Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Population
2.2. Ethics Approval
2.3. Computed Tomography Imaging
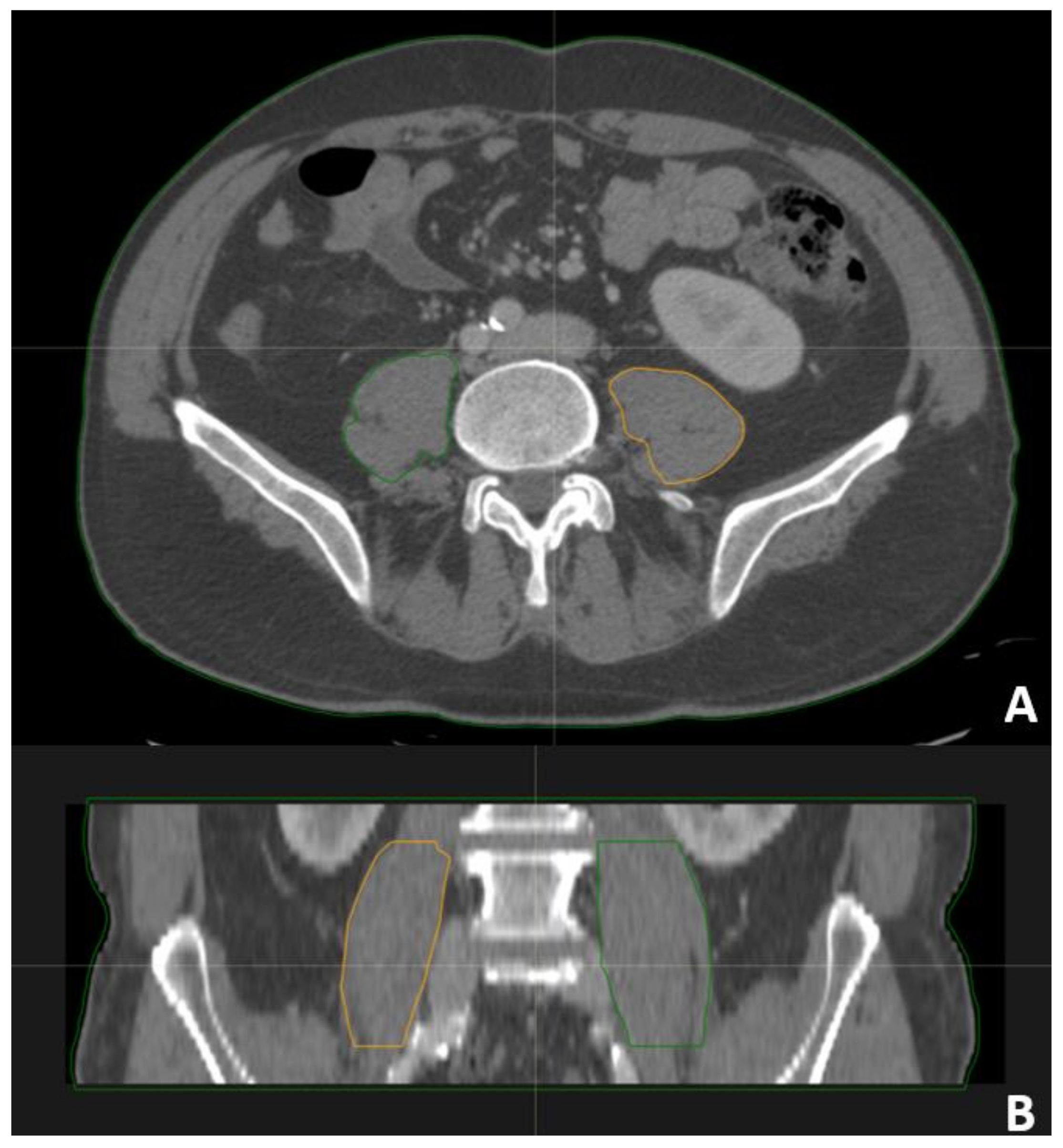
2.4. Psoas Contouring
2.5. Radiotherapy
2.6. Chemotherapy
2.7. Follow-Up
2.8. End Points and Statistical Analysis
3. Results
3.1. Radiotherapy Treatment
3.2. Clinical Outcome
3.3. Reliability of Volumetric Parameters and Cut-Offs
3.4. Factors Predicting bRFS and OS
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falcon, L.J.; Harris-Love, M.O. Sarcopenia and the New ICD-10-CM Code: Screening, Staging, and Diagnosis Considerations. Fed. Pract. 2017, 34, 24–32. [Google Scholar] [PubMed]
- Nakamura, R.; Inage, Y.; Tobita, R.; Yoneyama, S.; Numata, T.; Ota, K.; Yanai, H.; Endo, T.; Inadome, Y.; Sakashita, S.; et al. Sarcopenia in Resected NSCLC: Effect on Postoperative Outcomes. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Recio-Boiles, A.; Galeas, J.N.; Goldwasser, B.; Sanchez, K.; Man, L.M.W.; Gentzler, R.D.; Gildersleeve, J.; Hollen, P.J.; Gralla, R.J. Enhancing evaluation of sarcopenia in patients with non-small cell lung cancer (NSCLC) by assessing skeletal muscle index (SMI) at the first lumbar (L1) level on routine chest computed tomography (CT). Support Care Cancer 2018, 26, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, S.; Liu, S.; Wang, Q.; Che, X.; Wu, G. Frontiers in sarcopenia: Advancements in diagnostics, molecular mechanisms, and therapeutic strategies. Mol. Asp. Med. 2024, 97, 101270. [Google Scholar] [CrossRef]
- He, J.; Luo, W.; Huang, Y.; Song, L.; Mei, Y. Sarcopenia as a prognostic indicator in colorectal cancer: An updated meta-analysis. Front. Oncol. 2023, 13, 1247341. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. Radiol. Med. 2022, 127, 228–237. [Google Scholar] [CrossRef]
- Calvez, V.; Becherucci, G.; Covello, C.; Piccirilli, G.; Mignini, I.; Esposto, G.; Laterza, L.; Ainora, M.E.; Scaldaferri, F.; Gasbarrini, A.; et al. Navigating the Intersection: Sarcopenia and Sarcopenic Obesity in Inflammatory Bowel Disease. Biomedicines 2024, 12, 1218. [Google Scholar] [CrossRef]
- Kara, M.; Kaymak, B.; Frontera, W.; Ata, A.M.; Ricci, V.; Ekiz, T.; Chang, K.V.; Han, D.S.; Michail, X.; Quittan, M.; et al. Diagnosing sarcopenia: Functional perspectives and a new algorithm from the ISarcoPRM. J. Rehabil. Med. 2021, 53, jrm00209. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Tyrovolas, S.; Detopoulou, P.; Tsoumana, D.; Drakaki, M.; Apostolou, T.; Chatziprodromidou, I.P.; Papandreou, D.; Giaginis, C.; Papadopoulou, S.K. Diagnostic Criteria and Measurement Techniques of Sarcopenia: A Critical Evaluation of the Up-to-Date Evidence. Nutrients 2024, 16, 436. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Marino, F.Z.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison With Computed Tomography-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar] [CrossRef]
- Yoon, J.K.; Lee, S.; Kim, K.W.; Lee, J.E.; Hwang, J.A.; Park, T.; Lee, J. Reference Values for Skeletal Muscle Mass at the Third Lumbar Vertebral Level Measured by Computed Tomography in a Healthy Korean Population. Endocrinol. Metab. 2021, 36, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cheng, X.H.; Xu, M.; Zhao, R.; Wan, Q.Y.; Feng, W.H.; Gan, H.T. CT-determined low skeletal muscle index predicts poor prognosis in patients with colorectal cancer. Cancer Med. 2024, 13, e7328. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. Computed tomography-defined low skeletal muscle index and density in cancer patients: Observations from a systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Vogele, D.; Mueller, T.; Wolf, D.; Otto, S.; Manoj, S.; Goetz, M.; Ettrich, T.J.; Beer, M. Applicability of the CT Radiomics of Skeletal Muscle and Machine Learning for the Detection of Sarcopenia and Prognostic Assessment of Disease Progression in Patients with Gastric and Esophageal Tumors. Diagnostics 2024, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, F.; Di Palma, M.; Raynard, B.; Guibal, A.; Cohen, F.; Daidj, N.; Aziza, R.; El Hajjam, M.; Louis, G.; Goldwasser, F.; et al. Psoas muscle index is not representative of skeletal muscle index for evaluating cancer sarcopenia. J. Cachexia Sarcopenia Muscle 2023, 14, 1613–1620. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas 2012, 71, 109–114. [Google Scholar] [CrossRef]
- Ventura, C.; Baldassarre, S.; Cerimele, F.; Pepi, L.; Marconi, E.; Ercolani, P.; Floridi, C.; Argalia, G.; Goteri, G.; Giovagnoni, A. 2D shear wave elastography in evaluation of prognostic factors in breast cancer. Radiol. Med. 2022, 127, 1221–1227. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Costa, M.; Picone, C.; Cozzi, D.; Moroni, C.; La Casella, G.V.; Montanino, A.; Monti, R.; Mazzoni, F.; et al. Preliminary Report on Computed Tomography Radiomics Features as Biomarkers to Immunotherapy Selection in Lung Adenocarcinoma Patients. Cancers 2021, 13, 3992. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Paragliola, F.; Sparano, F.; Iacovino, M.L.; Castrichino, A.; Doria, F.; Sica, A.; et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med. Oncol. 2021, 13, 1758835920949418. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Dunne, R.F.; Giri, S.; Shachar, S.S.; Caan, B.J. Sarcopenia in the Older Adult With Cancer. J. Clin. Oncol. 2021, 39, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mega, S.; Fiore, M.; Carpenito, M.; Novembre, M.L.; Miele, M.; Trodella, L.E.; Grigioni, F.; Ippolito, E.; Ramella, S. Early GLS changes detection after chemoradiation in locally advanced non-small cell lung cancer (NSCLC). Radiol. Med. 2022, 127, 1355–1363. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Miele, V.; Larici, A.R.; Sverzellati, N.; Cappabianca, S.; Brunese, L.; Maggialetti, N.; Borghesi, A.; Fusco, R.; et al. Structured Reporting of Lung Cancer Staging: A Consensus Proposal. Diagnostics 2021, 11, 1569. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Sansone, M.; Monti, R.; Marrone, S.; Fusco, R.; Nardone, V.; Grassi, R.; Reginelli, A. Robustness of Radiomics in Pre-Surgical Computer Tomography of Non-Small-Cell Lung Cancer. J. Pers. Med. 2022, 13, 83. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef]
- Fiorelli, A.; Sagan, D.; Mackiewicz, L.; Cagini, L.; Scarnecchia, E.; Chiodini, P.; Caronia, F.P.; Puma, F.; Santini, M.; Ragusa, M. Incidence, Risk Factors, and Analysis of Survival of Unexpected N2 Disease in Stage I Non-Small Cell Lung Cancer. Thorac Cardiovasc. Surg. 2015, 63, 558–567. [Google Scholar] [CrossRef]
- Nardone, V.; Belfiore, M.P.; De Chiara, M.; De Marco, G.; Patanè, V.; Balestrucci, G.; Buono, M.; Salvarezza, M.; Di Guida, G.; D’Angiolella, D.; et al. CARdioimaging in Lung Cancer PatiEnts Undergoing Radical RadioTherapy: CARE-RT Trial. Diagnostics 2023, 13, 1717. [Google Scholar] [CrossRef]
- Nardone, V.; Nanni, S.; Pastina, P.; Vinciguerra, C.; Cerase, A.; Correale, P.; Guida, C.; Giordano, A.; Tini, P.; Reginelli, A.; et al. Role of perilesional edema and tumor volume in the prognosis of non-small cell lung cancer (NSCLC) undergoing radiosurgery (SRS) for brain metastases. Strahlenther. Onkol. 2019, 195, 734–744. [Google Scholar] [CrossRef]
- Insa, A.; Martín-Martorell, P.; Di Liello, R.; Fasano, M.; Martini, G.; Napolitano, S.; Vicidomini, G.; Cappabianca, S.; Franco, R.; Morgillo, F.; et al. Which treatment after first line therapy in NSCLC patients without genetic alterations in the era of immunotherapy? Crit. Rev. Oncol./Hematol. 2022, 169, 103538. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Xu, L.; Yang, J.; Zhang, Q. Prognostic significance of CT-determined sarcopenia in older patients with advanced squamous cell lung cancer treated with programmed death-1 inhibitors. Sci. Rep. 2024, 14, 12025. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; Tini, P.; Pastina, P.; Botta, C.; Reginelli, A.; Carbone, S.F.; Giannicola, R.; Calabrese, G.; Tebala, C.; Guida, C.; et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol. Lett. 2020, 19, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Lee, H.J.; Kim, J.; Park, K.M. Association between patients with migraine and sarcopenia: A retrospective study. Medicine 2024, 103, e38941. [Google Scholar] [CrossRef]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 361–376. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef]
- Zakaria, H.M.; Basheer, A.; Boyce-Fappiano, D.; Elibe, E.; Schultz, L.; Lee, I.; Siddiqui, F.; Griffith, B.; Chang, V. Application of morphometric analysis to patients with lung cancer metastasis to the spine: A clinical study. Neurosurg. Focus. 2016, 41, E12. [Google Scholar] [CrossRef]
- Swanson, S.; Patterson, R.B. The correlation between the psoas muscle/vertebral body ratio and the severity of peripheral artery disease. Ann. Vasc. Surg. 2015, 29, 520–525. [Google Scholar] [CrossRef]
- Sions, J.M.; Smith, A.C.; Hicks, G.E.; Elliott, J.M. Trunk Muscle Size and Composition Assessment in Older Adults with Chronic Low Back Pain: An Intra-Examiner and Inter-Examiner Reliability Study. Pain Med. 2016, 17, 1436–1446. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Ottaiano, A.; Grassi, F.; Sirica, R.; Genito, E.; Ciani, G.; Patanè, V.; Monti, R.; Belfiore, M.P.; Urraro, F.; Santorsola, M.; et al. Associations between Radiomics and Genomics in Non-Small Cell Lung Cancer Utilizing Computed Tomography and Next-Generation Sequencing: An Exploratory Study. Genes 2024, 15, 803. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Domingos, C.; Monteiro, D.; Morouço, P. A Review on Aging, Sarcopenia, Falls, and Resistance Training in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 874. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yuan, F.; Yang, L.; Guo, H.; Jiang, Y.; Cun, H.; Mou, Z.; Chen, J.; Li, C.; Zhang, Z.; et al. Computed tomography (CT)-based skeletal muscle vertebral-related index to assess low muscle mass in patients with non-small cell lung cancer. Quant Imaging Med. Surg. 2024, 14, 5737–5747. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, P.D.; Crawford, J.; Dunne, R.F.; Roeland, E.J.; Smoyer, K.E.; Siddiqui, M.K.; McRae, T.D.; Rossulek, M.I.; Revkin, J.H.; Tarasenko, L.C. Mortality burden of pre-treatment weight loss in patients with non-small-cell lung cancer: A systematic literature review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1226–1239. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, T.; Guo, Y.; Zheng, C. The impact of sarcopenia on the efficacy of PD-1 inhibitors in non-small cell lung cancer and potential strategies to overcome resistance. Front. Pharmacol. 2024, 15, 1377666. [Google Scholar] [CrossRef]
- Turcott, J.G.; Miyagui, S.M.; Gutiérrez Torres, S.; Cárdenas-Fernández, D.; Caballé-Perez, E.; Rios-Garcia, E.; Cardona, A.F.; Rolfo, C.; Arrieta, O. Sarcopenia as a Predictive Factor for Carboplatin Toxicity in Patients with Advanced Non-Small Cell Lung Cancer. Nutr. Cancer 2024, 1–9. [Google Scholar] [CrossRef]
- Nardone, V.; Correale, P.; Mutti, L.; Desideri, I.; Romeo, C.; Pastina, P.; Tagliaferri, P.; Caraglia, M.; Reginelli, A.; Pirtoli, L.; et al. Comparing Addition of Radiotherapy in EGFR- and ALK-Positive NSCLC with Brain Metastases: Are We Evaluating the Optimal End Point? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, e10–e12. [Google Scholar] [CrossRef]
- Carotti, M.; Salaffi, F.; Beci, G.; Giovagnoni, A. The application of dual-energy computed tomography in the diagnosis of musculoskeletal disorders: A review of current concepts and applications. Radiol. Med. 2019, 124, 1175–1183. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Poliseno, A.C.; Ceccarelli, L.; Farah, S.; Di Carlo, M.; Giovagnoni, A. Quantification of sarcopenia in patients with rheumatoid arthritis by measuring the cross-sectional area of the thigh muscles with magnetic resonance imaging. Radiol. Med. 2023, 128, 578–587. [Google Scholar] [CrossRef]
- Cortellini, A.; Palumbo, P.; Porzio, G.; Verna, L.; Giordano, A.V.; Masciocchi, C.; Parisi, A.; Cannita, K.; Ficorella, C.; Bozzetti, F. Single-institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non-small cell lung cancer patients treated with first-line chemotherapy. Thorac. Cancer 2018, 9, 1623–1630. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, O.S.; Huang, L.W.; Tsang, M.; Torka, P.; Loh, K.P.; Morrison, V.A.; Cordoba, R. Geriatric assessment in older adults with non-Hodgkin lymphoma: A Young International Society of Geriatric Oncology (YSIOG) review paper. J. Geriatr. Oncol. 2022, 13, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Chacon Castro, M.D.C.; Deese, A.R.; Zhang, L. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef] [PubMed]
- Gouran-Savadkoohi, M.; Mesci, A.; Pond, G.R.; Swaminath, A.; Quan, K.; Wright, J.; Tsakiridis, T. Contemporary real-world radiotherapy outcomes of unresected locally advanced non-small cell lung cancer. J. Thorac. Dis. 2023, 15, 423–433. [Google Scholar] [CrossRef]


| Parameter | Number | Percentage |
|---|---|---|
| Sex Male Female | 63 22 | 74.1% 25.9% |
| Age 70–79 ≥80 | 59 26 | 69.5% 30.6% |
| ECOG 0 1 2 3 | 35 15 30 5 | 41.2% 17.6% 35.3% 5.9% |
| Previous Surgery for Primary NSCLC Yes No | 14 71 | 16.5% 83.5% |
| Site of RT Primary NSCLS Bones WBI SRT Other | 9 33 13 27 3 | 10.6% 38.8% 15.3% 31.8% 3.5% |
| Load of Metastases Oligometastatic Disease Plurimetastatic Disease | 47 38 | 55.3% 44.7% |
| Parameter | OS (Median Value) |
|---|---|
| Sex Females Males | p-value: 0.55 10 ± 1.6 months 11 ± 3.3 months |
| Age | p-value: 0.13 HR: 1.03 95% CI: 0.99–1.07 |
| ECOG | p-value: 0.02 HR: 2.31 95% CI: 1.36–3.95 |
| Previous surgery for primary NSCLC Yes No | p-value: 0.011 Median not reached 8 ± 2 months |
| Palliative RT to primary NSCLC Yes No | p-value: 0.030 20 ± 6.2 months 8 ± 2.4 months |
| Primary NSCLC treated (either surgery or palliative RT) Yes No | p-value: 0.001 22 ± 3.5 months 7 ± 1.5 months |
| Load of metastases Oligometastatic disease Plurimetastatic disease | p-value: 0.010 16 ± 6 months 5 ± 1.9 months |
| MA | p-value: 0.001 HR: 0.59 95% CI: 0.44–0.78 |
| Endpoint | Parameter | p-Value | B | HR (95% CI) |
|---|---|---|---|---|
| OS | MA | 0.001 | −0.47 | 0.62 (0.46–0.83) |
| Treatment of primary NSCLC | 0.002 | −1.10 | 0.33 (0.16–0.66) | |
| ECOG | <0.001 | 1.05 | 2.87 (1.64–5.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, V.; Reginelli, A.; Patanè, V.; Sangiovanni, A.; Grassi, R.; Russo, A.; Correale, P.; Giordano, D.S.; Zaccaria, C.; Belfiore, M.P.; et al. Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy. Curr. Oncol. 2024, 31, 6673-6685. https://doi.org/10.3390/curroncol31110492
Nardone V, Reginelli A, Patanè V, Sangiovanni A, Grassi R, Russo A, Correale P, Giordano DS, Zaccaria C, Belfiore MP, et al. Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy. Current Oncology. 2024; 31(11):6673-6685. https://doi.org/10.3390/curroncol31110492
Chicago/Turabian StyleNardone, Valerio, Alfonso Reginelli, Vittorio Patanè, Angelo Sangiovanni, Roberta Grassi, Anna Russo, Pierpaolo Correale, Diego Sandro Giordano, Carmine Zaccaria, Maria Paola Belfiore, and et al. 2024. "Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy" Current Oncology 31, no. 11: 6673-6685. https://doi.org/10.3390/curroncol31110492
APA StyleNardone, V., Reginelli, A., Patanè, V., Sangiovanni, A., Grassi, R., Russo, A., Correale, P., Giordano, D. S., Zaccaria, C., Belfiore, M. P., & Cappabianca, S. (2024). Prognostic Value of Sarcopenia in Elderly Patients with Metastatic Non-Small-Cell Lung Cancer Undergoing Radiotherapy. Current Oncology, 31(11), 6673-6685. https://doi.org/10.3390/curroncol31110492






