastic leukemia treated with pediatric-type chemotherapy—A single institute experience. J. Formos. Med. Assoc. 2022, 121, 694–702. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Stevenson, K.E.; Dahlberg, S.E.; Silverman, L.B.; Couban, S.; Supko, J.G.; Amrein, P.C.; Ballen, K.K.; Seftel, M.D.; Turner, A.R.; et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18–50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 2015, 29, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.A.-O.; Jain, H.A.-O.; Bagal, B.; Subramanian, P.G.; George, B.A.-O.; Korula, A.A.-O.; Mehra, N.; Kalaiyarasi, J.P.; Bhurani, D.A.-O.; Agrawal, N.A.-O.; et al. Outcomes in adolescent and young adult acute lymphoblastic leukaemia: A report from the Indian Acute Leukaemia Research Database (INwARD) of the Hematology Cancer Consortium (HCC). Br. J. Haematol. 2021, 193, e1. [Google Scholar] [CrossRef]
- Ganesan, P.A.-O.; Sagar, T.G.; Kannan, K.; Radhakrishnan, V.; Dhanushkodi, M.; Swaminathan, R.; Sundersingh, S.; Ganesan, T.S. Acute Lymphoblastic Leukemia in Young Adults Treated with Intensive “Pediatric” Type Protocol. Indian J. Hematol. Blood Transfus. 2018, 34, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gómez-De León, A.; Varela-Constantino, A.L.; Colunga-Pedraza, P.R.; Sánchez-Arteaga, A.; García-Zárate, V.; Rodríguez-Zúñiga, A.C.; Méndez-Ramírez, N.; Cantú-Rodríguez, O.G.; Gutiérrez-Aguirre, C.H.; Tarín-Arzaga, L.; et al. Treatment of Ph-Negative Acute Lymphoblastic Leukemia in Adolescents and Young Adults with an Affordable Outpatient Pediatric Regimen. Clin. Lymphoma Myeloma Leuk. 2022, 22, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.A.-O.; Trahair, T.A.-O.X.; Sutton, R.A.-O.; Osborn, M.A.-O.; Kwan, J.; Mapp, S.; Howman, R.; Anazodo, A.A.-O.; Wylie, B.; D’Rozario, J.; et al. An MRD-stratified pediatric protocol is as deliverable in adolescents and young adults as in children with ALL. Blood Adv. 2021, 5, 5574–5583. [Google Scholar] [CrossRef]
- Gupta, S.A.-O.; Pole, J.D.; Baxter, N.A.-O.; Sutradhar, R.A.-O.; Lau, C.; Nagamuthu, C.; Nathan, P.C. The effect of adopting pediatric protocols in adolescents and young adults with acute lymphoblastic leukemia in pediatric vs adult centers: An IMPACT Cohort study. Cancer Med. 2019, 8, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, F.; Sakura, T.; Yujiri, T.; Kondo, E.; Fujimaki, K.; Sasaki, O.; Miyatake, J.; Handa, H.; Ueda, Y.; Aoyama, Y.; et al. Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: A phase II study by the Japan Adult Leukemia Study Group. Blood Cancer J. 2014, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Hough, R.; Rowntree, C.; Goulden, N.; Mitchell, C.; Moorman, A.; Wade, R.; Vora, A. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: Results from UKALL 2003. Br. J. Haematol. 2016, 172, 439–451. [Google Scholar] [CrossRef]
- Kliman, D.; Barnett, M.; Broady, R.; Forrest, D.; Gerrie, A.; Hogge, D.; Nantel, S.; Narayanan, S.; Nevill, T.; Power, M.; et al. Comparison of a pediatric-inspired treatment protocol versus standard-intensity chemotherapy for young adults with standard-risk BCR-ABL negative acute lymphoblastic leukemia. Leuk. Lymphoma 2017, 58, 909–915. [Google Scholar] [CrossRef]
- Quist-Paulsen, P.; Toft, N.; Heyman, M.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Jónsson, Ó.G.; Palk, K.; Vaitkeviciene, G.; Vettenranta, K.; et al. T-cell acute lymphoblastic leukemia in patients 1–45 years treated with the pediatric NOPHO ALL2008 protocol. Leukemia 2020, 34, 347–357. [Google Scholar] [CrossRef]
- Rank, C.A.-O.; Toft, N.; Tuckuviene, R.; Grell, K.; Nielsen, O.J.; Frandsen, T.L.; Marquart, H.V.H.; Albertsen, B.K.; Tedgård, U.; Hallböök, H.; et al. Thromboembolism in acute lymphoblastic leukemia: Results of NOPHO ALL2008 protocol treatment in patients aged 1 to 45 years. Blood 2018, 131, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.A.-O.; Morgades, M.; Montesinos, P.A.-O.; Tormo, M.; Martínez-Carballeira, D.; González-Campos, J.; Gil, C.; Barba, P.; García-Boyero, R.; Coll, R.; et al. A pediatric regimen for adolescents and young adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: Results of the ALLRE08 PETHEMA trial. Cancer Med. 2020, 9, 2317–2329. [Google Scholar] [CrossRef]
- Ribera, J.M.; Oriol, A.; Sanz, M.-A.; Tormo, M.; Fernández-Abellán, P.; del Potro, E.; Abella, E.; Bueno, J.; Parody, R.; Bastida, P.; et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J. Clin. Oncol. 2008, 26, 1843–1849. [Google Scholar] [CrossRef]
- Rytting, M.E.; Jabbour, E.J.; Jorgensen, J.L.; Ravandi, F.; Franklin, A.R.; Kadia, T.M.; Pemmaraju, N.; Daver, N.G.; Ferrajoli, A.; Garcia-Manero, G.; et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am. J. Hematol. 2016, 91, 819–823. [Google Scholar] [CrossRef]
- Rytting, M.E.; Thomas, D.A.; O’Brien, S.M.; Ravandi-Kashani, F.; Jabbour, E.J.; Franklin, A.R.; Kadia, T.M.; Pemmaraju, N.; Daver, N.G.; Ferrajoli, A.; et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer 2014, 120, 3660–3668. [Google Scholar] [CrossRef]
- Tantiworawit, A.; Rattanathammethee, T.A.-O.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Norasetthada, L. Outcomes of adult acute lymphoblastic leukemia in the era of pediatric-inspired regimens: A single-center experience. Int. J. Hematol. 2019, 110, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Toft, N.; Birgens, H.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Heyman, M.; Klausen, T.W.; Jónsson, Ó.G.; Palk, K.; Pruunsild, K.; et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 2018, 32, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Toft, N.; Birgens, H.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Heyman, M.; Klausen, T.W.; Jónsson, Ó.G.; Palk, K.; Pruunsild, K.; et al. Toxicity profile and treatment delays in NOPHO ALL2008-comparing adults and children with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur. J. Haematol. 2016, 96, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Valtis, Y.A.-O.; Stevenson, K.E.; Place, A.A.-O.; Silverman, L.B.; Vrooman, L.M.; Gotti, G.A.-O.; Brunner, A.M.; Nauffal, M.; DeAngelo, D.A.-O.; Luskin, M.A.-O. Orthopedic toxicities among adolescents and young adults treated in DFCI ALL Consortium Trials. Blood Adv. 2022, 6, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country | Years of Recruitment | Diagnosis | PIR | N of Pediatric Patients (%) | Adult Protocol | N of Adult Patients (%) | Median Age (Range) in Years | JBI Score |
| Studies comparing adolescents and young adult patients receiving a PIR or adult regimen | ||||||||
| Al-Khabori, 2010 Canada [11] | January 1990–March 2007 | T-ALL | DFCI regimen | 32 (44) | 9203ALL, Protocol C, Hyper-CVAD, MRC UKALL XII/ECOG E2993 | 40 (56) | 30.8 (17–69) | 1 |
| Alacacioglu, 2014 Turkey [10] | March 2006–October 2012 | ALL | BFM-Like | 20 (40) | Hyper-CVAD | 30 (60) | Overall: 27.5 (18–59) PIR: 25 Hyper-CVAD: 30.5 | 2 |
| Almanza-Huante, 2021 Mexico [12] | PIR: March 2016– June 2019 hyper-CVAD: February 2009–June 2015 | BCR–ABL negative ALL | modified ALL-BFM 90, modified CALGB C10403 | 73 (30) | Hyper-CVAD | 173 (70) | Overall: 22 (14–43) PIR: 24 Hyper-CVAD: 20 | 1 |
| Cheng, 2022 Taiwan [15] | 2008–2019 | VHR-ALL | TPOG-ALL-2002 protocol | 16 (59) | Hyper-CVAD/HD-Methotrexate and Cytarabine | 11 (41) | PIR: 24.3 (18–36) Hyper-CVAD: 33 (20–40) | 1 |
| Ganesan 2021 India [17] | 2012–2017 | ALL (including MPAL) | MCP-841, BFM-90, -95 or -2000, COG Protocols | 1002 (88) | GMALL, Hyper-CVAD, UKALL | 139 (12) | Overall: range 15–29 PIR: 20 Adult: 23 | 1 |
| Ganesan, 2018 India [18] | January 2000–December 2014 | BCR–ABL negative ALL | BFM 95, SR arm | 147 (63) | MCP-841 (22%) GMALL (9%) INCTR (4%) UKALL (2%) | 85 (37) | Overall: 21 (18–30) PIR: 21.8 Adult: 22.4 | 2 |
| Gupta, 2019, Canada [21] | 1992–2011 | ALL | DFCI Protocol 91-01 | 123 (54) | Not specified | 106 (46) | Range 15–21 Pediatric centers: mean 16 ± 1 Adult centers: mean 19 ± 1 | 1 |
| Hayakawa, 2014, Japan [22] | August 2002–October 2009 | BCR–ABL negative B-ALL | ALL202 | 139 (57) | ALL97-U | 104 (43) | 19 (15–24) | 1 |
| Tantiworawit, 2019, Thailand [31] | January 2007–December 2017 | ALL | TPOG protocol | 35 (33) | Hyper-CVAD or GMALL protocol | 75 (67) | Overall: 26 (15–63) Adult: 29.5 (16–63) PIR: 24 (15–39) | 2 |
| Rytting, 2014 USA [30] | October 2006–April 2012 | BCR–ABL negative ALL | Augmented BFM regimen | 85 | Historic Hyper-CVAD cohort | 71 | PIR: 21 (13–39) Adult: 26 (16–40) | 1 |
| Rytting, 2016, USA [29] | October 2006–March 2014 | BCR–ABL negative ALL | Augmented BFM regimen | 106 | Historic Hyper-CVAD cohort | 102 | PIR: 22 (13–39) Adult: 27 (15–40) | 1 |
| Kliman, 2017, Canada [24] | PIR: February 2008–November 2014 Adult: February 2003–July 2008 | SR BCR–ABL negative ALL | Modification of DFCI 01–175 | 22 | Comparative adult ALL protocols (not exactly specified) | 25 | Overall: 24.5 (18–40) PIR: 27.6 Adult: 23.5 | 2 |
| Studies comparing age-stratified outcomes and toxicity in adolescents and young adult patients receiving a PIR or adult regimen | ||||||||
| Ref. | Years of recruitment | Diagnosis | Protocol | Age groups and N of patients | JBI score | |||
| Advani, 2021, USA [9] | CALGB 10403: 2007–2012 COG AALL0232: 2004–2011 | B- or T-precursor ALL | PIR: CALGB 10403 and COG AALL0232 (arm identical to CALGB 10403) | CALGB 10403:
| 1 | |||
| Almanza-Huante, 2021 Mexico [12] | PIR: March 2016– June 2019 Adult: February 2009–June 2015 | BCR–ABL negative ALL | PIR: modified ALL-BFM 90, modified CALGB C10403 Adult: Hyper-CVAD | 14–20 years:
| 1 | |||
| Brandwein, 2014 Canada [13] | June 2000–June 2011 | BCR–ABL negative ALL | PIR: DFCI 91-01 | 17–<34 years: n = 73 (47%) 34–<50 years: n = 54 (35%) 50–60 years: n = 29 (19%) | 1 | |||
| Burke, 2022, USA [14] | January 2004–January 2011 | HR B-ALL, excluding DS patients | PIR: COG AALL0232 | <16 years: n = 2443 (=younger children) 16–30 years: n = 597 (=AYA population) | 1 | |||
| Cheng, 2022, Taiwan [15] | 2008–2019 | VHR-ALL | PIR: TPOG-ALL-2002 protocol | 18–25 years: n = 7 26–34 years: n = 9 | 1 | |||
| De Angelo, 2015, USA and Canada [16] | August 2002–February 2008 | ALL (excl. mature B-cell ALL) | PIR: DFCI Pediatric ALL Consortium regimen/DFCI Adult: ALL Consortium Protocol 01–175 | 18–29 years: n = 48 (52%) 30–50 years: n = 44 (48%) | 2 | |||
| Ganesan, 2021, India [17] | 2012–2017 | ALL (including MPAL) | PIR: MCP-8417, BFM-90, -95 or -2000), COG Adult: GMALL, Hyper-CVAD, UKALL | 15–17 years: n = 403 (29.1%) 18–24 years: n = 688 (49.7%) 25–29 years: n = 292 (21.2%) | 1 | |||
| Gómez-De León, 2022, Mexico [19] | 2016–2020 | BCR–ABL negative B-ALL | PIR: Modified BFM protocol | 16–19 years: n = 31 20–29 years: n = 33 30–39 years: n = 16 ≥40 years: n = 11 | 2 | |||
| Greenwood, 2021, Australia [20] | July 2012–June 2018 | ALL | PIR: ANZCHOG Study 8 protocol | Median 22.7 years (16–38 years), analyzed as a continuous variable | 1 | |||
| Hough, 2016 UK, Ireland [23] | October 2003–June 2011 | BCR–ABL negative B-ALL | PIR: UKALL2003 | 16–24 years: n = 2287 10–15 years: n = 610 <10 years: n = 229 | 1 | |||
| Valtis, 2022, USA [34] | 2000–2018 | ALL | PIR: DFCI ALL Consortium 00-001, 05-001, 01-175, 06-254 | 15–19 years: n = 138 (38%) 20–29 years: n = 110 (30%) 30–39 years: n = 62 (17%) 40–50 years: n = 57 (16%) | 1 | |||
| Toft, 2018, Denmark, Estonia, Finland, Iceland, Lithuania, Norway, Sweden [32] | July 2008–December 2014 | T-ALL or BCR–ABL negative B-ALL, excluding patients with DS | PIR: NOPHO ALL2008 protocol | 1–9 years: n = 1022 (68%) 10–17 years: n = 266 (18%) 18–45 years: n = 221 (14%) | 1 | |||
| Toft, 2016, Countries see above [33] | July 2008–April 2013 | T-ALL or BCR–ABL negative B-ALL, excluding patients with DS | PIR: NOPHO ALL2008 protocol | 1–9.9 years: n = 733 (69%) 10–14 years: n = 118 (11%) 15–17 years: n = 77 (7%) 18–26 years: n = 70 (7%) 27–45 years: n = 64 (6%) | 1 | |||
| Rytting, 2014, USA [30] | October 2006–April 2012 | BCR–ABL negative ALL | PIR: Augmented BFM regimen | 12–21 years: n = 44 (52%) 22–40 years: n = 41 (48%) | 1 | |||
| Rytting, 2016, USA [29] | October 2006–March 2014 | BCR–ABL negative ALL | PIR: Augmented BFM regimen Adult: Historical cohort (treated with Hyper-CVAD | 13–21 years:
| 1 | |||
| Ribera, 2008, Spain [28] | June 1996–June 2005 | SR ALL | PIR: PETHEMA ALL-96 protocol | 15–18 years: n = 35 (43%) 19–30 years: n = 46 (57%) | 1 | |||
| Ribera, 2020, Spain [27] | August 2008–April 2018 | SR BCR–ABL negative ALL | PIR: PETHEMA ALLRE08 | 15–18 years: n = 38 (43%) 19–30 years: n = 51 (57%) | 1–2 | |||
| Quist-Paulsen, 2020, Countries as Toft et al. [25] | July 2008–March 2016 | T-ALL | PIR: NOPHO ALL2008 protocol | 1–9: n = 117 (42%) 10–17: n = 78 (28%) 18–45: n = 83 (30%) | 1 | |||
| Rank, 2018, Countries same as Toft et al. [26] | July 2008–February 2016 | BCR–ABL negative ALL | PIR: NOPHO ALL2008 protocol | 1–9.9 years: n = 1192 (67%) 10–17.9 years: n = 306 (17%) 18–45 years: n = 274 (16%) | 1 | |||
| Ref. | CR Rate after Induction (PIR vs. Adult) | EFS/RFS/DFS/Relapse Rate (PIR vs. Adult) | OS (PIR vs. Adult) | Toxicity (PIR vs. Adult) | The Median Duration of Follow-Up in Months (Range) |
|---|---|---|---|---|---|
| AL-Khabouri, 2010 [11] | 84% vs. 93% | 3-year RFS: 89% vs. 24%; p < 0.0001 | 3-year OS: 81% vs. 44%; p = 0.0003 5-year OS: 75% (85% CI: 55–88%) vs. 25% (95% CI:13–39%); p = 0.0003 | NA | 54 (13–238) |
| Alacacioglu, 2014 [10] | 95% vs. 96% | Mean RFS: 53.9 ± 5.4 vs. 39.1 ± 6.8 months; p = 0.009 | Mean OS: 55.1 ± 4.9 vs. 41.5 ± 6.4 months; p = 0.012 5-year OS: 59% vs. 34% | No anaphylactic reactions to E. coli L-ASP, no pancreatitis, or venous complications. Mild elevation of liver enzymes. No complications caused a delay in either protocol. | 37 |
| Almanza-Huante, 2021 [12] | 79.5% vs. 64.2%; p = 0.02 | Relapse rate: 44.1% vs. 60%; p = 0.04 | OS: 18.5 [95% CI, 13.61–23.43] vs. 11.08 months [95% CI, 7.33–14.83]) 2-year OS: 41.5% vs. 28.1%; p = 0.01 | IRM: 1.4% PIR vs. 8% hyper-CVAD (p = 0.04) TRD due to infection: 3.3% PIR vs. 28.1% hyper-CVAD | Hyper-CVAD: 101 BFM: 32 CALGB: 22 |
| Cheng, 2022 [15] | NA | 5-year EFS: 71.6 ± 12.2% vs. 45.5 ± 15.0%; p = 0.152 HR: 0.42; p = 0.16 5-year EFS (untransplanted patients): 83.3% ± 10.8% vs. 28.6% ± 17.1%; p = 0.039 HR 4.19, p < 0.05 | NA | Toxic death: n = 1 in both groups | PIR: 60 months (6–108) Adult: 20 months (2–127) |
| Ganesan, 2021 [17] | NA | 2-year EFS: 56.6% vs. 52.1%; p = 0.730 HR with 95% CI: 1.05 (0.81–1.35); p = 0.736 2-year RFS: 75.1% vs. 75.4%; p = 0.702 | 2-year OS: 75.4% vs. 59.0%; p < 0.001 HR with 95% CI: 1.72 (1.29–2.29; p < 0.001 (univariate) 3.19 (1.95–5.22); p < 0.001 (multivariate) | NA | 23 months (95% CI 6–38) |
| Ganesan, 2018 [18] | 84% vs. 82% | 5-year RFS: 51% vs. 35%; p = 0.027 5-year EFS: 40% vs. 27% p = 0.054 | 5-year OS: 43% vs. 33%; p = 0.2 | IRM: 10% vs. 1% p = 0.001; major causes: sepsis, L-ASP associated thrombotic complications TRD: 12% vs. 2%; p = 0.031 | 21 months (0.3–165) |
| Gupta, 2019 [21] | NA | 5-year EFS, treated between 2006 and 2011: pediatric center AYA 80.8 ± 5.8% vs. adult center with PIR 71.8% ± 7.2% vs. adult centers with adult protocols 60.0% ± 11.0%; p = 0.02 | 5-year OS, treated between 2006 and 2011: pediatric center 90.9 ± 4.3%, adult center with PIR 76.9 ± 6.82%, adult centers with adult protocols 65.0 ± 10.7.0%; p = 0.004 | NA | NA |
| Hayakawa, 2014 [22] | 94% (95% CI 88–97%) vs. 84% (75–90%) | 5-year DFS: 67% (95% CI 58–75%) vs. 44% (33–55%), not statistically significant, but the p-value has not been shown | 5-year OS: 73% (95% CI 64–80%) vs. 45% (35–55%) not statistically significant, but the p-value has not been shown | Sepsis, hepatic toxicity, and neuropathy were more frequent in PIR. No toxic deaths occurred during post-remission therapy due to severe adverse events. | PIR: 61 Adult: 67 |
| Tantiworawit, 2019 [31] | 88.2% vs. 79.2%, p = 0.23 | 2-year DFS: 47.1% vs. 24.7% (HR 1.73, 1.22–3.03, p = 0.04) Relapse rate: 34.3% vs. 54.2%, p < 0.01 DFS for BCR–ABL negative ALL: 46.8% vs. 18.7% (HR 2.16, 1.16–4.01, p = 0.01) | 2-year OS 50.8% vs. 31.2% (HR 1.52, 0.83–2.78, p = 0.16) For BCR–ABL negative ALL 2-year OS of 59.4% vs. 31.8% (HR 2.03, 1.04–3.96, p = 0.03) | IRM: 2.9 vs. 5.6%, p = 0.53 | 11.6 (1–120) |
| Rytting, 2014 [30] | 94% vs. 99%, p = 0.14 | NA | 3-year OS rate: 74% vs. 71%, not statistically significant, but the p-value has not been shown | See Table 3 | 40 (4–75) |
| Rytting, 2016 [29] | 93% vs. 98%, p-value not shown | NA | 5-year OS: 60% vs. 60% | Toxicity (PIR vs. adult): No significant difference for allergic reactions, liver enzyme and bilirubin elevation, ON, thrombosis, stroke-like events, neuropathy, bleeding, or deaths in CR Hypofibrinogenemia 35% vs.14%, p < 0.001 Pancreatitis 11% vs. 3%, p = 0.02 Induction infections grade 3–4: 22% vs. 45%, p < 0.001 Infections in CR in the first 60 days: 30% vs. 60%, p < 0.001 | PIR: 66 months (17–107) Adult: 88 (1–152) |
| Kliman, 2017 [24] | 100% vs. 86%, p = 0.095 | 3-year EFS: 80% vs. 45%, p = 0.019 | 3-year OS: 80% vs. 59%, p = 0.12 | There were no significant differences between the incidence of candidemia, severe infection, thrombosis, pancreatitis, or toxic death. | Overall: 40.1 PIR: 36.8 Adult: 73.1 |
| Ref. | CR Rate after Induction | EFS/RFS/Relapse Rate | OS | Toxicity | Median Follow-Up in Months (Range) |
|---|---|---|---|---|---|
| Advani, 2021 [9] | NA | NA | NA | IRM with CALGB 10403 and COG AALL0232: 3.1% and 1.3%. Main Grade 3 and 4 toxicities with an incidence > 15%: hyperglycemia, bilirubin and ALT increases, febrile neutropenia, and infection. Post-induction mortality with CALGB 10403 and COG AALL0232: 1.3% and 0.8%. Main Grade 3 and 4 post-induction toxicities with an incidence > 15%: febrile neutropenia, infection, sensory neuropathy, hyperglycemia, bilirubin, AST and ALT increases, anaphylaxis. Increased age correlated with a decreased fibrinogen level and ALT increase in induction (OR 1.103; p = 0.0001 and OR 1.111; p = 0.0002) and post-induction therapy (OR 1.037; p = 0.039 and OR 1.045; p = 0.011). | NA |
| Almanza-Huante, 2021 [12] | Age group 14–20 years 71.0% PIR vs. 69.6% hyper-CVAD; p = 1.0 | NA | Median OS 27.4 months (95% CI 9.5–45.3) in PIR vs. 15.4 months (8.5–22.3) in hyper-CVAD (p = 0.30) | IRM: 0% PIR vs. 10.1% hyper-CVAD (p = 0.18) | Hyper-CVAD: n= 101 BFM: n = 32 CALGB. N = 22 |
| Age group 21–43 years 84% PIR vs. 57.4% hyper-CVAD p = 0.02 | NA | Median OS 16.9 months (95% CI 13.1–20.6) PIR vs. 9.2 months (95% CI 6–12.5) in hyper-CVAD (p < 0.01) | IRM: 2% PIR vs. 6.9% hyper-CVAD and 2% (p = 0.39) | ||
| Brandwein, 2014 [13] | 17–<34 years: 99% 34–50 years: 87% 50–60 years: 26% (p = 0.02) | NA | 5-year OS (95% CI), univariate: 17–<34 years: 80% (67–88%) 34–50 years: 50% (35–63%) 50–60 years: 62% (42–77%) p = 0.001 Age (cont. variable) as a predictor of OS (p = 0.0046) | 42 months (range 0.3–135 months) | |
| Burke, 2022 [14] | NA | 5-year EFS: 65.4 ± 2.2% for AYA vs. 78.1 ± 0.9% for younger patients (p < 0.0001) Age as a significant predictor of EFS as categorical (<16 vs., >16) and continuous variable in univariate and multivariable analysis (categorical univariate: p < 0.0001; continuious univariate: p < 0.0001; categorical multivariable: p = 0.018; continuous multivariable: p < 0.0001 respectively) | 5-year OS: 77.4 ± 2.0% for AYA vs. 87.3 ± 0.7% for younger patients (p < 0.0001) | IRM 2.2% in AYA versus 1.6% in younger patients (p = 0.366) Toxicity Grade ≥ 3 in induction (AYA vs. younger): Hyperglycemia: 23.6% vs. 15.4% (p < 0.0001) Hyperbilirubinemia: 6.9% vs. 3.7% (p = 0.0007) Febrile neutropenia: 7.4% vs. 13.8% (p < 0.0001) There was no significant difference in thrombosis or pancreatitis. Toxicity Grade ≥ 3 in post-induction (AYA vs. younger): Mucositis: 18.2% vs. 11.7% (p = 0.0002) Peripheral neuropathy: 12.1% vs.7.8% (p = 0.001) Febrile neutropenia: 45.2% vs. 56.8% (p < 0.0001) Hyperbilirubinemia: 17.3% vs. 9.5% (p < 0.0001) Hepatic failure: 1.3% vs. 0.3% (p = 0.009) Deaths in remission: 5.7% vs. 2.4% (p < 0.0001), mostly Grade 5 infections. | NA |
| Cheng, 2022 [15] | NA | 5-year EFS: 64.3 ± 21.0% for 18–25 years vs. 76.2 ± 14.8% for older group; p = 0.265 | NA | NA | 60 months (6–108) |
| DeAngelo, 2015 [16] | NA | 4-year EFS (95% CI) age 18–29 vs. 30–50: 55% (39–69%) vs. 61% (44–74%), p = 0.61 | 4-year OS (95% CI) age 18–29 vs. 30–50: 68% (52–80%) vs. 65% (49–77%), p = 0.93 | NA | 54 (95% CI 49–60) |
| Ganesan, 2021 [17] | NA | 2-year EFS, HR (95% CI) 15–17 years: 56.7%, ref. 18–24 years: 55.9%, 1.01 (0.83–1.23), p = 0.937 25–29 years: 55.4% 1.02 (0.80–1.30); p = 0.862 2-year RFS: 15–17 years: 74.8% 18–24 years: 75.3% 25–29 years: 75.4% p = 0.948 (log-rank) | 2-year OS; HR (95% CI) 15–17 years: 76.6%, ref. 18–24 years: 73.0%; 1.20 (0.91–1.58), p = 0.203 25–29 years: 69.3%; 1.37 (0.99–1.89); p = 0.057 | NA | 23 (95% CI 6–38) |
| Gomez, 2022 [19] | NA | ≥40 years with lower EFS: 8.3 months (95% CI 0–21.2; p = 0.006); no difference in the AYA groups Age as continuous variable HR (95% CI): 1.93 (0.99–1.07) | There was no statistically significant difference in OS between age groups. Age as continuous variable HR (95% CI): 1.03 (0.9–1.07) | Induction deaths: bleeding (n = 4), severe pancreatitis (n = 1), and a sudden unwitnessed event (n = 1) | 18 (1–52.8) |
| Greenwood, 2021 [20] | NA | NA | HR: 0.85 (95% CI 0.36–2.10) for OS (p = 0.751) | 44 (1–96) | |
| Hough, 2016 [23] | NA | 5-year EFS 16–24 years: 72.3% (66.2–78.4) 10–15 years: 83.6% (80.5–86.7) <10 years: 89.8% (88.4–91.2) OR = 2.1 (95% CI: 1.7–2.4), p (trend) < 0.00005, p (10–15 vs. ≥16) = 0.00004 | 5-year OS 16–24 years: 76.4% (70.5–82.3) 10–15 years: 87.5% (84.8–90.2) <10 years: 94.2% (93.2–95.2) OR = 2.7 (2.2–3.4), p (trend) < 0.00005, p (10–15 vs. ≥16) = 0.0004 | 5-year risk of DIR 16–24 years: 6.1% (2.8–9.4) 10–15 years: 3.4% (1.8–5.0) <10 years: 2.1% (1.5–2.7) OR = 2.0 (1.4–3.9), p (trend) = 0.0007 SAE incidence < 10 years vs. 10–24 years 2.58 (95% CI: 2.24–2.95), p < 0.00005 The time to first SAE was significantly shorter, and the cumulative incidence of SAEs was significantly higher in >10 years. | 70 (1–121) |
| Valtis, 2022 [34] | NA | NA | NA | ON 5-year cumulative incidence (95% CI) < 30 years vs. 30–50 years: 21% (95% CI, 16–27) vs. 8% (CI, 4–14); univariate HR 2.77 (95% CI, 1.35–5.65); p = 0.004 ON 5-year cumulative incidence (95% CI) with peg-asparaginase vs. E. coli asparaginase 24% (95% CI, 18–30) vs. 5% (95% CI, 2–10); HR 5.28 (95% CI, 2.24–12.48); p = 0.001 ON 5-year cumulative incidence (95% CI): 15–19 years: 18 (12–25) 20–29 years: 25 (17–36) 30–39 years: 12 (5–23) 40–50 years: 4 (1–11) p = 0.003 (Gray test) | 59 (1–169) |
| Toft, 2018 [32] | NA | 5-year EFS (HR, 95% CI) 1–9 years: 0.89 ± 0.01 (ref.) 10–17 years: 0.80 ± 0.03 (2.0; 1.4–2.8) p < 0.001 18–45 years: 0.74 ± 0.04 (2.8; 2.0–4.0) p < 0.001 | 5-years OS (HR, 95% CI) 1–9 years: 0.94 ± 0.01 (ref.) 10–17 years: 0.87 ± 0.02 (2.3; 1.5–3.5) p < 0.001 18–45 years: 0.78 ± 0.03 (3.8; 2.5–5.7) p < 0.001 | IRM 0.01 in all groups; p = 0.87 Adverse events in 1–9 vs. 10–17 vs. 18–45 years: No sig. difference in ICU14 admission, peripheral paralysis, anaphylactic reaction to ASP, invasive fungal infection, pancreatitis, hyperlipidemia, seizures Thrombosis: 3.6% vs. 15.3% vs.17.5% (p < 0.001) ON: 2.3% vs. 13.4% vs.8.5% (p < 0.001) | 55 (36–77) 1–9 years: 59 10–17 years: 55 18–45 years: 38 |
| Toft, 2016 [33] | NA | NA | NA | Increasing incidence of at least one toxic event (p < 0.0001): 1–9.9 years: 44.5% 10–14 years: 57.6% 15–17 years: 62.3% 18–26 years: 64.0% 27–45 years: 64.2% Toxic events during induction: There was no significant difference in ICU admissions, septic shock, heart failure, anaphylactic reactions, pancreatitis, seizures, coma, VOD, PRES, abdominal surgery, ON, liver or kidney dysfunction, bleeding, or peripheral paralysis. Hyperglycemia was more common >9 years (overall p < 0.0001) and 18–28 years (OR = 11.3 (95% CI: (2.9;43.5); p = 0.0002). Thrombosis was more frequent in 15–17 years (OR 10.2 (2.6;39.1), p = 0.0004) and 18–28 years (OR 7.3 (1.5;31.7), p = 0.007). Toxic events after induction: There was no significant difference in heart failure, pancreatitis, hyperglycemia, abdominal catastrophe, CNS catastrophe/bleeding, anaphylactic reaction, VOD, liver or kidney dysfunction, hypertension, Pneumocystis jiroveci pneumonia, PRES, coma, seizures, peripheral paralysis, or ICU admission. Increasing incidence of ON, thrombosis, and fungal infections with age (p < 0.0001, p < 0.0001, p = 0.006, respectively). OR (95% CI) for thrombosis was 5.4 (2.6–11.0), 5.1 (2.4–10.4), and 5.0 (2.2–10.8) for patients 15–17, 18–26, and 27–45 years, respectively, compared with children 1–9 years (all p < 0.0001). OR (95% CI) for avascular osteonecrosis for patients 10–14, 15–17, 18–26, and 27–45 years 10.4 (4.4–24.9, p < 0.0001), 6.3 (1.9–18.3, p = 0.001), 4.9 (1.3–15.0; p = 0.009), and 6.6 (1.8–21.2, p = 0.003) compared to 1–9 years, respectively. | 40 (12–71) |
| Tantiworawit, 2019 [30] | NA | 3-year OS (≤21 years vs. >21 years): 85% vs. 60%, p = 0.055 | Toxicity (≤21 years vs. >21 years): There was no significant difference in the incidence of allergic reactions to ASP, pancreatitis, elevated liver enzymes or bilirubin, ON, thrombosis, stroke-like events, or neuropathy. Grade 3 hypofibrinogenemia: 10 vs. 21%, p = 0.006 | 40 (4–75) | |
| Rytting, 2016 [29] | NA | NA | 5-year OS ≤ 21 years (PIR vs. adult): 65% vs. 68% | NA | PIR: 66 months (17–107) Adult: 88 (1–152) |
| 5-year OS > 21 years (PIR vs. adult): 57% and 58% Differences between protocols were not statistically significant, and the p-value was not shown. | |||||
| Ribera, 2008 [28] | NA | EFS 15–18 vs. 19–30 years : 60% (95% CI : 43% –77%) vs. 63% (48%–78%), p = 0.97 | OS 15–18 vs. 19–30 years: 77% (95% CI, 63%–91%) vs. 63% (46%–80%) p = 0.44 | Toxicity (15–18 vs. 19–30 years): Grade 1 infections: 2.9 vs. 28%, p = 0.007 Grade 4 neutropenia: 44% vs. 59% Grade 4 thrombocytopenia: 10% vs. 33% Delays during reinduction were significantly more frequent in young adults than in adolescents, p = 0.04 Modifications in L-ASP or VCR were performed in 19% of cycles in adolescents vs. 33% in young adults, p = 0.03 | 50 (24–120) |
| Ribera, 2020 [27] | NA | 5-year EFS 15–18 vs. 19–30 years: 78% (95% CI: 59–89) vs. 49% (31–65%), p = 0.151 | 5 years OS 15–18 vs. 19–30 years: 87% (95% CI: 74%–100%) vs. 63% (46%–80%), p = 0.021 | There were no differences between adolescents and YA in drug modifications and delays | 50 (0.5–114) |
| Quist-Paulsen, 2020 [25] | NA | 5-year EFS (increasing age groups): 0.80 (95% CI: 0.72–0.88, ref.) vs. 0.75 (0.65–0.85) vs. 0.64 (0.52–0.76), p-values not shown | 5-year OS (increasing age groups): 0.82 (95% CI: 0.74–0.88, ref.) vs. 0.76 (0.66–0.86, p = 0.3) vs. 0.65 (0.55–0.75, p = 0.01) | NA | Overall: 71 1–9 years: 76 (48–100) 10–17 years: 71 (53–91) 18–45 years: 68 (56–82) |
| Rank, 2018 [26] | NA | NA | NA | 2.5-year cumulative incidence of any TE 1–9.9 years: 3.7% (2.64–4.8) 10–17.9 years: 15.5% (11.3–19.4) 18–45 years: 18.1% (13.2–22.8) p < 0.0001 he adjusted TE-specific hazard significantly increased in patients aged 6.0 to 14.9 years (HRa, 2.0; 95% CI, 1.2–3.5; p = 0.01), 15.0 to 20.9 years (HRa, 7.74; 95% CI, 4.52–13.2; p < 0.0001), and 21.0 to 45.9 years (HRa, 6.54; 95% CI, 3.69–11.6; p < 0.0001), using 1.0 to 5.9 years as reference. Patients aged 18.0–45.9: increased hazard of PE compared with children younger than 10.0 years (HRa, 11.6, 95% CI: 4.02–33.7; p < 0.0001). Adolescents aged 10.0 to 17.9 years: increased hazard of CSVT compared with children younger than 10.0 years (HRa 3.3, 95% CI: 1.5–7.3; p = 0.003). | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeckanovic, A.; Fuchs, P.; Heesen, P.; Bodmer, N.; Otth, M.; Scheinemann, K. Pediatric-Inspired Regimens in the Treatment of Acute Lymphoblastic Leukemia in Adolescents and Young Adults: A Systematic Review. Curr. Oncol. 2023, 30, 8612-8632. https://doi.org/10.3390/curroncol30090625
Zeckanovic A, Fuchs P, Heesen P, Bodmer N, Otth M, Scheinemann K. Pediatric-Inspired Regimens in the Treatment of Acute Lymphoblastic Leukemia in Adolescents and Young Adults: A Systematic Review. Current Oncology. 2023; 30(9):8612-8632. https://doi.org/10.3390/curroncol30090625
Chicago/Turabian StyleZeckanovic, Aida, Philipp Fuchs, Philip Heesen, Nicole Bodmer, Maria Otth, and Katrin Scheinemann. 2023. "Pediatric-Inspired Regimens in the Treatment of Acute Lymphoblastic Leukemia in Adolescents and Young Adults: A Systematic Review" Current Oncology 30, no. 9: 8612-8632. https://doi.org/10.3390/curroncol30090625
APA StyleZeckanovic, A., Fuchs, P., Heesen, P., Bodmer, N., Otth, M., & Scheinemann, K. (2023). Pediatric-Inspired Regimens in the Treatment of Acute Lymphoblastic Leukemia in Adolescents and Young Adults: A Systematic Review. Current Oncology, 30(9), 8612-8632. https://doi.org/10.3390/curroncol30090625







