The Efficacy of Fat Grafting on Treating Post-Mastectomy Pain with and without Breast Reconstruction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility
2.2. Screening
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Outcomes
2.6. Data Analysis
3. Results
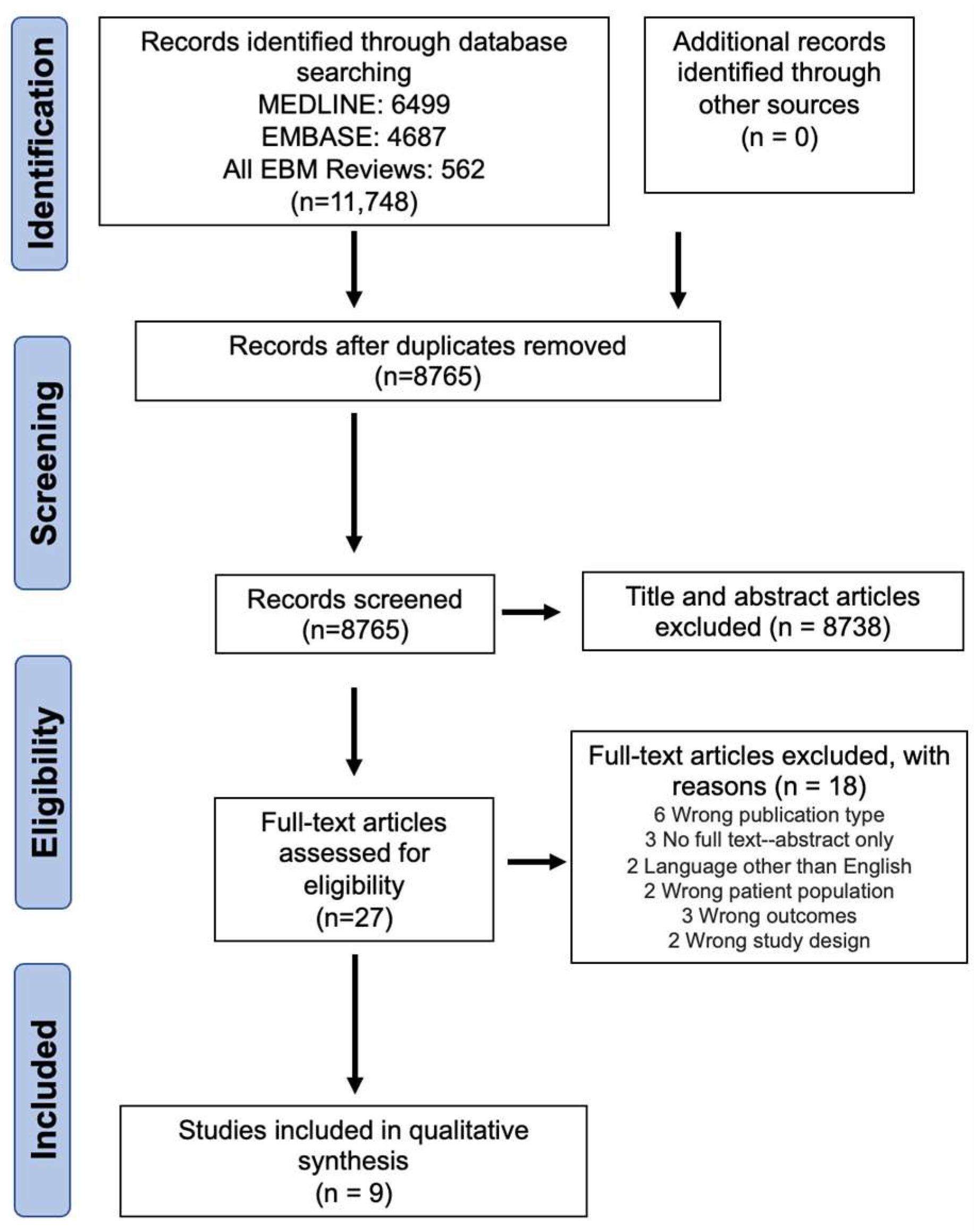
3.1. Study Selection
3.2. Screening Agreeability
3.3. Study Characteristics
3.4. Risk of Bias Assessment
3.5. Post-Mastectomy Pain Syndrome
3.6. VAS
3.7. NPSI
3.8. Complications
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, F.; Kuo, Y.F.; Shih, Y.C.T.; Giordano, S.H.; Berenson, A.B. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer 2018, 124, 3500–3509. [Google Scholar] [CrossRef] [PubMed]
- Fefferman, M.; Nicholson, K.; Kuchta, K.; Pesce, C.; Kopkash, K.; Yao, K. Rates of Bilateral Mastectomy in Patients with Early-Stage Breast Cancer. JAMA Netw. Open 2023, 6, e2251348. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, S.S.; Chappell, A.G.; Jackson, B.T.; Wescott, A.B.; Ellis, M.F. Post Mastectomy Pain Syndrome: A Systematic Review of Prevention Modalities. JPRAS Open 2022, 31, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.C.; Zoberi, K.; Ferguson, M.; Levenhagen, K.; Luebbert, R.A.; Rowland, K.; Salsich, G.B.; Herndon, C. Persistent Post-Mastectomy Pain: Risk Factors and Current Approaches to Treatment. J. Pain 2018, 19, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Poleshuck, E.L.; Katz, J.; Andrus, C.H.; Hogan, L.A.; Jung, B.F.; Kulick, D.I.; Dworkin, R.H. Risk factors for chronic pain following breast cancer surgery: A prospective study. J. Pain 2006, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Appelgren, M.; Sackey, H.; Wengström, Y.; Johansson, K.; Ahlgren, J.; Andersson, Y.; Bergkvist, L.; Frisell, J.; Lundstedt, D.; Rydén, L.; et al. Patient-reported outcomes one year after positive sentinel lymph node biopsy with or without axillary lymph node dissection in the randomized SENOMAC trial. Breast 2022, 63, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.G.; Yuksel, S.; Sasson, D.C.; Wescott, A.B.; Connor, L.M.; Ellis, M.F. Post-Mastectomy Pain Syndrome: An Up-to-Date Review of Treatment Outcomes. JPRAS Open 2021, 30, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lisa, A.V.E.; Murolo, M.; Maione, L.; Vinci, V.; Battistini, A.; Morenghi, E.; De Santis, G.; Klinger, M. Autologous fat grafting efficacy in treating PostMastectomy pain syndrome: A prospective multicenter trial of two Senonetwork Italia breast centers. Breast J. 2020, 26, 1652–1658. [Google Scholar] [CrossRef]
- Klomparens, K.; Simman, R. Autologous Fat Grafting: Evaluation of Efficacy in Pain Relief. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4543. [Google Scholar] [CrossRef]
- Caviggioli, F.; Maione, L.; Forcellini, D.; Klinger, F.; Klinger, M. Autologous fat graft in postmastectomy pain syndrome. Plast. Reconstr. Surg. 2011, 128, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Sollie, M.; Toyserkani, N.M.; Bille, C.; Thomsen, J.B.; Sorensen, J.A. Autologous Fat Grafting as Treatment of Postmastectomy Pain Syndrome: A Randomized Controlled Trial. Plast. Reconstr. Surg. 2022, 149, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Walter, S.D.; Yao, X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J. Clin. Epidemiol. 2007, 60, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 29 February 2024).
- Myles, P.S.; Myles, D.B.; Galagher, W.; Boyd, D.; Chew, C.; MacDonald, N.; Dennis, A. Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 2017, 118, 424–429. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Caviggioli, F.; Maione, L.; Klinger, F.; Lisa, A.; Klinger, M. Autologous Fat Grafting Reduces Pain in Irradiated Breast: A Review of Our Experience. Stem Cells Int. 2016, 2016, 2527349. [Google Scholar] [CrossRef]
- Calabrese, S.; Zingaretti, N.; De Francesco, F.; Riccio, M.; De Biasio, F.; Massarut, S.; Almesberger, D.; Parodi, P.C. Long-term impact of lipofilling in hybrid breast reconstruction: Retrospective analysis of two cohorts. Eur. J. Plast. Surg. 2020, 43, 257–268. [Google Scholar] [CrossRef]
- Cogliandro, A.; Barone, M.; Tenna, S.; Morelli Coppola, M.; Persichetti, P. The Role of Lipofilling after Breast Reconstruction: Evaluation of Outcomes and Patient Satisfaction with BREAST-Q. Aesthetic Plast. Surg. 2017, 41, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Juhl, A.A.; Karlsson, P.; Damsgaard, T.E. Fat grafting for alleviating persistent pain after breast cancer treatment: A randomized controlled trial. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2016, 69, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Maione, L.; Vinci, V.; Caviggioli, F.; Klinger, F.; Banzatti, B.; Catania, B.; Lisa, A.; Klinger, M. Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Aesthetic Plast. Surg. 2014, 38, 528–532. [Google Scholar] [CrossRef]
- Panettiere, P.; Marchetti, L.; Accorsi, D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast. Surg. 2009, 33, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Riyat, H.; Touil, L.L.; Briggs, M.; Shokrollahi, K. Autologous fat grafting for scars, healing and pain: A review. Scars Burn. Heal. 2017, 3, 2059513117728200. [Google Scholar] [CrossRef]
- Alessandri-Bonetti, M.; Egro, F.M.; Persichetti, P.; Coleman, S.R.; Peter Rubin, J. The Role of Fat Grafting in Alleviating Neuropathic Pain: A Critical Review of the Literature. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2216. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Y. Objective Pain Assessment: A Key for the Management of Chronic Pain. F1000Research 2020, 9, 35. [Google Scholar] [CrossRef]
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef]
- Fang, H.A.; Soto, E.; Pigg, R.; Smith, M.; Boyd, C.J.; Ananthasekar, S.; Fix, R.J.; Kilic, A.; Denney, B.; Patcha, P.; et al. The Safety of Fat Grafting: An Institutional Retrospective Review. Ann. Plast. Surg. 2022, 88, S473–S477. [Google Scholar] [CrossRef]
- Ørholt, M.; Larsen, A.; Hemmingsen, M.N.; Mirian, C.; Zocchi, M.L.; Vester-Glowinski, P.V.; Herly, M. Complications after Breast Augmentation with Fat Grafting: A Systematic Review. Plast. Reconstr. Surg. 2020, 145, 530e–537e. [Google Scholar] [CrossRef]
- Scardina, L.; Di Leone, A.; Biondi, E.; Carnassale, B.; Sanchez, A.M.; D’Archi, S.; Franco, A.; Moschella, F.; Magno, S.; Terribile, D.; et al. Prepectoral vs. Submuscular Immediate Breast Reconstruction in Patients Undergoing Mastectomy after Neoadjuvant Chemotherapy: Our Early Experience. J. Pers. Med. 2022, 12, 1533. [Google Scholar] [CrossRef] [PubMed]

| Query | Results from 29 April 2023 | ||
|---|---|---|---|
| Medline | EMBASE | EBM | |
| 1. Breast Neoplasms/or breast surgery.mp. | 330,797 | 44,072 | 19,463 |
| 2. mastectomy.mp. or Mastectomy/ | 46,860 | 76,214 | 6068 |
| 3. breast reconstruction.mp. or Mammaplasty/ | 19,265 | 23,371 | 1056 |
| 4. lumpectomy.mp. or Mastectomy, Segmental/ | 12,004 | 23,079 | 1300 |
| 5. postmastectomy.mp. | 2504 | 3393 | 389 |
| 6. post-mastectomy.mp. | 1429 | 2787 | 411 |
| 7. post-mastectomy pain syndrome.mp. | 54 | 108 | 44 |
| 8. postmastectomy pain syndrome.mp. | 74 | 104 | 33 |
| 9. fat.mp. | 325,443 | 493,736 | 41,334 |
| 10. fat transfer.mp. | 659 | 795 | 46 |
| 11. fat transplantation.mp. | 510 | 654 | 35 |
| 12. fat graft.mp. | 1675 | 2143 | 106 |
| 13. fat grafting.mp. | 2591 | 2955 | 127 |
| 14. Tissue Expansion/ | 2476 | 3916 | 53 |
| 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 | 349,324 | 123,617 | 23,147 |
| 16. 9 or 10 or 11 or 12 or 13 or 14 | 327,807 | 497,180 | 41,385 |
| 17. 15 and 16 | 6499 | 4687 | 562 |
| Cohort Studies | |||
| Study | Selection | Comparability | Outcome |
| Caviggioli 2011 [11] | **** | *** | |
| Caviggioli 2015 [20] | **** | *** | |
| Cogliandro 2017 [22] | **** | * | *** |
| Lisa 2020 [9] | **** | *** | |
| Maione 2014 [24] | **** | * | *** |
| Panettiere 2009 [25] | *** | ** | |
| Case–Control Studies | |||
| Study | Selection | Comparability | Exposure |
| Calabrese 2019 [21] | **** | * | * |
| Complications | Overall Rate in Intervention Group |
|---|---|
| Revision surgery | 4.1% (n = 18/435) |
| Capsular contracture | 1.4% (n = 6/435) |
| Hematoma | 0.5% (n = 2/435) |
| Seroma | 0.5% (n = 2/435) |
| Implant infection/dehiscence | 0.5% (n = 2/435) |
| Implant rupture | 0.2% (n = 1/435) |
| Implant exposure | 0% (n = 0/435) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Alghamdi, A.A.; Wong, C.Y.; Alnaim, M.F.; Kuper, G.; Zhang, J. The Efficacy of Fat Grafting on Treating Post-Mastectomy Pain with and without Breast Reconstruction: A Systematic Review and Meta-Analysis. Curr. Oncol. 2024, 31, 2057-2066. https://doi.org/10.3390/curroncol31040152
Chen J, Alghamdi AA, Wong CY, Alnaim MF, Kuper G, Zhang J. The Efficacy of Fat Grafting on Treating Post-Mastectomy Pain with and without Breast Reconstruction: A Systematic Review and Meta-Analysis. Current Oncology. 2024; 31(4):2057-2066. https://doi.org/10.3390/curroncol31040152
Chicago/Turabian StyleChen, Jeffrey, Abdulrahman A. Alghamdi, Chi Yi Wong, Muna F. Alnaim, Gabriel Kuper, and Jing Zhang. 2024. "The Efficacy of Fat Grafting on Treating Post-Mastectomy Pain with and without Breast Reconstruction: A Systematic Review and Meta-Analysis" Current Oncology 31, no. 4: 2057-2066. https://doi.org/10.3390/curroncol31040152
APA StyleChen, J., Alghamdi, A. A., Wong, C. Y., Alnaim, M. F., Kuper, G., & Zhang, J. (2024). The Efficacy of Fat Grafting on Treating Post-Mastectomy Pain with and without Breast Reconstruction: A Systematic Review and Meta-Analysis. Current Oncology, 31(4), 2057-2066. https://doi.org/10.3390/curroncol31040152






