Unlocking the Potential of Therapy-Induced Cytokine Responses: Illuminating New Pathways in Cancer Precision Medicine
Abstract
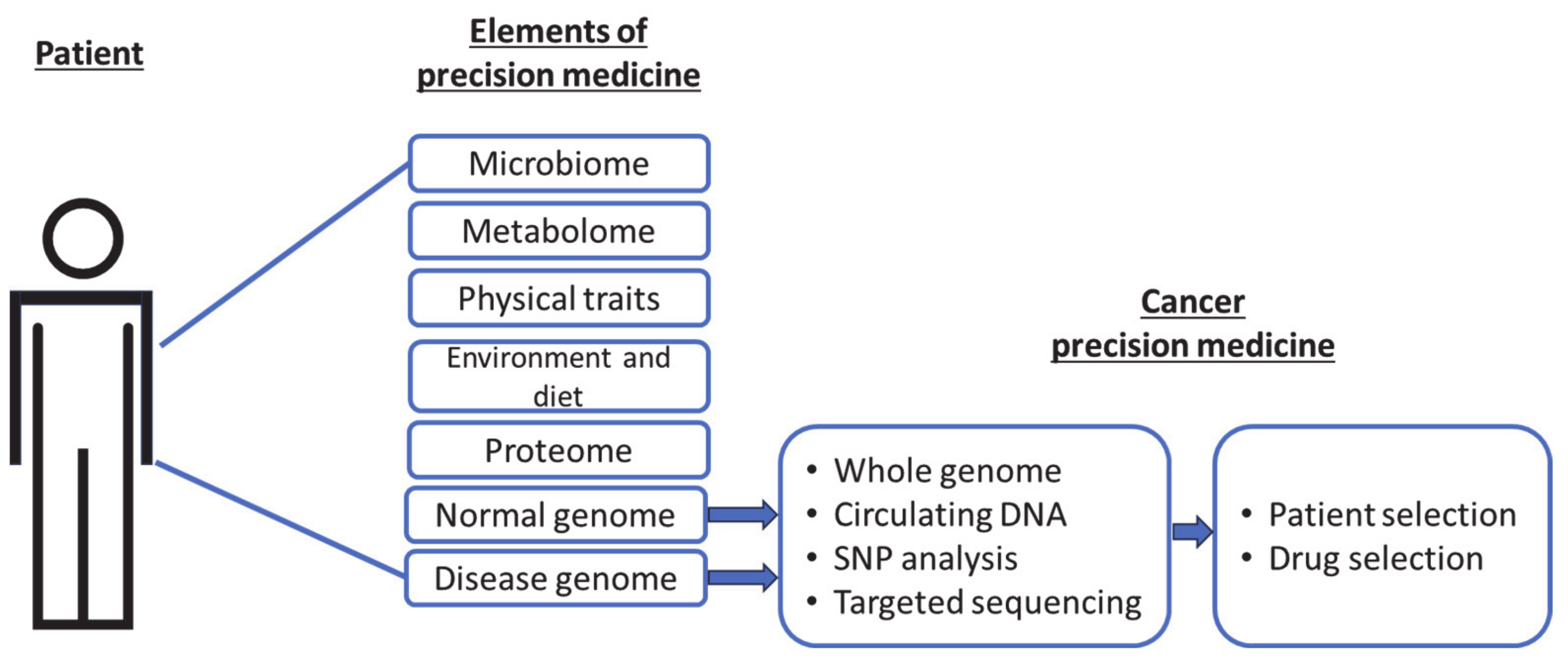
:1. Precision Cancer Medicine
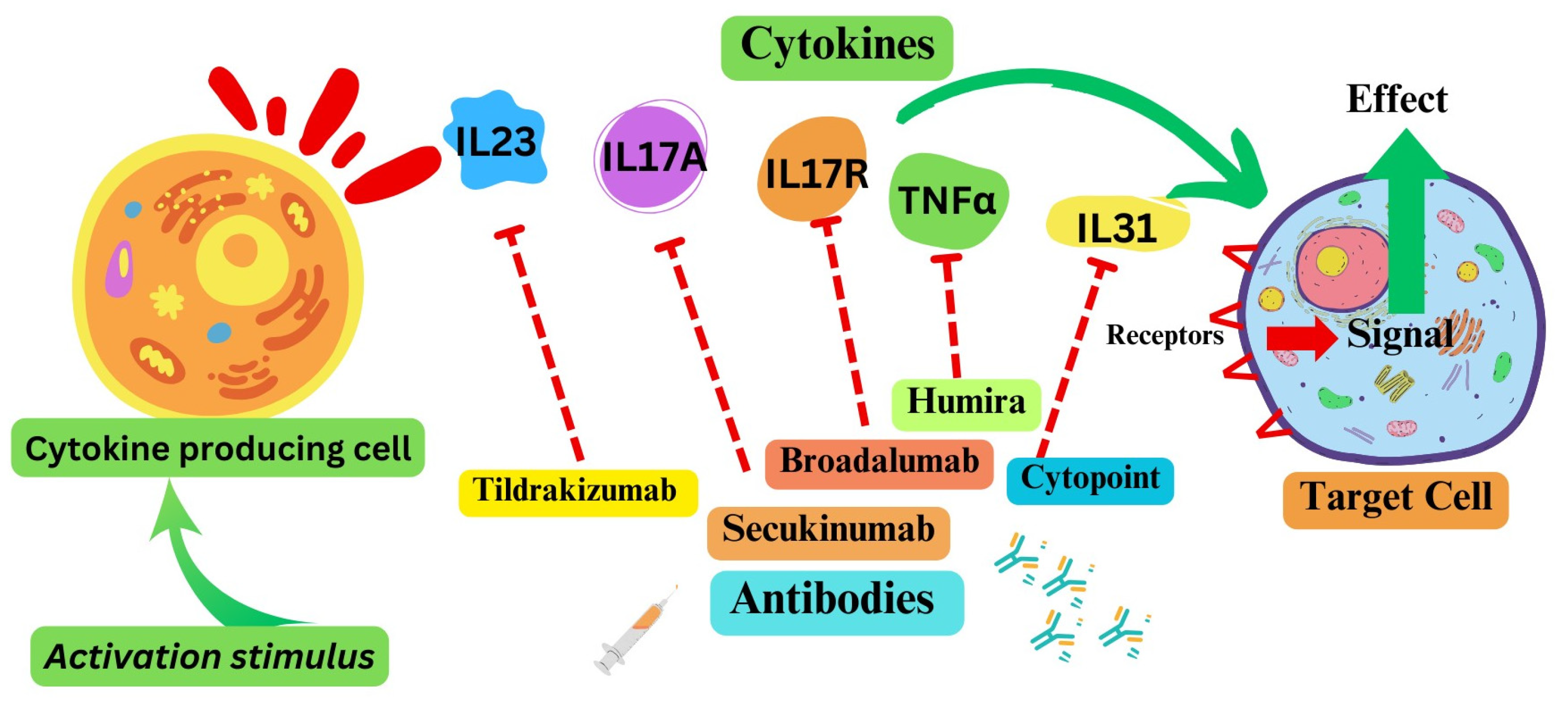
2. Cytokine Pathways as Therapeutic Targets
3. Cells of Cytokine Origin
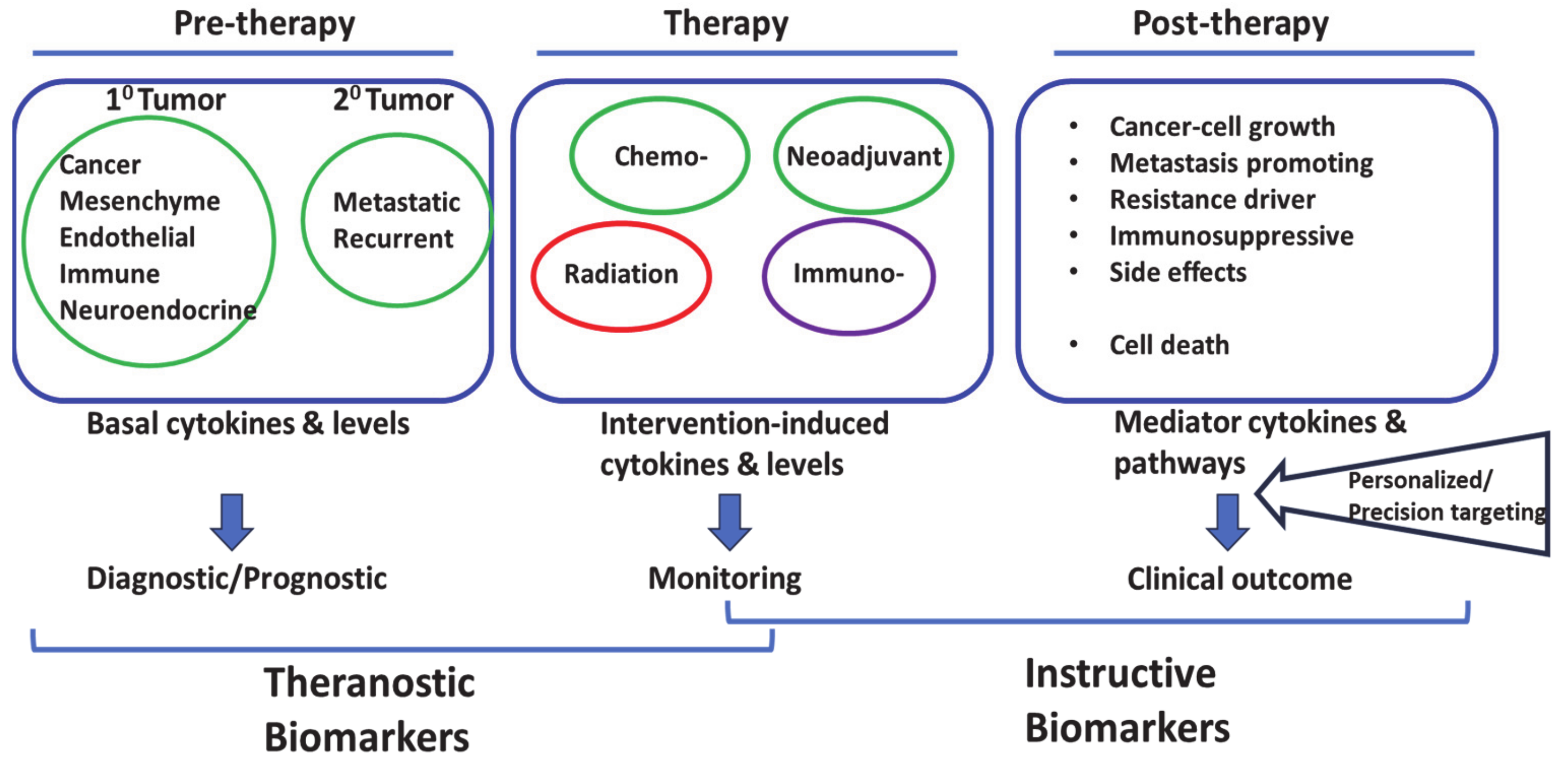
4. Cytokine Responses in Solid-Tumors Therapy: An Emerging Theme in Cancer Precision Medicine
5. Cytokine Responses to Chemotherapy and Radiation
6. Role of Cytokines Post Chemo- or Radiotherapy
7. Challenges in Cytokine-Directed Precision Approach
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef]
- Sadée, W.; Dai, Z. Pharmacogenetics/genomics and personalized medicine. Hum. Mol. Genet. 2005, 14, R207–R214. [Google Scholar] [CrossRef]
- Agúndez, J.A.G.; García-Martín, E. Editorial: Insights in Pharmacogenetics and Pharmacogenomics: 2021. Front. Pharmacol. 2022, 13, 907131. [Google Scholar] [CrossRef]
- Wilke, R.A.; Reif, D.M.; Moore, J.H. Combinatorial Pharmacogenetics. Nat. Rev. Drug Discov. 2005, 4, 911–918. [Google Scholar] [CrossRef]
- Shastry, B.S. Pharmacogenetics and the concept of individualized medicine. Pharmacogenomics J. 2005, 6, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Warner, J. Giving Up on Precision Oncology? Not So Fast! Clin. Transl. Sci. 2017, 10, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Miossec, P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Agüero, R.; Woodbury, M.J.; Lee, K.; Johnsson, H.J.; Merola, J.F.; Armstrong, A.W. Interleukin (IL)-17 Versus IL-23 Inhibitors: Which Is Better to Treat Patients with Moderate-to-Severe Psoriasis and Mild Psoriatic Arthritis in Dermatology Clinics? J. Rheumatol. 2023, 50, 11–13. [Google Scholar] [CrossRef]
- Moyaert, H.; Van Brussel, L.; Borowski, S.; Escalada, M.; Mahabir, S.P.; Walters, R.R.; Stegemann, M.R. A blinded, randomized clinical trial evaluating the efficacy and safety of lokivetmab compared to ciclosporin in client-owned dogs with atopic dermatitis. Vet. Dermatol. 2017, 28, 593-e145. [Google Scholar] [CrossRef]
- Maini, R.N.; Taylor, P.C.; Szechinski, J.; Pavelka, K.; Bröll, J.; Balint, G.; Emery, P.; Raemen, F.; Petersen, J.; Smolen, J.; et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006, 54, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Pascual, I.; Berlana, D.; Martinez-Valle, F.; Campany-Herrero, D.; Montoro-Ronsano, J.B. Effect of tocilizumab versus standard of care in adults hospitalized with moderate-severe COVID-19 pneumonia. Med. Clin. (Engl. Ed.) 2022, 158, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Amoabeng, D.; Kanji, Z.; Ford, B.; Beutler, B.D.; Riddle, M.S.; Siddiqui, F. Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review. J. Med. Virol. 2020, 92, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- WHO. A living WHO guideline on drugs to prevent COVID-19. Br. Med. J. 2021, 372, n526. [Google Scholar]
- West, W. Continuous infusion recombinant interleukin-2 (rIL-2) in adoptive cellular therapy of renal carcinoma and other malignancies. Cancer Treat. Rev. 1989, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Sayana, S.; Khanlou, H. Maraviroc: A new CCR5 antagonist. Expert Rev. Anti-Infect. Ther. 2009, 7, 9–19. [Google Scholar] [CrossRef]
- Ndegwa, S. Maraviroc (Celsentri) for multidrug-resistant human immunodeficiency virus (HIV)-1. Issues Emerg. Health Technol. 2007, 110, 1–8. [Google Scholar]
- Rallis, K.S.; Corrigan, A.E.; Dadah, H.; George, A.M.; Keshwara, S.M.; Sideris, M.; Szabados, B. Cytokine-based Cancer Immunotherapy: Challenges and Opportunities for IL-10. Anticancer. Res. 2021, 41, 3247–3252. [Google Scholar] [CrossRef]
- Roufas, C.; Chasiotis, D.; Makris, A.; Efstathiades, C.; Dimopoulos, C.; Zaravinos, A. The Expression and Prognostic Impact of Immune Cytolytic Activity-Related Markers in Human Malignancies: A Comprehensive Meta-analysis. Front. Oncol. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Song, Y.; Li, W.; Xu, L. Targeting macrophages: A novel treatment strategy in solid tumors. J. Transl. Med. 2022, 20, 586. [Google Scholar] [CrossRef]
- Choudhry, H.; Helmi, N.; Abdulaal, W.H.; Zeyadi, M.; Zamzami, M.A.; Wu, W.; Mahmoud, M.M.; Warsi, M.K.; Rasool, M.; Jamal, M.S. Prospects of IL-2 in Cancer Immunotherapy. BioMed Res. Int. 2018, 2018, 9056173. [Google Scholar] [CrossRef]
- Yui, M.A.; Sharp, L.L.; Havran, W.L.; Rothenberg, E.V. Preferential Activation of an IL-2 Regulatory Sequence Transgene in TCRγδ and NKT Cells: Subset-Specific Differences in IL-2 Regulation. J. Immunol. 2004, 172, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
- Paliard, X.; Malefijt, R.d.W.; Yssel, H.; Blanchard, D.; Chrétien, I.; Abrams, J.; de Vries, J.; Spits, H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J. Immunol. 1988, 141, 849–855. [Google Scholar] [CrossRef]
- Hershko, A.Y.; Suzuki, R.; Charles, N.; Alvarez-Errico, D.; Sargent, J.L.; Laurence, A.; Rivera, J. Mast Cell Interleukin-2 Production Contributes to Suppression of Chronic Allergic Dermatitis. Immunity 2011, 35, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Granucci, F.; Vizzardelli, C.; Pavelka, N.; Feau, S.; Persico, M.; Virzi, E.; Rescigno, M.; Moro, G.; Ricciardi-Castagnoli, P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2001, 2, 882–888. [Google Scholar] [CrossRef]
- Hinrichs, C.S.; Spolski, R.; Paulos, C.M.; Gattinoni, L.; Kerstann, K.W.; Palmer, D.C.; Klebanoff, C.A.; Rosenberg, S.A.; Leonard, W.J.; Restifo, N.P. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008, 111, 5326–5333. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Li, S.; Kotlabova, K.; Dickinson, A.M. Influence of In Vitro IL-2 or IL-15 Alone or in Combination with Hsp 70 Derived 14-Mer Peptide (TKD) on the Expression of NK Cell Activatory and Inhibitory Receptors on Peripheral Blood T Cells, B Cells and NKT Cells. PLoS ONE 2016, 11, e0151535. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef]
- Deng, H.; Liu, R.; Ellmeier, W.; Choe, S.; Unutmaz, D.; Burkhart, M.; Marzio, P.D.; Marmon, S.; Sutton, R.E.; Hill, C.M.; et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996, 381, 661–666. [Google Scholar] [CrossRef]
- Simmons, G.; Wilkinson, D.; Reeves, J.D.; Dittmar, M.T.; Beddows, S.; Weber, J.; Carnegie, G.; Desselberger, U.; Gray, P.W.; Weiss, R.A.; et al. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 1996, 70, 8355–8360. [Google Scholar] [CrossRef]
- Wu, L.; Gerard, N.P.; Wyatt, R.; Choe, H.; Parolin, C.; Ruffing, N.; Borsetti, A.; Cardoso, A.A.; Desjardin, E.; Newman, W.; et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 1996, 384, 179–183. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Riedesser, J.E.; Ebert, M.P.; Betge, J. Precision medicine for metastatic colorectal cancer in clinical practice. Ther. Adv. Med. Oncol. 2022, 14, 17588359211072703. [Google Scholar] [CrossRef]
- Ganesh, K. Optimizing immunotherapy for colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 19, 93–94. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, L.; Liang, D.; Chen, H.; Ji, S.; Zhang, G.; Yang, K.; Hu, Y.; Mao, B.; Liu, T.; et al. Identification of immune checkpoint and cytokine signatures associated with the response to immune checkpoint blockade in gastrointestinal cancers. Cancer Immunol. Immunother. 2021, 70, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Pomati, G.; Cirillo, A.; Scagnoli, S.; Pisegna, S.; Chiavassa, A.; Rossi, E.; Schinzari, G.; Tortora, G.; Di Pietro, F.R.; et al. The role of immune profile in predicting outcomes in cancer patients treated with immunotherapy. Front. Immunol. 2022, 13, 974087. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Yoon, H.K. Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine 2020, 138, 155363. [Google Scholar] [CrossRef] [PubMed]
- Achyut, B.R.; Yang, L. Transforming Growth Factor-β in the Gastrointestinal and Hepatic Tumor Microenvironment. Gastroenterology 2011, 141, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Desoteux, M.; Maillot, B.; Bévant, K.; Ferlier, T.; Leroux, R.; Angenard, G.; Louis, C.; Sulpice, L.; Boudjema, K.; Coulouarn, C. Transcriptomic evidence for tumor-specific beneficial or adverse effects of TGFβ pathway inhibition on the prognosis of patients with liver cancer. FEBS Open Bio 2023, 13, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- DeMaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Petit, C.; Toutain, B.; Guette, C.; Lelièvre, E.; Coqueret, O. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity. Cell Cycle 2019, 18, 2385–2397. [Google Scholar] [CrossRef]
- Sriram, G.; Milling, L.E.; Chen, J.-K.; Kong, Y.W.; Joughin, B.A.; Abraham, W.; Swartwout, S.; Handly, E.D.; Irvine, D.J.; Yaffe, M.B. The injury response to DNA damage in live tumor cells promotes antitumor immunity. Sci. Signal. 2021, 14, eabc4764. [Google Scholar] [CrossRef]
- Lotti, F.; Jarrar, A.M.; Pai, R.K.; Hitomi, M.; Lathia, J.; Mace, A.; Gantt, G.A., Jr.; Sukhdeo, K.; DeVecchio, J.; Vasanji, A.; et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013, 210, 2851–2872. [Google Scholar] [CrossRef]
- Reers, S.; Pfannerstill, A.C.; Rades, D.; Maushagen, R.; Andratschke, M.; Pries, R.; Wollenberg, B. Cytokine changes in response to radio-/chemotherapeutic treatment in head and neck cancer. Anticancer. Res. 2013, 33, 2481–2489. [Google Scholar]
- Edwardson, D.W.; Parissenti, A.M.; Kovala, A.T. Chemotherapy and Inflammatory Cytokine Signalling in Cancer Cells and the Tumour Microenvironment. Adv. Exp. Med. Biol. 2019, 1152, 173–215. [Google Scholar] [CrossRef]
- Van der Sijde, F.; Dik, W.A.; Mustafa, D.A.M.; Vietsch, E.E.; Besselink, M.G.; Debets, R.; Koerkamp, B.G.; Haberkorn, B.C.M.; Homs, M.Y.V.; Janssen, Q.P.; et al. Serum cytokine levels are associated with tumor progression during FOLFIRINOX chemotherapy and overall survival in pancreatic cancer patients. Front. Immunol. 2022, 13, 898498. [Google Scholar] [CrossRef]
- Gu, C.; Xiong, X.; Liu, W. Prognostic Significance of the CXCLs and Its Impact on the Immune Microenvironment in Ovarian Cancer. Dis. Markers 2023, 2023, 5223657. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y. Comprehensive Analysis and Identification of an Immune-Related Gene Signature with Prognostic Value for Prostate Cancer. Int. J. Gen. Med. 2021, 14, 2931–2942. [Google Scholar] [CrossRef]
- Hincu, M.-A.; Zonda, G.-I.; Stanciu, G.D.; Nemescu, D.; Paduraru, L. Relevance of Biomarkers Currently in Use or Research for Practical Diagnosis Approach of Neonatal Early-Onset Sepsis. Children 2020, 7, 309. [Google Scholar] [CrossRef]
- Pernot, S.; Evrard, S.; Khatib, A.-M. The Give-and-Take Interaction Between the Tumor Microenvironment and Immune Cells Regulating Tumor Progression and Repression. Front. Immunol. 2022, 13, 850856. [Google Scholar] [CrossRef]
- Groysman, L.; Carlsen, L.; Huntington, K.E.; Shen, W.H.; Zhou, L.; El-Deiry, W.S. Chemotherapy-induced cytokines and prognostic gene signatures vary across breast and colorectal cancer. Am. J. Cancer Res. 2021, 11, 6086–6106. [Google Scholar]
- Carlsen, L.; Schorl, C.; Huntington, K.; Hernandez-Borrero, L.; Jhaveri, A.; Zhang, S.; Zhou, L.; El-Deiry, W.S. Pan-drug and drug-specific mechanisms of 5-FU, irinotecan (CPT-11), oxaliplatin, and cisplatin identified by comparison of transcriptomic and cytokine responses of colorectal cancer cells. Oncotarget 2021, 12, 2006–2021. [Google Scholar] [CrossRef]
- Bedi, D.; Henderson, H.J.; Manne, U.; Samuel, T. Camptothecin Induces PD-L1 and Immunomodulatory Cytokines in Colon Cancer Cells. Medicines 2019, 6, 51. [Google Scholar] [CrossRef]
- Bravatà, V.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Russo, G.; Di Maggio, F.M.; Augello, G.; Lio, D.; Gilardi, M.C. Cytokine profile of breast cell lines after different radiation doses. Int. J. Radiat. Biol. 2017, 93, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, L.; Yu, W.; Wu, Q.; Lian, J.; Li, F.; Liu, S.; Li, A.; He, Z.; Liu, J.; et al. Colorectal cancer cell-derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF-κB signaling. J. Immunother. Cancer 2019, 7, 215. [Google Scholar] [CrossRef]
- Stojanovska, V.; Prakash, M.; McQuade, R.; Fraser, S.; Apostolopoulos, V.; Sakkal, S.; Nurgali, K. Oxaliplatin Treatment Alters Systemic Immune Responses. BioMed Res. Int. 2019, 2019, 4650695. [Google Scholar] [CrossRef]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remédios, C.; et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in Radiobiological Responses: A Review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef]
- Palata, O.; Podzimkova, N.H.; Nedvedova, E.; Umprecht, A.; Sadilkova, L.; Jelinkova, L.P.; Spisek, R.; Adkins, I. Radiotherapy in Combination with Cytokine Treatment. Front. Oncol. 2019, 9, 367. [Google Scholar] [CrossRef]
- Tada, N.; Tsuno, N.H.; Kawai, K.; Murono, K.; Nirei, T.; Ishihara, S.; Sunami, E.; Kitayama, J.; Watanabe, T. Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients. Oncol. Rep. 2013, 31, 463–471. [Google Scholar] [CrossRef]
- Sato, M.; Kasai, C.; Takeuchi, S.; Takemura, M.; Shimokawa, K.; Noma, A. Changes in serum cytokine levels in patients with malignant bone and soft tissue tumors in the course of chemotherapy. Gan To Kagaku Ryoho 1992, 19 (Suppl. 10), 1449–1452. [Google Scholar]
- Kim, M.J.; Jang, J.W.; Oh, B.S.; Kwon, J.H.; Chung, K.W.; Jung, H.S.; Jekarl, D.W.; Lee, S. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine 2013, 64, 516–522. [Google Scholar] [CrossRef]
- Deniz, D.; Gürbilek, M.; Koç, M. Prognostic value of interferon-gamma, interleukin-6, and tumor necrosis factor-alpha in the radiation response of patients diagnosed with locally advanced non-small-cell lung cancer and glioblastoma multiforme. Turk. J. Med. Sci. 2018, 48, 117–123. [Google Scholar] [CrossRef]
- Xiao, M. The Role of Proinflammatory Cytokine Interleukin-18 in Radiation Injury. Heal. Phys. 2016, 111, 212–217. [Google Scholar] [CrossRef]
- Ha, C.T.; Li, X.-H.; Fu, D.; Moroni, M.; Fisher, C.; Arnott, R.; Srinivasan, V.; Xiao, M. Circulating Interleukin-18 as a Biomarker of Total-Body Radiation Exposure in Mice, Minipigs, and Nonhuman Primates (NHP). PLoS ONE 2014, 9, e109249. [Google Scholar] [CrossRef]
- Elgström, E.; Ohlsson, T.G.; E Eriksson, S. Cytokine evaluation in untreated and radioimmunotherapy-treated tumors in an immunocompetent rat model. Tumor Biol. 2017, 39, 1010428317697550. [Google Scholar] [CrossRef]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.-X.; Auh, S.L. The Efficacy of Radiotherapy Relies upon Induction of Type I Interferon–Dependent Innate and Adaptive Immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef]
- Hsu, L.-H.; Soong, T.C.; Chu, N.-M.; Huang, C.-Y.; Kao, S.-H.; Lin, Y.-F. The Inflammatory Cytokine Profile of Patients with Malignant Pleural Effusion Treated with Pleurodesis. J. Clin. Med. 2020, 9, 4010. [Google Scholar] [CrossRef]
- Chiamulera, M.M.A.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef]
- Liu, J.; Wan, M.; Lyon, C.J.; Hu, T.Y. Nanomedicine therapies modulating Macrophage Dysfunction: A potential strategy to attenuate Cytokine Storms in severe infections. Theranostics 2020, 10, 9591–9600. [Google Scholar] [CrossRef]
- Di Caro, G.; Carvello, M.; Pesce, S.; Erreni, M.; Marchesi, F.; Todoric, J.; Sacchi, M.; Montorsi, M.; Allavena, P.; Spinelli, A. Circulating Inflammatory Mediators as Potential Prognostic Markers of Human Colorectal Cancer. PLoS ONE 2016, 11, e0148186. [Google Scholar] [CrossRef]
- Silveira, C.R.F.; Corveloni, A.C.; Caruso, S.R.; Macêdo, N.A.; Brussolo, N.M.; Haddad, F.; Fernandes, T.R.; de Andrade, P.V.; Orellana, M.D.; Guerino-Cunha, R.L. Cytokines as an important player in the context of CAR-T cell therapy for cancer: Their role in tumor immunomodulation, manufacture, and clinical implications. Front. Immunol. 2022, 13, 947648. [Google Scholar] [CrossRef]
- Deckers, J.; Anbergen, T.; Hokke, A.M.; de Dreu, A.; Schrijver, D.P.; de Bruin, K.; Toner, Y.C.; Beldman, T.J.; Spangler, J.B.; de Greef, T.F.A.; et al. Engineering cytokine therapeutics. Nat. Rev. Bioeng. 2023, 1, 286–303. [Google Scholar] [CrossRef]
| Study Reference | Cell Lines/Models | Treatment | Key Findings |
|---|---|---|---|
| [55] | MCF7 (BC), HCT116 (CRC) | Oxaliplatin, Cisplatin, 5-FU, Doxorubicin, Paclitaxel, Docetaxel, Carboplatin | Varying potential for prognostic significance in CRC vs. BC. Drug-specific and tissue-specific cytokine regulation after chemotherapy. Upregulation of TRAIL-R2 and chitinase 3-like 1 in BC cell line; downregulation of TRAIL and IFN-β. |
| [56] | CRC cell lines | Various chemotherapy drugs | Drug- and p53-dependent signatures, suggesting a personalized approach considering p53-status. |
| [57] | CRC cell lines | Topoisomerase inhibitor treatment | Induced cytokines clinically associated with patient overall survival. |
| [58] | MCF10A, MCF7, MDA-MB-231 | Ionizing radiation (9 Gy and 23 Gy) | Dose-independent, cell-line dependent release of cytokines and growth factors. |
| [59] | CRC models (in vitro and in vivo) | 5-FU chemotherapy | CCL20 induced after 5-FU, mediator of Treg recruitment and drug resistance. |
| [60] | Mice | Chemotherapy (Oxaliplatin) | Altered cytokine abundance in the hippocampus, potential toxic side-effects in the brain. Increased splenic populations of CD4, CD8, and Treg cells; altered splenic cytokine expression. |
| [61] | Not specified | Anthracyclines | Type-I interferon response, upregulation of CXCL10, resulting in antitumor outcome. |
| [62] | Solid cancers | Radiotherapy | Alters local or systemic cytokines. CXCL9, CXCL10, CXCL16 mediate anticancer effects; TGF-β, CCL2, CSF1, CXCL12, and insulin-like growth factor 1 may create an immunosuppressive microenvironment. |
| [63] | Solid cancers | Radiotherapy combined with cytokine treatments | CXCL9, CXCL10, CXCL16 mediate anticancer effects; other induced molecules may create an immunosuppressive microenvironment. |
| [64] | Rectal cancer patients | Chemoradiation therapy (CRT) | sCD40L and CCL5 levels associated with malignant tumor behaviors; higher post-CRT IL6 associated with a poor response. |
| [65] | Patients with bone and soft tissue tumors | Chemotherapy | Increases in IL6 and TNFα production, decreases in neutrophil counts after antitumor drug infusion. |
| [66] | Patients with hepatocellular carcinoma | Trans arterial chemoembolization (TACE) | IL-6 peaks on day 3, decreases thereafter; IL4, IL5, IL10 increase at two months after TACE. |
| [67] | Patients with NSCLC or GBM | CRT | TNF-α levels decrease in NSCLC, IFN-γ levels decrease in GBM after CRT. |
| [68] | Not specified | Gamma radiation-induced injury | Role of IL18 in response to gamma radiation-induced injury. |
| [69] | Mice, minipigs, nonhuman primates | Gamma radiation exposure | IL18 as a potential biomarker for radiation injury. |
| [70] | Tumor microenvironment | Radioimmunotherapy (177Lu) | Tumor regression not accompanied by a significant increase in attracting immune cell cytokines. |
| [71] | Not specified | Local high-dose radiotherapy | IFN-β production involved in ablative local radiotherapy-mediated tumor control. The antitumor effect of radiotherapy diminished in mice deficient in type I IFN response. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunturu, D.R.; Hassan, M.; Bedi, D.; Datta, P.; Manne, U.; Samuel, T. Unlocking the Potential of Therapy-Induced Cytokine Responses: Illuminating New Pathways in Cancer Precision Medicine. Curr. Oncol. 2024, 31, 1195-1206. https://doi.org/10.3390/curroncol31030089
Gunturu DR, Hassan M, Bedi D, Datta P, Manne U, Samuel T. Unlocking the Potential of Therapy-Induced Cytokine Responses: Illuminating New Pathways in Cancer Precision Medicine. Current Oncology. 2024; 31(3):1195-1206. https://doi.org/10.3390/curroncol31030089
Chicago/Turabian StyleGunturu, Dilip R., Mohammed Hassan, Deepa Bedi, Pran Datta, Upender Manne, and Temesgen Samuel. 2024. "Unlocking the Potential of Therapy-Induced Cytokine Responses: Illuminating New Pathways in Cancer Precision Medicine" Current Oncology 31, no. 3: 1195-1206. https://doi.org/10.3390/curroncol31030089
APA StyleGunturu, D. R., Hassan, M., Bedi, D., Datta, P., Manne, U., & Samuel, T. (2024). Unlocking the Potential of Therapy-Induced Cytokine Responses: Illuminating New Pathways in Cancer Precision Medicine. Current Oncology, 31(3), 1195-1206. https://doi.org/10.3390/curroncol31030089









