The Role of Frailty and Myosteatosis in Predicting All-Cause Mortality in Older Adults with Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
2.2. Data Collection
2.3. Overall Mortality
2.4. Assessment of Frailty and Myosteatosis
2.5. Statistical Analysis
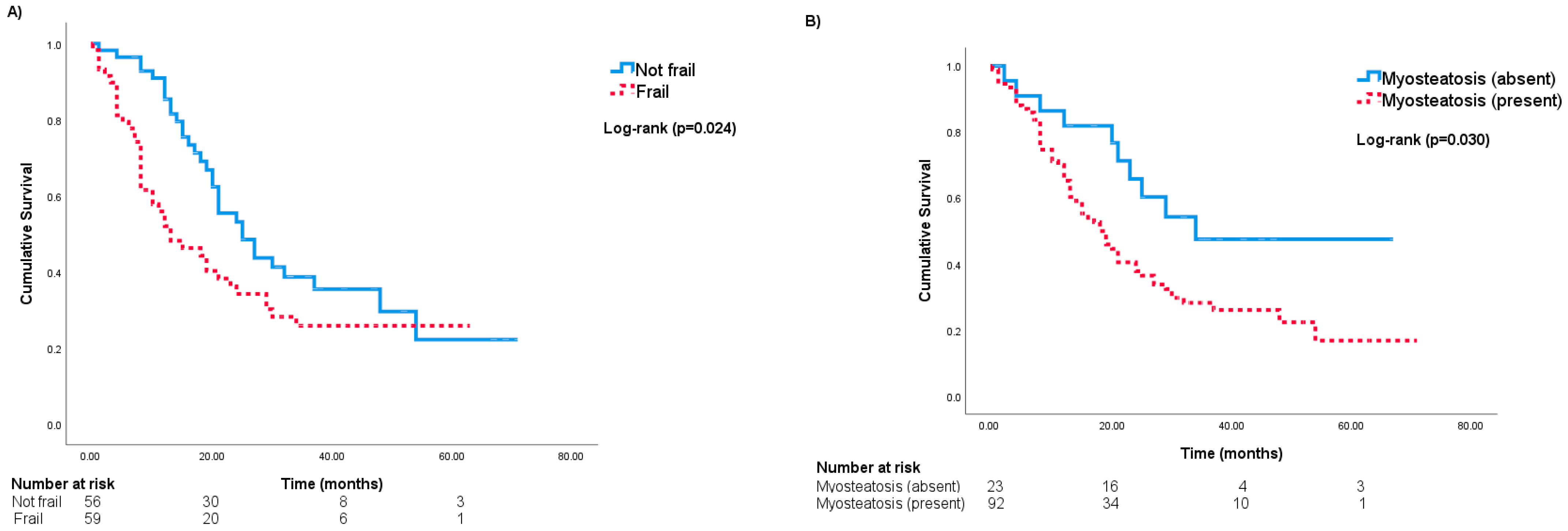
3. Results
Risk of All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Baltussen, J.C.; de Glas, N.A.; van Holstein, Y.; van der Elst, M.; Trompet, S.; Uit den Boogaard, A.; van der Plas-Krijgsman, W.; Labots, G.; Holterhues, C.; van der Bol, J.M.; et al. Chemotherapy-Related Toxic Effects and Quality of Life and Physical Functioning in Older Patients. JAMA Netw. Open 2023, 6, e2339116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Greenhaff, P.L.; Bartlett, D.B.; Jackson, T.A.; Duggal, N.A.; Lord, J.M. Multisystem physiological perspective of human frailty and its modulation by physical activity. Physiol. Rev. 2023, 103, 1137–1191. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Rockwood, K.; Fox, R.A.; Stolee, P.; Robertson, D.; Beattie, B.L. Frailty in elderly people: An evolving concept. Can. Med. Assoc. J. 1994, 150, 489–495. [Google Scholar]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Guerard, E.J.; Deal, A.M.; Chang, Y.; Williams, G.R.; Nyrop, K.A.; Pergolotti, M.; Muss, H.B.; Sanoff, H.K.; Lund, J.L. Frailty Index Developed from a Cancer-Specific Geriatric Assessment and the Association with Mortality Among Older Adults with Cancer. J. Natl. Compr. Cancer Netw. 2017, 15, 894–902. [Google Scholar] [CrossRef]
- Gordon, E.H.; Reid, N.; Khetani, I.S.; Hubbard, R.E. How frail is frail? A systematic scoping review and synthesis of high impact studies. BMC Geriatr. 2021, 21, 719. [Google Scholar] [CrossRef]
- Goede, V.; Neuendorff, N.R.; Schulz, R.J.; Hormigo, A.I.; Martinez-Peromingo, F.J.; Cordoba, R. Frailty assessment in the care of older people with haematological malignancies. Lancet Healthy Longev. 2021, 2, e736–e745. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.; Gonzalez, M.C.; Martucci, R.B.; Rodrigues, V.D.; de Pinho, N.B.; Ponce de Leon, A.; Avesani, C.M. Frailty is associated with myosteatosis in obese patients with colorectal cancer. Clin. Nutr. 2020, 39, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Leeuwenburgh, C.; Lauretani, F.; Onder, G.; Bandinelli, S.; Maraldi, C.; Guralnik, J.M.; Pahor, M.; Ferrucci, L. Frailty syndrome and skeletal muscle: Results from the Invecchiare in Chianti study. Am. J. Clin. Nutr. 2006, 83, 1142–1148. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, J.I.; Blaum, C.; Moore, T.; Xue, Q.L.; Hirsch, C.H.; Walston, J.D.; Fried, L.P. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch. Intern. Med. 2007, 167, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.S.; Kao, T.W.; Chang, P.K.; Chen, W.L.; Peng, P.J.; Wu, L.W. Association between HOMA-IR and Frailty among U.S. Middle-aged and Elderly Population. Sci. Rep. 2019, 9, 4238. [Google Scholar] [CrossRef]
- Kim, M.J.; Cho, Y.K.; Jung, H.N.; Kim, E.H.; Lee, M.J.; Jung, C.H.; Park, J.Y.; Kim, H.K.; Lee, W.J. Association Between Insulin Resistance and Myosteatosis Measured by Abdominal Computed Tomography. J. Clin. Endocrinol. Metab. 2023, 108, 3100–3110. [Google Scholar] [CrossRef]
- Ingram, K.H.; Hill, H.; Moellering, D.R.; Hill, B.G.; Lara-Castro, C.; Newcomer, B.; Brandon, L.J.; Ingalls, C.P.; Penumetcha, M.; Rupp, J.C.; et al. Skeletal muscle lipid peroxidation and insulin resistance in humans. J. Clin. Endocrinol. Metab. 2012, 97, E1182-6. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Xie, J. Circulating interleukin-6 levels and cardiovascular and all-cause mortality in the elderly population: A meta-analysis. Arch. Gerontol. Geriatr. 2017, 73, 257–262. [Google Scholar] [CrossRef]
- Williams, G.R.; Deal, A.M.; Muss, H.B.; Weinberg, M.S.; Sanoff, H.K.; Guerard, E.J.; Nyrop, K.A.; Pergolotti, M.; Shachar, S.S. Frailty and skeletal muscle in older adults with cancer. J. Geriatr. Oncol. 2018, 9, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.; Wong, A.K.O.; Law, S.H.C.; Zhang, L.Z.J.; Breunis, H.; Emmenegger, U.; Alibhai, S.M.H. The impact of sarcopenia on clinical outcomes in men with metastatic castrate-resistant prostate cancer. PLoS ONE 2023, 18, e0286381. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.; Helal, A.A.; Jin, R.; Monginot, S.; Berger, A.; Romanovsky, L.; Alibhai, S.M.H. The impact of pre-treatment muscle strength and physical performance on treatment modification in older adults with cancer following comprehensive geriatric assessment. Age Ageing 2022, 51, afac152. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Helal, A.A.; Jin, R.; Monginot, S.; Berger, A.; Romanovsky, L.; Alibhai, S.M.H. Do clinicians address impairments in muscle strength and physical performance for older adults with cancer? J. Geriatr. Oncol. 2023, 14, 101426. [Google Scholar] [CrossRef]
- Alibhai, S.M.H.; Breunis, H.; Hansen, A.R.; Gregg, R.; Warde, P.; Timilshina, N.; Tomlinson, G.; Joshua, A.M.; Hotte, S.; Fleshner, N.; et al. Examining the ability of the Cancer and Aging Research Group tool to predict toxicity in older men receiving chemotherapy or androgen-receptor-targeted therapy for metastatic castration-resistant prostate cancer. Cancer 2021, 127, 2587–2594. [Google Scholar] [CrossRef]
- Wong, A.K.; Beattie, K.A.; Min, K.K.; Gordon, C.; Pickard, L.; Papaioannou, A.; Adachi, J.D.; Canadian Multicentre Osteoporosis Study Research Group. Peripheral quantitative computed tomography-derived muscle density and peripheral magnetic resonance imaging-derived muscle adiposity: Precision and associations with fragility fractures in women. J. Musculoskelet Neuronal Interact. 2014, 14, 401–410. [Google Scholar]
- Wong, A.K.O.; Szabo, E.; Erlandson, M.; Sussman, M.S.; Duggina, S.; Song, A.; Reitsma, S.; Gillick, H.; Adachi, J.D.; Cheung, A.M. A Valid and Precise Semiautomated Method for Quantifying Intermuscular Fat Intramuscular Fat in Lower Leg Magnetic Resonance Images. J. Clin. Densitom. 2020, 23, 611–622. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; Lastayo, P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef] [PubMed]
- Dondero, K.; Friedman, B.; Rekant, J.; Landers-Ramos, R.; Addison, O. The effects of myosteatosis on skeletal muscle function in older adults. Physiol. Rep. 2024, 12, e16042. [Google Scholar] [CrossRef] [PubMed]
- Biltz, N.K.; Collins, K.H.; Shen, K.C.; Schwartz, K.; Harris, C.A.; Meyer, G.A. Infiltration of intramuscular adipose tissue impairs skeletal muscle contraction. J. Physiol. 2020, 598, 2669–2683. [Google Scholar] [CrossRef]
- Hardin, B.J.; Campbell, K.S.; Smith, J.D.; Arbogast, S.; Smith, J.; Moylan, J.S.; Reid, M.B. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J. Appl. Physiol. 2008, 104, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Cao, H.; Gong, Y.; Wang, Y. The prognostic impact of myosteatosis on overall survival in gynecological cancer patients: A meta-analysis and trial sequential analysis. Int. J. Cancer 2022, 151, 1997–2003. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Henwood, T.R.; Nalls, M.A.; Walker, D.G.; Lang, T.F.; Harris, T.B. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology 2009, 55, 217–223. [Google Scholar] [CrossRef]
- Zoico, E.; Corzato, F.; Bambace, C.; Rossi, A.P.; Micciolo, R.; Cinti, S.; Harris, T.B.; Zamboni, M. Myosteatosis and myofibrosis: Relationship with aging, inflammation and insulin resistance. Arch. Gerontol. Geriatr. 2013, 57, 411–416. [Google Scholar] [CrossRef]
- Miljkovic, I.; Cauley, J.A.; Wang, P.Y.; Holton, K.F.; Lee, C.G.; Sheu, Y.; Barrett-Connor, E.; Hoffman, A.R.; Lewis, C.B.; Orwoll, E.S.; et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 2013, 21, 2118–2125. [Google Scholar] [CrossRef]
- Malietzis, G.; Johns, N.; Al-Hassi, H.O.; Knight, S.C.; Kennedy, R.H.; Fearon, K.C.; Aziz, O.; Jenkins, J.T. Low Muscularity and Myosteatosis is Related to the Host Systemic Inflammatory Response in Patients Undergoing Surgery for Colorectal Cancer. Ann. Surg. 2016, 263, 320–325. [Google Scholar] [CrossRef]
- Kim, D.S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Z.; Shen, Z.L.; Zhang, F.M.; Zhang, X.Z.; Chen, W.H.; Yan, X.L.; Zhuang, C.L.; Chen, X.L.; Yu, Z. Prognostic value of myosteatosis and sarcopenia for elderly patients with colorectal cancer: A large-scale double-center study. Surgery 2022, 172, 1185–1193. [Google Scholar] [CrossRef]
- Mishra, M.; Wu, J.; Kane, A.E.; Howlett, S.E. The intersection of frailty and metabolism. Cell Metab. 2024, 36, 893–911. [Google Scholar] [CrossRef]
- Miljkovic, I.; Vella, C.A.; Allison, M. Computed Tomography-Derived Myosteatosis and Metabolic Disorders. Diabetes Metab. J. 2021, 45, 482–491. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Qasawa, A.H.; Ferrara, P.J.; Malik, A.N.; Funai, K.; McDonagh, B.; Mendias, C.L. Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. FASEB J. 2019, 33, 7863–7881. [Google Scholar] [CrossRef]
- Andreux, P.A.; van Diemen, M.P.J.; Heezen, M.R.; Auwerx, J.; Rinsch, C.; Groeneveld, G.J.; Singh, A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci. Rep. 2018, 8, 8548. [Google Scholar] [CrossRef]
- Kochlik, B.; Franz, K.; Henning, T.; Weber, D.; Wernitz, A.; Herpich, C.; Jannasch, F.; Aykac, V.; Muller-Werdan, U.; Schulze, M.B.; et al. Frailty is characterized by biomarker patterns reflecting inflammation or muscle catabolism in multi-morbid patients. J. Cachexia Sarcopenia Muscle 2023, 14, 157–166. [Google Scholar] [CrossRef]
- Ezzatvar, Y.; Ramirez-Velez, R.; Saez de Asteasu, M.L.; Martinez-Velilla, N.; Zambom-Ferraresi, F.; Izquierdo, M.; Garcia-Hermoso, A. Physical Function and All-Cause Mortality in Older Adults Diagnosed With Cancer: A Systematic Review and Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Beetz, N.L.; Maier, C.; Segger, L.; Shnayien, S.; Trippel, T.D.; Lindow, N.; Bousabarah, K.; Westerhoff, M.; Fehrenbach, U.; Geisel, D. First PACS-integrated artificial intelligence-based software tool for rapid and fully automatic analysis of body composition from CT in clinical routine. JCSM Clin. Rep. 2021, 7, 3–11. [Google Scholar] [CrossRef]
- Just, I.A.; Schoenrath, F.; Roehrich, L.; Heil, E.; Stein, J.; Auer, T.A.; Fehrenbach, U.; Potapov, E.; Solowjowa, N.; Balzer, F.; et al. Artificial intelligence-based analysis of body composition predicts outcome in patients receiving long-term mechanical circulatory support. J. Cachexia Sarcopenia Muscle 2024, 15, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, W.; Gao, Y.; Qin, L.; Feng, H.; Tan, H.; Chen, Q.; Peng, L.; Wu, I.X.Y. Comparative effectiveness of non-pharmacological interventions for frailty: A systematic review and network meta-analysis. Age Ageing 2023, 52, afad004. [Google Scholar] [CrossRef]
- Ramirez-Velez, R.; Ezzatvar, Y.; Izquierdo, M.; Garcia-Hermoso, A. Effect of exercise on myosteatosis in adults: A systematic review and meta-analysis. J. Appl. Physiol. 2021, 130, 245–255. [Google Scholar] [CrossRef]
| Variable | Frail (n = 59) | Non-Frail (n = 56) | p | Missing Data (%) |
|---|---|---|---|---|
| Age (years), mean (SD) | 78.1 (7.0) | 76.1 (5.9) | 0.10 | 0 (0) |
| Sex (males), n (%) | 43 (72.9) | 39 (69.6) | 0.70 | 0 (0) |
| Treatment intent (palliative), n (%) | 41 (69.5) | 37 (66.1) | 0.69 | 0 (0) |
| Disease site, n (%) | 0.41 | 0 (0) | ||
| Genitourinary | 33 (55.9) | 29 (51.8) | ||
| Gastrointestinal | 6 (10.2) | 11 (19.6) | ||
| Gynecological | 7 (11.9) | 8 (14.3) | ||
| Lymphoma | 13 (22.0) | 8 (14.3) | ||
| Disease stage, n (%) | 0.27 | 0 (0) | ||
| Localized | 4 (6.8) | 7 (12.5) | ||
| Locally advanced | 3 (5.1) | 7 (12.5) | ||
| Metastatic | 39 (66.1) | 34 (60.7) | ||
| Hematologic | 13 (22.0) | 8 (14.3) | ||
| Chemotherapy agent(s) | 0.41 | 0 (0) | ||
| Alkylating | 7 (11.9) | 7 (12.5) | ||
| Alkylating and monoclonal antibody | 10 (16.9) | 7 (12.5) | ||
| Alkylating and taxanes | 4 (6.8) | 4 (7.1) | ||
| Antimetabolites | 5 (8.5) | 2 (3.6) | ||
| Antimetabolites and alkylating | 4 (6.8) | 9 (16.1) | ||
| Antimetabolite and monoclonal antibody | 0 (0) | 1 (1.8) | ||
| Antimetabolite and taxanes | 0 (0) | 2 (3.6) | ||
| Taxanes | 29 (49.2) | 24 (42.9) | ||
| Body mass index, mean (SD) | 27.8 (4.5) | 25.6 (5.4) | 0.021 | 0 (0) |
| Comorbidities | 0 (0) | |||
| Anxiety, n (%) | 6 (10.2) | 2 (3.6) | 0.16 | |
| Arrythmias, n (%) | 10 (16.9) | 1 (1.8) | 0.006 | |
| Arthritis, n (%) | 29 (49.2) | 13 (23.2) | 0.004 | |
| COPD, n (%) | 6 (10.2) | 5 (8.9) | 0.82 | |
| Coronary artery disease, n (%) | 12 (20.3) | 4 (7.1) | 0.041 | |
| Congestive heart failure, n (%) | 4 (6.8) | 0 (0) | 0.047 | |
| Depression, n (%) | 5 (8.5) | 1 (1.8) | 0.10 | |
| Diabetes, n (%) | 22 (37.3) | 5 (8.9) | <0.001 | |
| Hearing impairment, n (%) | 11 (18.6) | 4 (7.1) | 0.067 | |
| Hyperlipidemia, n (%) | 25 (42.4) | 21 (37.5) | 0.59 | |
| Hypertension, n (%) | 42 (71.2) | 24 (42.9) | 0.002 | |
| Visual impairment, n (%) | 9 (15.3) | 2 (3.6) | 0.033 | |
| Osteoporosis, n (%) | 13 (22.0) | 7 (12.5) | 0.18 | |
| Valvular disease, n (%) | 3 (5.1) | 1 (1.8) | 0.33 | |
| Dependent in one or more IADLs, n (%) | 41 (69.5) | 20 (35.7) | <0.001 | 0 (0) |
| VES-13 (≥3), n (%) | 42 (71.2) | 23 (41.1) | 0.001 | 0 (0) |
| Cognitive impairment a, n (%) | 30 (51.7) | 18 (32.1) | 0.034 | 1 (0.9) |
| Albumin (g/L), mean (SD) | 37.2 (3.2) | 39.7 (2.8) | <0.001 | 23 (20.0) |
| Alkaline phosphatase (u/L), Median (IQR) | 101.5 (76.0–165.75) | 82.5 (66.0–107.5) | 0.11 | 1 (0.9) |
| Hemoglobin (g/L), mean (SD) | 109.7 (19.9) | 123.1 (15.2) | <0.001 | 1 (0.9) |
| Lactate dehydrogenase (u/L), mean (SD) | 312.1 (121.8) | 233.3 (62.8) | <0.001 | 1 (0.9) |
| Neutrophil-to-lymphocyte ratio (>3), n (%) | 5.8 (4.7) | 3.3 (1.9) | <0.001 | 1 (0.9) |
| Low grip strength per EWGSOP2, n (%) | 32 (54.2) | 8 (14.3) | <0.001 | 0 (0) |
| Low physical performance b n (%) | 33 (55.9) | 13 (23.2) | <0.001 | 0 (0) |
| Skeletal muscle density (HU), mean (SD) | 26.4 (8.8) | 29.2 (10.9) | 0.066 | 0 (0) |
| Myosteatosis (present), n (%) | 48 (81.4) | 44 (78.6) | 0.71 | 0 (0) |
| Variable | Univariable HR (95%CI) | p | MV#1 c aHR (95%CI) | p | MV#2 d aHR (95%CI) | p | MV#3 e aHR (95%CI) | p |
|---|---|---|---|---|---|---|---|---|
| Age per 10-year increase | 1.18 (0.83–1.67) | 0.35 | 1.35 (0.96–1.90) | 0.085 | 1.35 (0.95–1.91) | 0.095 | 1.23 (0.87–1.73) | 0.23 |
| Sex (males) | 1.53 (0.86–2.71) | 0.14 | 1.26 (0.69–2.30) | 0.46 | 1.08 (0.58–2.03) | 0.81 | 1.06 (0.57–1.99) | 0.85 |
| Body mass index kg/m2 | 0.97 (0.92–1.02) | 0.21 | 0.98 (0.93–1.02) | 0.30 | 0.97 (0.92–1.01) | 0.16 | ||
| Treatment intent (palliative) | 2.28 (1.27–4.10) | 0.006 | 2.53 (1.35–4.74) | 0.004 | 2.84 (1.49–5.40) | 0.001 | 2.85 (1.49–5.45) | 0.002 |
| Frail (>0.25) | 1.69 (1.06–2.69) a | 0.028 | 1.68 (1.03–2.72) | 0.037 | Not included | 1.81 (1.11–2.94) | 0.018 | |
| Non-frail (≤0.25) | ref. | ref. | ref. | ref. | ||||
| Myosteatosis (present) | 2.04 (1.05–3.99) b | 0.036 | Not included | 2.14 (1.07–4.30) | 0.032 | 2.33 (1.16–4.69) | 0.018 | |
| Myosteatosis (absent) | ref. | ref. | ref. | ref. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, E.; Wong, A.K.O.; Law, S.H.C.; Costa, S.; Cheung, A.M.; Rozenberg, D.; Alibhai, S.M.H. The Role of Frailty and Myosteatosis in Predicting All-Cause Mortality in Older Adults with Cancer. Curr. Oncol. 2024, 31, 7852-7862. https://doi.org/10.3390/curroncol31120578
Papadopoulos E, Wong AKO, Law SHC, Costa S, Cheung AM, Rozenberg D, Alibhai SMH. The Role of Frailty and Myosteatosis in Predicting All-Cause Mortality in Older Adults with Cancer. Current Oncology. 2024; 31(12):7852-7862. https://doi.org/10.3390/curroncol31120578
Chicago/Turabian StylePapadopoulos, Efthymios, Andy Kin On Wong, Sharon Hiu Ching Law, Sarah Costa, Angela M. Cheung, Dmitry Rozenberg, and Shabbir M. H. Alibhai. 2024. "The Role of Frailty and Myosteatosis in Predicting All-Cause Mortality in Older Adults with Cancer" Current Oncology 31, no. 12: 7852-7862. https://doi.org/10.3390/curroncol31120578
APA StylePapadopoulos, E., Wong, A. K. O., Law, S. H. C., Costa, S., Cheung, A. M., Rozenberg, D., & Alibhai, S. M. H. (2024). The Role of Frailty and Myosteatosis in Predicting All-Cause Mortality in Older Adults with Cancer. Current Oncology, 31(12), 7852-7862. https://doi.org/10.3390/curroncol31120578





