Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update
Abstract
:1. Introduction
2. Methods
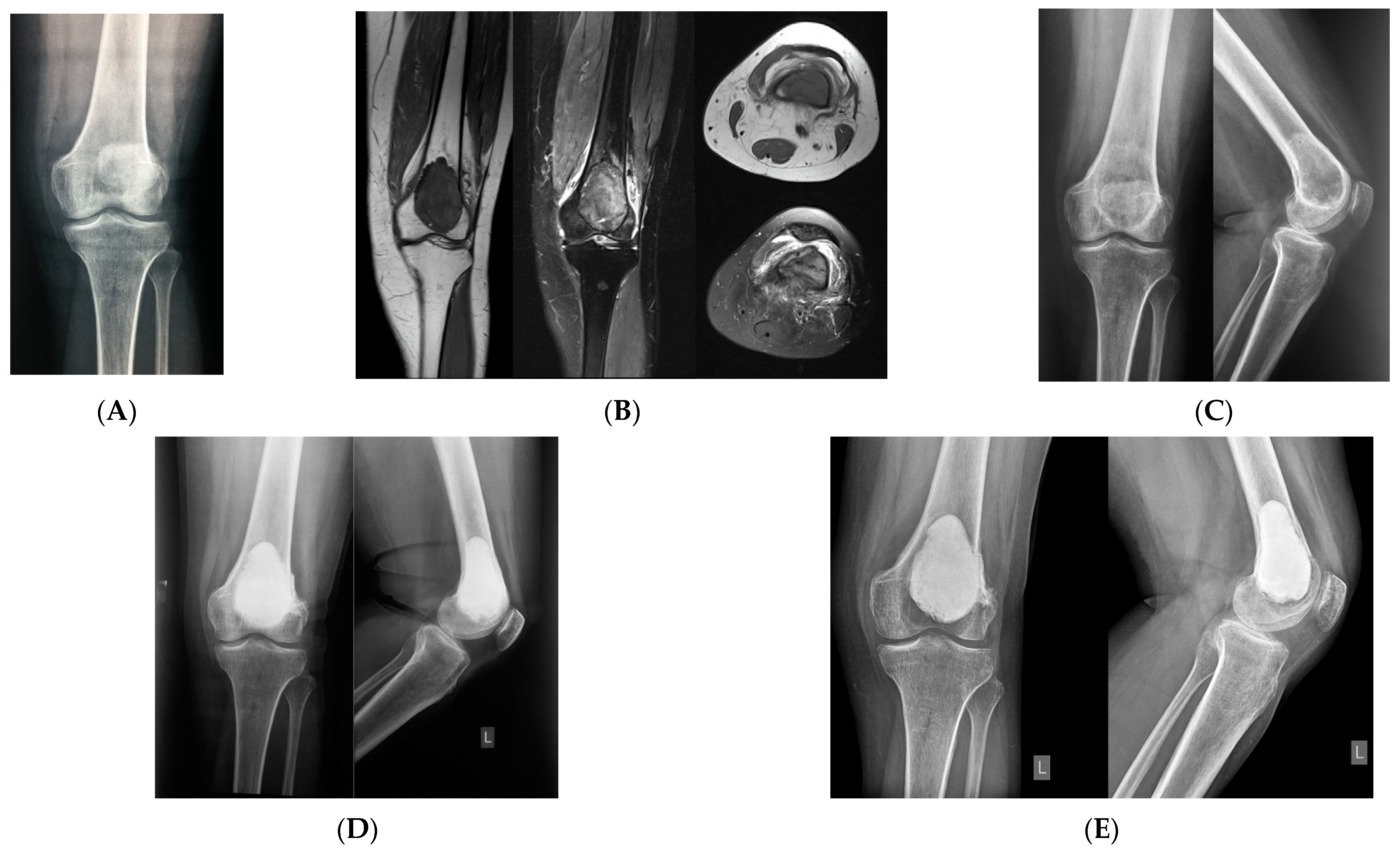
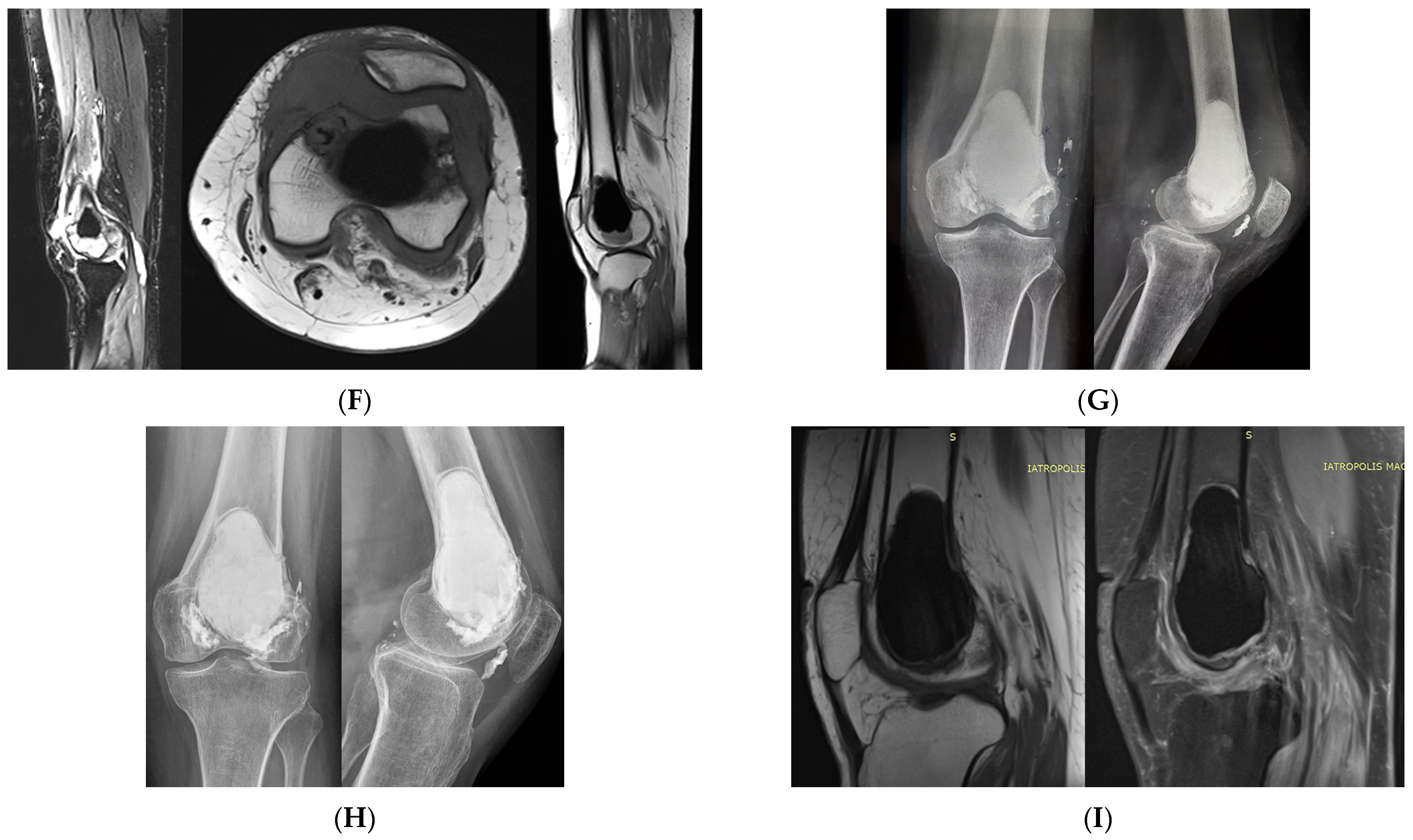
3. Extremities
4. Pelvis and Sacrum
5. Spine
6. Lung Metastasis
7. Multicentric GCTB
8. Denosumab Monotherapy
9. Malignant GCTB
10. Genomic Profiling
11. Research Implication
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flanagan, A.M.; Larousserie, F.; O’Donnell, P.G.; Yoshida, A. Giant Cell Tumour of Bone. In WHO Classification of Tumours, 5th ed.; Soft Tissue and Bone Tumours; The WHO Classification of Tumours Editorial Board; International Arctic Research Center: Lyon, France, 2020; pp. 440–446. [Google Scholar]
- Tabarestani, T.Q.; Levine, N.; Sachs, E.; Scholl, A.; Colglazier, R.; French, R.; Al-Rohil, R.; Brigman, B.; Eward, W.; Visgauss, J. Giant Cell Tumor of Bone in the Pediatric Population: A Retrospective Study Highlighting Cases of Metaphyseal Only Location and Increased Local Recurrence Rates in Skeletally Immature Patients. Skeletal. Radiol. 2023, 52, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Iwasaki, T.; Yamada, Y.; Matsumoto, Y.; Otsuka, H.; Yoshimoto, M.; Kohashi, K.; Taguchi, K.; Yokoyama, R.; Nakashima, Y.; et al. Diagnostic Utility of Histone H3.3 G34W, G34R, and G34V Mutant-Specific Antibodies for Giant Cell Tumors of Bone. Hum. Pathol. 2018, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Amary, F.; Berisha, F.; Ye, H.; Gupta, M.; Gutteridge, A.; Baumhoer, D.; Gibbons, R.; Tirabosco, R.; O’Donnell, P.; Flanagan, A.M. H3F3A (Histone 3.3) G34W Immunohistochemistry: A Reliable Marker Defining Benign and Malignant Giant Cell Tumor of Bone. Am. J. Surg. Pathol. 2017, 41, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Cleven, A.H.G.; Höcker, S.; Briaire-de Bruijn, I.; Szuhai, K.; Cleton-Jansen, A.-M.; Bovée, J.V.M.G. Mutation Analysis of H3F3A and H3F3B as a Diagnostic Tool for Giant Cell Tumor of Bone and Chondroblastoma. Am. J. Surg. Pathol. 2015, 39, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanaka, Y.; Kido, A.; Honoki, K.; Tanaka, Y.; Errani, C. Metastasectomy Versus Non-Metastasectomy for Giant Cell Tumor of Bone Lung Metastases. Orthopedics 2021, 44, e707–e712. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Picci, P.; Reichardt, P.; Downey, G. Malignancy in Giant Cell Tumor of Bone: A Review of the Literature. Technol. Cancer Res. Treat. 2019, 18, 1533033819840000. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-Cell Tumor of Bone. J. Bone Jt. Surg. Am. 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Chawla, S.; Henshaw, R.; Seeger, L.; Choy, E.; Blay, J.-Y.; Ferrari, S.; Kroep, J.; Grimer, R.; Reichardt, P.; Rutkowski, P.; et al. Safety and Efficacy of Denosumab for Adults and Skeletally Mature Adolescents with Giant Cell Tumour of Bone: Interim Analysis of an Open-Label, Parallel-Group, Phase 2 Study. Lancet Oncol. 2013, 14, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Ferrari, S.; Grimer, R.J.; Stalley, P.D.; Dijkstra, S.P.D.; Pienkowski, A.; Vaz, G.; Wunder, J.S.; Seeger, L.L.; Feng, A.; et al. Surgical Downstaging in an Open-Label Phase II Trial of Denosumab in Patients with Giant Cell Tumor of Bone. Ann. Surg. Oncol. 2015, 22, 2860–2868. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Kido, A.; Errani, C. Current Concepts in the Treatment of Giant Cell Tumors of Bone. Cancers 2021, 13, 3647. [Google Scholar] [CrossRef]
- Errani, C.; Ruggieri, P.; Asenzio, M.A.N.; Toscano, A.; Colangeli, S.; Rimondi, E.; Rossi, G.; Longhi, A.; Mercuri, M. Giant Cell Tumor of the Extremity: A Review of 349 Cases from a Single Institution. Cancer Treat. Rev. 2010, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Machak, G.N.; Snetkov, A.I. The Impact of Curettage Technique on Local Control in Giant Cell Tumour of Bone. Int. Orthop. 2021, 45, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, J.; Zhou, W.; Zhang, C.; Wang, G.; Dong, D.; Xia, P.; Liu, X.; Xu, F. Microwave in Situ Inactivation in the Treatment of Bone Giant Cell Tumor: A Mid-Term Descriptive Study. J. Cancer Res. Clin. Oncol. 2023, 149, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, B.; Haase, D.; Sweeney, K.; Friedman, E.; Poppe, T.; Hughes, N. A Comparison of Depth of Necrosis among Adjuvant Therapies Used for the Treatment of Benign Bone Tumors. J. Surg. Oncol. 2021, 123, 1299–1303. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanzi, P.; Leone, G.; Akahane, M.; Tanaka, Y.; Errani, C. Curettage as First Surgery for Bone Giant Cell Tumor : Adequate Surgery Is More Important than Oncology Training or Surgical Management by High Volume Specialized Teams. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, P.; Tian, Q.; Li, H.; Li, J.; Ren, P.; Lv, Z.; Lv, J.; Bai, J.; Feng, Y. “Triple Clear”: A Systematic and Comprehensive Surgical Process for Campanacci Grades II and III Giant Cell Tumors of the Bone, with or without Pathological Fracture and Slight Joint Invasion. World J. Surg. Oncol. 2023, 21, 114. [Google Scholar] [CrossRef]
- Furuta, T.; Kubo, T.; Sakuda, T.; Saito, T.; Kurisu, K.; Muragaki, Y.; Adachi, N. Utility of Intraoperative Magnetic Resonance Imaging for Giant Cell Tumor of Bone after Denosumab Treatment: A Pilot Study. Acta Radiol. 2022, 63, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Fernandes, R.J.; Manrique, J.; Polissar, N.L.; Miljacic, L.; Hippe, D.S.; Vaux, J.; Thompson, M.J. Cytotoxic Effects of Common Irrigation Solutions on Chondrosarcoma and Giant Cell Tumors of Bone. J. Bone Jt. Surg. Am. 2022, 104, 2153–2159. [Google Scholar] [CrossRef]
- Benevenia, J.; Rivero, S.M.; Moore, J.; Ippolito, J.A.; Siegerman, D.A.; Beebe, K.S.; Patterson, F.R. Supplemental Bone Grafting in Giant Cell Tumor of the Extremity Reduces Nononcologic Complications. Clin. Orthop. Relat. Res. 2017, 475, 776–783. [Google Scholar] [CrossRef]
- Teng, W.; Lin, P.; Li, Y.; Yan, X.; Li, H.; Li, B.; Wang, Z.; Wu, Y.; Wang, S.; Zhou, X.; et al. Bone Combined Cement Grafting in Giant Cell Tumor around the Knee Reduces Mechanical Failure. Int. Orthop. 2019, 43, 475–482. [Google Scholar] [CrossRef]
- Jamshidi, K.; Bagherifard, A.; Mohaghegh, M.R.; Mirzaei, A. Fibular Strut Allograft or Bone Cement for Reconstruction after Curettage of a Giant Cell Tumour of the Proximal Femur: A Retrospective Cohort Study. Bone Jt. J. 2022, 104-B, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Mavrogenis, A.F.; Akahane, M.; Honoki, K.; Kido, A.; Tanaka, Y.; Donati, D.M.; Errani, C. Risk Factors of Fracture Following Curettage for Bone Giant Cell Tumors of the Extremities. BMC Musculoskelet. Disord. 2022, 23, 477. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, T.; Yasunaga, Y.; Ikuta, Y.; Fujimoto, Y. Effects on Articular Cartilage of Subchondral Replacement with Polymethylmethacrylate and Calcium Phosphate Cement. J. Biomed. Mater. Res. 2002, 59, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Damron, T.A.; Green, J.K.; Morgan, H.D.; Werner, F.W. Contained Femoral Defects: Biomechanical Analysis of Pin Augmentation in Cement. Clin. Orthop. Relat. Res. 2004, 420, 251–256. [Google Scholar] [CrossRef]
- Welch, R.D.; Berry, B.H.; Crawford, K.; Zhang, H.; Zobitz, M.; Bronson, D.; Krishnan, S. Subchondral Defects in Caprine Femora Augmented with in Situ Setting Hydroxyapatite Cement, Polymethylmethacrylate, or Autogenous Bone Graft: Biomechanical and Histomorphological Analysis after Two-Years. J. Orthop. Res. 2002, 20, 464–472. [Google Scholar] [CrossRef]
- Manley, P.A.; McKeown, D.B.; Schatzker, J.; Palmer, N.C.; Carman, S. Replacement of Epiphyseal Bone with Methylmethacrylate: Its Effect on Articular Cartilage. Arch. Orthop. Trauma Surg. 1982, 100, 3–10. [Google Scholar] [CrossRef]
- van der Heijden, L.; van de Sande, M.A.J.; Heineken, A.C.; Fiocco, M.; Nelissen, R.G.H.H.; Dijkstra, P.D.S. Mid-Term Outcome after Curettage with Polymethylmethacrylate for Giant Cell Tumor around the Knee: Higher Risk of Radiographic Osteoarthritis? J. Bone Jt. Surg. Am. 2013, 95, e159. [Google Scholar] [CrossRef]
- Takeuchi, A.; Suwanpramote, P.; Yamamoto, N.; Shirai, T.; Hayashi, K.; Kimura, H.; Miwa, S.; Higuchi, T.; Abe, K.; Tsuchiya, H. Mid- to Long-Term Clinical Outcome of Giant Cell Tumor of Bone Treated with Calcium Phosphate Cement Following Thorough Curettage and Phenolization. J. Surg. Oncol. 2018, 117, 1232–1238. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; Hu, X.; Lu, M.; Zhang, Y.; Gan, Y.; Tu, C.; Min, L. Clinical Evaluation of the Three-Dimensional Printed Strut-Type Prosthesis Combined with Autograft Reconstruction for Giant Cell Tumor of the Distal Femur. Front. Oncol. 2023, 13, 1206765. [Google Scholar] [CrossRef]
- Errani, C.; Tsukamoto, S.; Ciani, G.; Donati, D.M. Present Day Controversies and Consensus in Curettage for Giant Cell Tumor of Bone. J. Clin. Orthop. Trauma 2019, 10, 1015–1020. [Google Scholar] [CrossRef]
- Arrigoni, F.; Zoccali, C.; Evangelista, L.; Giuliani, L.; Daffinà, J.; Zugaro, L.; Masciocchi, C. CT-Guided RFA for Management of Surgical Relapses of Giant Cell Tumour of Bone. CardioVasc. Interv. Radiol. 2023, 46, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Errani, C.; Tsukamoto, S.; Leone, G.; Righi, A.; Akahane, M.; Tanaka, Y.; Donati, D.M. Denosumab May Increase the Risk of Local Recurrence in Patients with Giant-Cell Tumor of Bone Treated with Curettage. J. Bone Jt. Surg. Am. 2018, 100, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Tanaka, Y.; Mavrogenis, A.F.; Kido, A.; Kawaguchi, M.; Errani, C. Is Treatment with Denosumab Associated with Local Recurrence in Patients with Giant Cell Tumor of Bone Treated with Curettage? A Systematic Review. Clin. Orthop. Relat. Res. 2020, 478, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.G.; Gundavda, M.K.; Gupta, R.; Reddy, R. Does Denosumab Change the Giant Cell Tumor Treatment Strategy? Lessons Learned from Early Experience. Clin. Orthop. Relat. Res. 2018, 476, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Scoccianti, G.; Totti, F.; Scorianz, M.; Baldi, G.; Roselli, G.; Beltrami, G.; Franchi, A.; Capanna, R.; Campanacci, D.A. Preoperative Denosumab with Curettage and Cryotherapy in Giant Cell Tumor of Bone: Is There an Increased Risk of Local Recurrence? Clin. Orthop. Relat. Res. 2018, 476, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Liu, W.; Xu, H.; Niu, X. A Nonrandomized Controlled Study of Sacral Giant Cell Tumors with Preoperative Treatment of Denosumab. Medicine 2018, 97, e13139. [Google Scholar] [CrossRef] [PubMed]
- Medellin, M.R.; Fujiwara, T.; Tillman, R.M.; Jeys, L.M.; Gregory, J.; Stevenson, J.D.; Parry, M.; Abudu, A. Prognostic Factors for Local Recurrence in Extremity-Located Giant Cell Tumours of Bone with Pathological Fracture. Bone Jt. J. 2018, 100-B, 1626–1632. [Google Scholar] [CrossRef]
- Asano, N.; Saito, M.; Kobayashi, E.; Morii, T.; Kikuta, K.; Watanabe, I.; Anazawa, U.; Takeuchi, K.; Suzuki, Y.; Susa, M.; et al. Preoperative Denosumab Therapy Against Giant Cell Tumor of Bone Is Associated with an Increased Risk of Local Recurrence After Curettage Surgery. Ann. Surg. Oncol. 2022, 29, 3992–4000. [Google Scholar] [CrossRef]
- Mak, I.W.Y.; Evaniew, N.; Popovic, S.; Tozer, R.; Ghert, M. A Translational Study of the Neoplastic Cells of Giant Cell Tumor of Bone Following Neoadjuvant Denosumab. J. Bone Jt. Surg. Am. 2014, 96, e127. [Google Scholar] [CrossRef]
- Traub, F.; Singh, J.; Dickson, B.C.; Leung, S.; Mohankumar, R.; Blackstein, M.E.; Razak, A.R.; Griffin, A.M.; Ferguson, P.C.; Wunder, J.S. Efficacy of Denosumab in Joint Preservation for Patients with Giant Cell Tumour of the Bone. Eur. J. Cancer 2016, 59, 1–12. [Google Scholar] [CrossRef]
- Lau, C.P.Y.; Huang, L.; Wong, K.C.; Kumta, S.M. Comparison of the Anti-Tumor Effects of Denosumab and Zoledronic Acid on the Neoplastic Stromal Cells of Giant Cell Tumor of Bone. Connect. Tissue Res. 2013, 54, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, I.; Takami, M.; Miyamoto, A.; Karakawa, A.; Dezawa, A.; Nakamura, S.; Kamijo, R. In Vitro Study of the Effects of Denosumab on Giant Cell Tumor of Bone: Comparison with Zoledronic Acid. Pathol. Oncol. Res. 2019, 25, 409–419. [Google Scholar] [CrossRef]
- Treffel, M.; Lardenois, E.; Larousserie, F.; Karanian, M.; Gomez-Brouchet, A.; Bouvier, C.; Le Loarer, F.; Aubert, S.; de Pinieux, G.; Audard, V.; et al. Denosumab-Treated Giant Cell Tumors of Bone: A Clinicopathologic Analysis of 35 Cases from the French Group of Bone Pathology. Am. J. Surg. Pathol. 2020, 44, 1–10. [Google Scholar] [CrossRef]
- Ud Din, N.; Umer, M.; Park, Y.-K. Histomorphometric Analysis of Pre- and Post-Denosumab-Treated Giant Cell Tumor of Bone. Int. J. Surg. Pathol. 2020, 28, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, H.; Zhang, X.; Tang, Y.; Wu, Z.; Wang, Y.; Huang, H.; Fu, X.; Liu, J.; Hogendoorn, P.C.W.; et al. Clinicopathologic and Molecular Features of Denosumab-Treated Giant Cell Tumour of Bone (GCTB): Analysis of 21 Cases. Ann. Diagn. Pathol. 2022, 57, 151882. [Google Scholar] [CrossRef]
- Girolami, I.; Mancini, I.; Simoni, A.; Baldi, G.G.; Simi, L.; Campanacci, D.; Beltrami, G.; Scoccianti, G.; D’Arienzo, A.; Capanna, R.; et al. Denosumab Treated Giant Cell Tumour of Bone: A Morphological, Immunohistochemical and Molecular Analysis of a Series. J. Clin. Pathol. 2016, 69, 240–247. [Google Scholar] [CrossRef]
- Kato, I.; Furuya, M.; Matsuo, K.; Kawabata, Y.; Tanaka, R.; Ohashi, K. Giant Cell Tumours of Bone Treated with Denosumab: Histological, Immunohistochemical and H3F3A Mutation Analyses. Histopathology 2018, 72, 914–922. [Google Scholar] [CrossRef]
- Arndt, S.; Hartmann, W.; Rókusz, A.; Leinauer, B.; von Baer, A.; Schultheiss, M.; Pablik, J.; Fritzsche, H.; Mogler, C.; Antal, I.; et al. Histomorphometric Analysis of 38 Giant Cell Tumors of Bone after Recurrence as Compared to Changes Following Denosumab Treatment. Cancers 2023, 15, 4249. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, M.; Wang, B.; Zhao, Z.; Lin, T.; Huang, G.; Yin, J.; Xie, X.; Shen, J.; Zou, C. Preoperative Denosumab Treatment in Patients with Giant Cell Bone Tumors in Limbs: A Retrospective Study Using Propensity Score Matching. Cancer Med. 2023, 12, 12041–12049. [Google Scholar] [CrossRef]
- Deventer, N.; Budny, T.; Gosheger, G.; Rachbauer, A.; Puetzler, J.; Theil, J.C.; Kovtun, D.; de Vaal, M.; Deventer, N. Giant Cell Tumor of Bone: A Single Center Study of 115 Cases. J. Bone Oncol. 2022, 33, 100417. [Google Scholar] [CrossRef]
- Urakawa, H.; Mizusawa, J.; Tanaka, K.; Eba, J.; Hiraga, H.; Kawai, A.; Nishida, Y.; Hosaka, M.; Iwamoto, Y.; Fukuda, H.; et al. A Randomized Phase III Trial of Denosumab before Curettage for Giant Cell Tumor of Bone: Japan Clinical Oncology Group Study JCOG1610. Jpn. J. Clin. Oncol. 2019, 49, 379–382. [Google Scholar] [CrossRef]
- Urakawa, H.; Nagano, A.; Machida, R.; Tanaka, K.; Kataoka, T.; Sekino, Y.; Nishida, Y.; Takahashi, M.; Kunisada, T.; Kawano, M.; et al. A Randomized Phase III Trial of Denosumab before Curettage for Giant Cell Tumor of Bone. JCOG1610. Jpn. J. Clin. Oncol. 2022, 52, 1021–1028. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Hindiskere, S.; Honoki, K.; Mavrogenis, A.F.; Tanaka, Y.; Chinder, P.S.; Donati, D.M.; Errani, C. Outcome of Re-Operation for Local Recurrence Following Pre-Operative Denosumab Administration and Curettage for Giant Cell Tumour of Bone with Difficult Joint Preservation. Int. Orthop. 2023, 47, 265–273. [Google Scholar] [CrossRef]
- Perrin, D.L.; Visgauss, J.D.; Wilson, D.A.; Griffin, A.M.; Abdul Razak, A.R.; Ferguson, P.C.; Wunder, J.S. The Role of Denosumab in Joint Preservation for Patients with Giant Cell Tumour of Bone. Bone Jt. J. 2021, 103-B, 184–191. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, H.; Lin, S.; Jin, H.; Zhang, Z.; Dong, Y.; Yang, Q.; Zhang, C.; Yuan, T. Computerised Tomography Features of Giant Cell Tumour of the Knee Are Associated with Local Recurrence after Extended Curettage. Int. Orthop. 2022, 46, 381–390. [Google Scholar] [CrossRef]
- Tang, H.; Moro, A.; Feng, W.; Lai, Y.; Xiao, Z.; Liu, Y.; Wang, K. Giant Cell Tumors Combined with Secondary Aneurysmal Bone Cysts Are More Likely to Develop Postoperative Recurrence: A Retrospective Study of 256 Cases. J. Surg. Oncol. 2019, 120, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Errani, C.; Facchini, F.; Papagelopoulos, P.; Mavrogenis, A.F. Fluid-Fluid Levels in Musculoskeletal Tumor Imaging. Curr. Med. Imaging 2021, 17, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rapp, T.B.; Ward, J.P.; Alaia, M.J. Aneurysmal Bone Cyst. J. Am. Acad. Orthop. Surg. 2012, 20, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, M.J.; Sweet, D.E. Aneurysmal Bone Cyst: Concept, Controversy, Clinical Presentation, and Imaging. AJR Am. J. Roentgenol. 1995, 164, 573–580. [Google Scholar] [CrossRef]
- Vergel De Dios, A.M.; Bond, J.R.; Shives, T.C.; McLeod, R.A.; Unni, K.K. Aneurysmal Bone Cyst. A Clinicopathologic Study of 238 Cases. Cancer 1992, 69, 2921–2931. [Google Scholar] [CrossRef]
- Mankin, H.J.; Hornicek, F.J.; Ortiz-Cruz, E.; Villafuerte, J.; Gebhardt, M.C. Aneurysmal Bone Cyst: A Review of 150 Patients. J. Clin. Oncol. 2005, 23, 6756–6762. [Google Scholar] [CrossRef] [PubMed]
- Bonakdarpour, A.; Levy, W.M.; Aegerter, E. Primary and Secondary Aneurysmal Bone Cyst: A Radiological Study of 75 Cases. Radiology 1978, 126, 75–83. [Google Scholar] [CrossRef]
- Martinez, V.; Sissons, H.A. Aneurysmal Bone Cyst. A Review of 123 Cases Including Primary Lesions and Those Secondary to Other Bone Pathology. Cancer 1988, 61, 2291–2304. [Google Scholar] [CrossRef]
- Gutierrez, L.B.; Link, T.M.; Horvai, A.E.; Joseph, G.B.; O’Donnell, R.J.; Motamedi, D. Secondary Aneurysmal Bone Cysts and Associated Primary Lesions: Imaging Features of 49 Cases. Clin. Imaging 2020, 62, 23–32. [Google Scholar] [CrossRef]
- Tsai, J.C.; Dalinka, M.K.; Fallon, M.D.; Zlatkin, M.B.; Kressel, H.Y. Fluid-Fluid Level: A Nonspecific Finding in Tumors of Bone and Soft Tissue. Radiology 1990, 175, 779–782. [Google Scholar] [CrossRef]
- Van Dyck, P.; Vanhoenacker, F.M.; Vogel, J.; Venstermans, C.; Kroon, H.M.; Gielen, J.; Parizel, P.M.; Bloem, J.L.; De Schepper, A.M.A. Prevalence, Extension and Characteristics of Fluid-Fluid Levels in Bone and Soft Tissue Tumors. Eur. Radiol. 2006, 16, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Aiba, H.; Righi, A.; Nitta, Y.; Traversari, M.; Mavrogenis, A.F.; Honoki, K.; Tanaka, Y.; Donati, D.M.; Errani, C. Giant Cell Tumor of Bone with Secondary Aneurysmal Bone Cyst Does Not Have a Higher Risk of Local Recurrence. J. Surg. Oncol. 2023, 128, 350–358. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, S.; Zhu, H.; Dong, Y.; Yang, Q.; Yuan, T. The Blood Pressure and Use of Tourniquet Are Related to Local Recurrence after Intralesional Curettage of Primary Benign Bone Tumors: A Retrospective and Hypothesis-Generating Study. BMC Musculoskelet. Disord. 2022, 23, 201. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, G.; Chen, F.; Xia, J.; Jiang, L. The Prognostic Significance of the Neutrophil-to-Lymphocyte Ratio and the Platelet-to-Lymphocyte Ratio in Giant Cell Tumor of the Extremities. BMC Cancer 2019, 19, 329. [Google Scholar] [CrossRef]
- Liang, S.; Li, Y.; Liu, H.; Wang, B. Pre-Operative Prognostic Nutritional Index Was Associated with Recurrence after Surgery in Giant Cell Tumor of Bone Patients. J. Bone Oncol. 2020, 25, 100324. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Alvarado, R.A.; Traversari, M.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; Errani, C. Association between Inflammatory Markers and Local Recurrence in Patients with Giant Cell Tumor of Bone: A Preliminary Result. Curr. Oncol. 2023, 30, 1116–1131. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanzi, P.; Leone, G.; Righi, A.; Akahane, M.; Kido, A.; Honoki, K.; Tanaka, Y.; Donati, D.M.; et al. Similar Local Recurrence but Better Function with Curettage versus Resection for Bone Giant Cell Tumor and Pathological Fracture at Presentation. J. Surg. Oncol. 2019, 119, 864–872. [Google Scholar] [CrossRef]
- Kuruoglu, D.; Rizzo, M.; Rose, P.S.; Moran, S.L.; Houdek, M.T. Treatment of Giant Cell Tumors of the Distal Radius: A Long-Term Patient-Reported Outcomes Study. J. Surg. Oncol. 2022, 126, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tang, J.; Shen, L.; Zhang, Z.; Yuan, T. Giant Cell Tumors of the Distal Ulna: Long-Term Recurrence Rate and Functional Outcomes of En Bloc Resection versus Curettage in a Multicenter Study. J. Orthop. Surg. Res. 2023, 18, 743. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, W.; Li, D.; Yang, Y.; Wei, R.; Xu, J. Functional Results of Wrist Arthrodesis versus Arthroplasty with Proximal Fibula Following Giant Cell Tumour Excision of the Distal Radius. J. Hand Surg. Eur. Vol. 2018, 44, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Bali, K.; Bachhal, V.; Mootha, A.K.; Dhillon, M.S.; Gill, S.S. En Bloc Excision and Autogenous Fibular Reconstruction for Aggressive Giant Cell Tumor of Distal Radius: A Report of 12 Cases and Review of Literature. J. Orthop. Surg. Res. 2011, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Wang, J.-W.; Huang, P.; Xu, Z.-H. Distal Radius Reconstruction with Vascularized Proximal Fibular Autograft after En-Bloc Resection of Recurrent Giant Cell Tumor. BMC Musculoskelet. Disord. 2016, 17, 346. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanzi, P.; Leone, G.; Ciani, G.; Righi, A.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; et al. Denosumab for Bone Giant Cell Tumor of the Distal Radius. Orthopedics 2020, 43, 284–291. [Google Scholar] [CrossRef]
- Rabitsch, K.; Maurer-Ertl, W.; Pirker-Frühauf, U.; Lovse, T.; Windhager, R.; Leithner, A. Reconstruction of the Distal Radius Following Tumour Resection Using an Osteoarticular Allograft. Sarcoma 2013, 2013, 318767. [Google Scholar] [CrossRef]
- Scoccianti, G.; Campanacci, D.A.; Beltrami, G.; Caldora, P.; Capanna, R. The Use of Osteo-Articular Allografts for Reconstruction after Resection of the Distal Radius for Tumour. J. Bone Jt. Surg. Br. 2010, 92, 1690–1694. [Google Scholar] [CrossRef]
- Bianchi, G.; Donati, D.; Staals, E.L.; Mercuri, M. Osteoarticular Allograft Reconstruction of the Distal Radius after Bone Tumour Resection. J. Hand Surg. Br. 2005, 30, 369–373. [Google Scholar] [CrossRef]
- Lans, J.; Ballatori, S.E.; Castelein, R.M.; Chen, N.C.; Lozano Calderon, S.A. Osteoarticular Allograft Reconstruction after Distal Radius Tumor Resection: Reoperation and Patient Reported Outcomes. J. Surg Oncol. 2021, 123, 1304–1315. [Google Scholar] [CrossRef]
- Lu, M.; Min, L.; Xiao, C.; Li, Y.; Luo, Y.; Zhou, Y.; Zhang, W.; Tu, C. Uncemented Three-Dimensional-Printed Prosthetic Replacement for Giant Cell Tumor of Distal Radius: A New Design of Prosthesis and Surgical Techniques. Cancer Manag. Res. 2018, 10, 265–277. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Liu, J.; Chen, S.; Zhang, Z.; Shao, Z. What Are the Functional Results, Complications, and Outcomes of Using a Custom Unipolar Wrist Hemiarthroplasty for Treatment of Grade III Giant Cell Tumors of the Distal Radius? Clin. Orthop. Relat. Res. 2016, 474, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, M.A.J.; van Geldorp, N.H.W.; Dijkstra, P.D.S.; Taminiau, A.H.M. Surgical Technique: Tibia Cortical Strut Autograft Interposition Arthrodesis after Distal Radius Resection. Clin. Orthop. Relat. Res. 2013, 471, 803–813. [Google Scholar] [CrossRef] [PubMed]
- van Isacker, T.; Barbier, O.; Traore, A.; Cornu, O.; Mazzeo, F.; Delloye, C. Forearm Reconstruction with Bone Allograft Following Tumor Excision: A Series of 10 Patients with a Mean Follow-up of 10 Years. Orthop. Traumatol. Surg. Res. 2011, 97, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Sambri, A.; Marini, E.; Piana, R.; Campanacci, D.A.; Donati, D.M. Wrist Arthrodesis and Osteoarticular Reconstruction in Giant Cell Tumor of the Distal Radius. J. Hand Surg. Am. 2020, 45, 882.e1–882.e6. [Google Scholar] [CrossRef] [PubMed]
- Gundavda, M.K.; Agarwal, M.G.; Reddy, R.; Katariya, A.; Bhadiyadra, R. Does a Modified Technique to Achieve Arthrodesis of the Wrist After Resection of the Distal Radius and Translocating the Ipsilateral Ulna as a Vascularized Graft to Reconstruct the Defect Improve Grip Strength and Outcomes Scores? Clin. Orthop. Relat. Res. 2021, 479, 1285–1293. [Google Scholar] [CrossRef]
- Puri, A.; Gulia, A.; Agarwal, M.G.; Reddy, K. Ulnar Translocation after Excision of a Campanacci Grade-3 Giant-Cell Tumour of the Distal Radius: An Effective Method of Reconstruction. J. Bone Jt. Surg. Br. 2010, 92, 875–879. [Google Scholar] [CrossRef]
- Wang, T.; Chan, C.M.; Yu, F.; Li, Y.; Niu, X. Does Wrist Arthrodesis with Structural Iliac Crest Bone Graft after Wide Resection of Distal Radius Giant Cell Tumor Result in Satisfactory Function and Local Control? Clin. Orthop. Relat. Res. 2017, 475, 767–775. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, D.; Wen, J.; Sun, W.; Cai, Z.; Zhang, W.; Zhang, Z.; Dong, Y.; Yang, Q.; Zhu, H.; et al. Reduced Recurrence Rate and Comparable Functionality after Wide Resection and Reverse Total Shoulder Arthroplasty with Allograft-Prosthetic Composite versus Curettage for Proximal Humeral Giant Cell Tumor: A Multicenter Retrospective Study. J. Shoulder Elbow Surg. 2023, in press. [Google Scholar] [CrossRef]
- Boriani, S.; Cecchinato, R.; Cuzzocrea, F.; Bandiera, S.; Gambarotti, M.; Gasbarrini, A. Denosumab in the Treatment of Giant Cell Tumor of the Spine. Preliminary Report, Review of the Literature and Protocol Proposal. Eur. Spine J. 2020, 29, 257–271. [Google Scholar] [CrossRef]
- Engellau, J.; Seeger, L.; Grimer, R.; Henshaw, R.; Gelderblom, H.; Choy, E.; Chawla, S.; Reichardt, P.; O’Neal, M.; Feng, A.; et al. Assessment of Denosumab Treatment Effects and Imaging Response in Patients with Giant Cell Tumor of Bone. World J. Surg. Oncol. 2018, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Hindiskere, S.; Errani, C.; Doddarangappa, S.; Ramaswamy, V.; Rai, M.; Chinder, P.S. Is a Short-Course of Preoperative Denosumab as Effective as Prolonged Therapy for Giant Cell Tumor of Bone? Clin. Orthop. Relat. Res. 2020, 478, 2522–2533. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liu, X.; Yang, Y.; Guo, W.; Yang, R.; Tang, X.; Yan, T.; Li, Y.; Tang, S.; Li, D.; et al. Ultra-Short Course of Neo-Adjuvant Denosumab for Nerve-Sparing Surgery for Giant Cell Tumor of Bone in Sacrum. Spine 2022, 47, 691–701. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Tanaka, Y.; Kido, A.; Kawaguchi, M.; Errani, C. Denosumab Does Not Decrease Local Recurrence in Giant Cell Tumor of Bone Treated with En Bloc Resection. Orthopedics 2021, 44, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kanwat, H.; Banjara, R.; Kumar, V.S.; Majeed, A.; Gamnagatti, S.; Khan, S.A. Comparison of Denosumab and Zoledronic Acid as Neoadjuvant Therapy in Patients with Giant Cell Tumor of Bone. J. Orthop. Surg. 2021, 29, 23094990211007565. [Google Scholar] [CrossRef]
- Balke, M.; Streitbuerger, A.; Budny, T.; Henrichs, M.; Gosheger, G.; Hardes, J. Treatment and Outcome of Giant Cell Tumors of the Pelvis. Acta Orthop. 2009, 80, 590–596. [Google Scholar] [CrossRef]
- Donati, D.; Wafa, H.; Di Bella, C.; Colangeli, M.; Colangeli, S.; Bertoni, F. Management of Pelvic Giant Cell Tumours Involving the Acetabular Bone. Acta Orthop. Belg. 2008, 74, 773–778. [Google Scholar]
- Kattapuram, A.S.; O’Donnell, R.J.; Huszar, M.; Rosenberg, A.E.; Kattapuram, S.V.; Mankin, H.J. Surgical Management of Innominate Giant Cell Tumor. Clin. Orthop. Relat. Res. 1996, 329, 281–287. [Google Scholar] [CrossRef]
- Leggon, R.E.; Zlotecki, R.; Reith, J.; Scarborough, M.T. Giant Cell Tumor of the Pelvis and Sacrum: 17 Cases and Analysis of the Literature. Clin. Orthop. Relat. Res. 2004, 423, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Osaka, S.; Toriyama, S. Surgical Treatment of Giant Cell Tumors of the Pelvis. Clin. Orthop. Relat. Res. 1987, 222, 123–131. [Google Scholar] [CrossRef]
- Sanjay, B.K.; Frassica, F.J.; Frassica, D.A.; Unni, K.K.; McLeod, R.A.; Sim, F.H. Treatment of Giant-Cell Tumor of the Pelvis. J. Bone Jt. Surg. Am. 1993, 75, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, X.; Zang, J.; Qu, H. Intralesional Excision versus Wide Resection for Giant Cell Tumor Involving the Acetabulum: Which Is Better? Clin. Orthop. Relat. Res. 2012, 470, 1213–1220. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.K. Resection and Reconstruction for Primary Neoplasms Involving the Innominate Bone. J. Bone Jt. Surg. Am. 1978, 60, 731–746. [Google Scholar] [CrossRef]
- Gradinger, R.; Rechl, H.; Hipp, E. Pelvic Osteosarcoma. Resection, Reconstruction, Local Control, and Survival Statistics. Clin. Orthop. Relat. Res. 1991, 270, 149–158. [Google Scholar] [CrossRef]
- Hillmann, A.; Hoffmann, C.; Gosheger, G.; Rödl, R.; Winkelmann, W.; Ozaki, T. Tumors of the Pelvis: Complications after Reconstruction. Arch. Orthop. Trauma. Surg. 2003, 123, 340–344. [Google Scholar] [CrossRef]
- Nilsonne, U.; Kreicbergs, A.; Olsson, E.; Stark, A. Function after Pelvic Tumour Resection Involving the Acetabular Ring. Int. Orthop. 1982, 6, 27–33. [Google Scholar] [CrossRef]
- Sambri, A.; Medellin, M.R.; Errani, C.; Campanacci, L.; Fujiwara, T.; Donati, D.; Parry, M.; Grimer, R. Denosumab in Giant Cell Tumour of Bone in the Pelvis and Sacrum: Long-Term Therapy or Bone Resection? J. Orthop. Sci. 2020, 25, 513–519. [Google Scholar] [CrossRef]
- Li, Z.; Lu, M.; Min, L.; Luo, Y.; Tu, C. Treatment of Pelvic Giant Cell Tumor by Wide Resection with Patient-Specific Bone-Cutting Guide and Reconstruction with 3D-Printed Personalized Implant. J. Orthop. Surg. Res. 2023, 18, 648. [Google Scholar] [CrossRef]
- Wei, R.; Guo, W.; Ji, T.; Zhang, Y.; Liang, H. One-Step Reconstruction with a 3D-Printed, Custom-Made Prosthesis after Total En Bloc Sacrectomy: A Technical Note. Eur. Spine J. 2017, 26, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, W.; Tang, X.; Yang, R.; Tang, S.; Qu, H.; Yang, Y.; Sun, X.; Du, Z. Preservation of the Contralateral Sacral Nerves during Hemisacrectomy for Sacral Malignancies. Eur. Spine J. 2014, 23, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, W.; Tang, X.; Ji, T.; Zhang, Y. Surgical Classification of Different Types of En Bloc Resection for Primary Malignant Sacral Tumors. Eur. Spine J. 2011, 20, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, W.; Yang, R.; Tang, X.; Ji, T. Proposed Scoring System for Evaluating Neurologic Deficit after Sacral Resection: Functional Outcomes of 170 Consecutive Patients. Spine 2016, 41, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, X.; Yan, T.; Ji, T.; Yang, R.; Guo, W. Risk Factors for the Local Recurrence of Giant Cell Tumours of the Sacrum Treated with Nerve-Sparing Surgery. Bone Jt. J. 2020, 102-B, 1392–1398. [Google Scholar] [CrossRef]
- Guo, W.; Ji, T.; Tang, X.; Yang, Y. Outcome of Conservative Surgery for Giant Cell Tumor of the Sacrum. Spine 2009, 34, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, W.; Tang, X.; Yang, R.; Yan, T.; Dong, S.; Wang, S.; Zaphiros, N. Can Aortic Balloon Occlusion Reduce Blood Loss During Resection of Sacral Tumors That Extend into the Lower Lumber Spine? Clin. Orthop. Relat. Res. 2018, 476, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Liu, X.; He, F.; Liang, H.; Yang, Y.; Ji, T.; Yang, R.; Guo, W. Retrospective Cohort Study of 68 Sacral Giant Cell Tumours Treated with Nerve-Sparing Surgery and Evaluation on Therapeutic Benefits of Denosumab Therapy. Bone Jt. J. 2020, 102-B, 177–185. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, H.; Liu, W.; Niu, X. Study of Imaging Changes Following Preoperative Denosumab for Giant Cell Tumor of Bone. J. Bone Oncol. 2022, 32, 100410. [Google Scholar] [CrossRef]
- Puri, A.; Gupta, S.M.; Gulia, A.; Shetty, N.; Laskar, S. Giant Cell Tumors of the Sacrum: Is Non-Operative Treatment Effective? Eur. Spine J. 2021, 30, 2881–2886. [Google Scholar] [CrossRef]
- Lin, P.P.; Guzel, V.B.; Moura, M.F.; Wallace, S.; Benjamin, R.S.; Weber, K.L.; Morello, F.A.; Gokaslan, Z.L.; Yasko, A.W. Long-Term Follow-up of Patients with Giant Cell Tumor of the Sacrum Treated with Selective Arterial Embolization. Cancer 2002, 95, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- He, S.-H.; Xu, W.; Sun, Z.-W.; Liu, W.-B.; Liu, Y.-J.; Wei, H.-F.; Xiao, J.-R. Selective Arterial Embolization for the Treatment of Sacral and Pelvic Giant Cell Tumor: A Systematic Review. Orthop. Surg. 2017, 9, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, Y.; Guo, W.; Yang, R.; Tang, X.; Yan, T.; Ji, T.; Xie, L.; Xu, J.; Wang, J. Therapeutic Benefits of Neoadjuvant and Post-Operative Denosumab on Sacral Giant Cell Tumor: A Retrospective Cohort Study of 30 Cases. J. BU ON. Off. J. Balk. Union Oncol. 2018, 23, 453–459. [Google Scholar]
- Tsukamoto, S.; Ali, N.; Mavrogenis, A.F.; Honoki, K.; Tanaka, Y.; Spinnato, P.; Donati, D.M.; Errani, C. Intralesional Nerve-Sparing Surgery versus Non-Surgical Treatment for Giant Cell Tumor of the Sacrum. BMC Musculoskelet. Disord. 2021, 22, 1023. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A System for the Surgical Staging of Musculoskeletal Sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Boriani, S.; Bandiera, S.; Casadei, R.; Boriani, L.; Donthineni, R.; Gasbarrini, A.; Pignotti, E.; Biagini, R.; Schwab, J.H. Giant Cell Tumor of the Mobile Spine: A Review of 49 Cases. Spine 2012, 37, E37–E45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, F.; Dang, L.; Li, Y.; Liu, X.; Liu, Z.; Wei, F. Comparison of the Prognostic Factors of Total En Bloc Spondylectomy and Total Piecemeal Spondylectomy in Patients with Enneking Stage III Giant Cell Tumor in the Thoracic and Lumbar Spine. Eur. Spine J. 2023, 32, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Lu, J.; Zhu, X.; Song, G.; Wu, H.; Xu, H.; Wang, A.; Wang, J. The Efficacy and Safety of Short-Course Neoadjuvant Denosumab for En Bloc Spondylectomy in Spinal Giant Cell Tumor of Bone: A Preliminary Report. Eur. Spine J. 2023, 32, 4297–4305. [Google Scholar] [CrossRef]
- Tubbs, W.S.; Brown, L.R.; Beabout, J.W.; Rock, M.G.; Unni, K.K. Benign Giant-Cell Tumor of Bone with Pulmonary Metastases: Clinical Findings and Radiologic Appearance of Metastases in 13 Cases. AJR Am. J. Roentgenol. 1992, 158, 331–334. [Google Scholar] [CrossRef]
- Siebenrock, K.A.; Unni, K.K.; Rock, M.G. Giant-Cell Tumour of Bone Metastasising to the Lungs. A Long-Term Follow-Up. J. Bone Jt. Surg. Br. 1998, 80, 43–47. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Niu, X.; Xu, H.; Li, Y.; Liu, W. Clinical Characteristics and Risk Factors Analysis of Lung Metastasis of Benign Giant Cell Tumor of Bone. J. Bone Oncol. 2017, 7, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Poosiripinyo, T.; Sukpanichyingyong, S.; Salang, K.; Mahikul, W.; Chobpenthai, T. Non-surgical Outcomes and Risk Factors for Pulmonary Metastasis from Giant Cell Tumor of Bone. Oncol. Lett. 2023, 26, 508. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Mavrogenis, A.F.; Leone, G.; Righi, A.; Akahane, M.; Tanzi, P.; Kido, A.; Honoki, K.; Tanaka, Y.; Donati, D.M.; et al. Denosumab Does Not Decrease the Risk of Lung Metastases from Bone Giant Cell Tumour. Int. Orthop. 2019, 43, 483–489. [Google Scholar] [CrossRef]
- Chan, C.M.; Adler, Z.; Reith, J.D.; Gibbs, C.P. Risk Factors for Pulmonary Metastases from Giant Cell Tumor of Bone. J. Bone Jt. Surg. Am. 2015, 97, 420–428. [Google Scholar] [CrossRef]
- Rosario, M.; Kim, H.-S.; Yun, J.Y.; Han, I. Surveillance for Lung Metastasis from Giant Cell Tumor of Bone. J. Surg. Oncol. 2017, 116, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, W.; Xie, X.; Tu, J.; Huang, G.; Zou, C.; Yin, J.; Wen, L.; Shen, J. Development and Validation of a Prognostic Index to Predict Pulmonary Metastasis of Giant Cell Tumor of Bone. Oncotarget 2017, 8, 108054–108063. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Yang, Y.; Yang, R.; Tang, X.; Yan, T.; Guo, W. Pulmonary Metastasis of Giant Cell Tumour: A Retrospective Study of Three Hundred and Ten Cases. Int. Orthop. 2021, 45, 769–778. [Google Scholar] [CrossRef]
- Itkin, B.; Straminsky, S.; De Ronato, G.; Lewi, D.; Marantz, A.; Bardach, A. Prognosis of Metastatic Giant Cell Tumor of Bone in the Pre-Denosumab Era. A Systematic Review and a Meta-Analysis. Jpn. J. Clin. Oncol. 2018, 48, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Ciani, G.; Mavrogenis, A.F.; Ferrari, C.; Akahane, M.; Tanaka, Y.; Rocca, M.; Longhi, A.; Errani, C. Outcome of Lung Metastases Due to Bone Giant Cell Tumor Initially Managed with Observation. J. Orthop. Surg. Res. 2020, 15, 510. [Google Scholar] [CrossRef]
- Kito, M.; Matusmoto, S.; Ae, K.; Tanizawa, T.; Gokita, T.; Kobayashi, H.; Hayakawa, K.; Funauchi, Y. Pulmonary Metastasis from Giant Cell Tumor of Bone: Clinical Outcome Prior to the Introduction of Molecular Target Therapy. Jpn. J. Clin. Oncol. 2017, 47, 529–534. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Zhao, L.; Schuetze, S.M.; Chugh, R. Giant Cell Tumor of Bone: Effect of Longer Dosing Intervals of Denosumab on Tumor Control and Bone-Related Complications. Oncologist 2022, 27, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Chawla, N.S.; Ferrari, S.; Sudan, M.; Picci, P.; Marchesi, E.; Leopardi, M.P.; Syed, I.; Sankhala, K.K.; Parthasarathy, P.; et al. Denosumab in Advanced/Unresectable Giant-Cell Tumour of Bone (GCTB): For How Long? Eur. J. Cancer 2017, 76, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Law, G.W.; Yeo, N.E.M.; Howe, T.S.; Tan, Y.Z.; Tan, S.B.; Siddiqui, M.M.A. Recommencement of Denosumab for Unresectable Giant Cell Tumor of the Cervical Spine: A Case Report. Spine 2018, 43, E551–E556. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, A.; Simeone, N.; Guzzo, M.; Maniezzo, M.; Collini, P.; Morosi, C.; Greco, F.G.; Frezza, A.M.; Casali, P.G.; Stacchiotti, S. Rechallenge of Denosumab in Jaw Osteonecrosis of Patients with Unresectable Giant Cell Tumour of Bone: A Case Series Analysis and Literature Review. ESMO Open 2020, 5, e000663. [Google Scholar] [CrossRef] [PubMed]
- Nasca, V.; Frezza, A.M.; Morosi, C.; Buonomenna, C.; Parafioriti, A.; Zappalà, G.; Bini, F.; Casali, P.G.; Loppini, M.; Stacchiotti, S. Rechallenge of Denosumab in Advanced Giant Cell Tumor of the Bone after Atypical Femur Fracture: A Case Report and Review of Literature. Front. Oncol. 2022, 12, 953149. [Google Scholar] [CrossRef] [PubMed]
- Trovarelli, G.; Pala, E.; Angelini, A.; Ruggieri, P. A Systematic Review of Multicentric Giant Cell Tumour with the Presentation of Three Cases at Long-Term Follow-Up. Bone Jt. J. 2022, 104-B, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Errani, C.; Tsukamoto, S.; Angulo Alvarado, R.; Righi, A.; Nitta, Y.; Donati, D.M.; Mavrogenis, A.F. Multicentric Giant Cell Tumor of Bone. Orthopedics 2023, 46, e376–e380. [Google Scholar] [CrossRef]
- Chawla, S.; Blay, J.-Y.; Rutkowski, P.; Le Cesne, A.; Reichardt, P.; Gelderblom, H.; Grimer, R.J.; Choy, E.; Skubitz, K.; Seeger, L.; et al. Denosumab in Patients with Giant-Cell Tumour of Bone: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2019, 20, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Dubory, A.; Missenard, G.; Domont, J.; Court, C. Interest of Denosumab for the Treatment of Giant-Cells Tumors and Aneurysmal Bone Cysts of the Spine. About Nine Cases. Spine 2016, 41, E654–E660. [Google Scholar] [CrossRef]
- Thornley, P.; Habib, A.; Bozzo, A.; Evaniew, N.; Ghert, M. The Role of Denosumab in the Modern Treatment of Giant Cell Tumor of Bone. JBJS Rev. 2017, 5, e4. [Google Scholar] [CrossRef]
- Chandler, A.; Bartelstein, M.K.; Fujiwara, T.; Antonescu, C.R.; Healey, J.H.; Vaynrub, M. Anti-IL17 Antibody Secukinumab Therapy Is Associated with Ossification in Giant Cell Tumor of Bone: A Case Report of Pathologic Similarities and Therapeutic Potential Similar to Denosumab. BMC Musculoskelet. Disord. 2021, 22, 320. [Google Scholar] [CrossRef]
- Genovese, M.C.; Durez, P.; Richards, H.B.; Supronik, J.; Dokoupilova, E.; Mazurov, V.; Aelion, J.A.; Lee, S.-H.; Codding, C.E.; Kellner, H.; et al. Efficacy and Safety of Secukinumab in Patients with Rheumatoid Arthritis: A Phase II, Dose-Finding, Double-Blind, Randomised, Placebo Controlled Study. Ann. Rheum. Dis. 2013, 72, 863–869. [Google Scholar] [CrossRef]
- Baeten, D.; Baraliakos, X.; Braun, J.; Sieper, J.; Emery, P.; van der Heijde, D.; McInnes, I.; van Laar, J.M.; Landewé, R.; Wordsworth, P.; et al. Anti-Interleukin-17A Monoclonal Antibody Secukinumab in Treatment of Ankylosing Spondylitis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2013, 382, 1705–1713. [Google Scholar] [CrossRef]
- Yue, J.; Sun, W.; Li, S. Denosumab versus Zoledronic Acid in Cases of Surgically Unsalvageable Giant Cell Tumor of Bone: A Randomized Clinical Trial. J. Bone Oncol. 2022, 35, 100441. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, W.; Yin, H.; Huang, Q.; Liu, T.; Yang, X.; Wei, H.; Xiao, J. Therapeutic Radiotherapy for Giant Cell Tumor of the Spine: A Systemic Review. Eur. Spine J. 2015, 24, 1754–1760. [Google Scholar] [CrossRef]
- Chen, Z.X.; Gu, D.Z.; Yu, Z.H.; Qian, T.N.; Huang, Y.R.; Hu, Y.H.; Gu, X.Z. Radiation Therapy of Giant Cell Tumor of Bone: Analysis of 35 Patients. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 329–334. [Google Scholar] [CrossRef]
- van der Heijden, L.; Dijkstra, P.D.S.; Blay, J.-Y.; Gelderblom, H. Giant Cell Tumour of Bone in the Denosumab Era. Eur. J. Cancer 2017, 77, 75–83. [Google Scholar] [CrossRef]
- Mahdal, M.; Neradil, J.; Mudry, P.; Paukovcekova, S.; Staniczkova Zambo, I.; Urban, J.; Macsek, P.; Pazourek, L.; Tomas, T.; Veselska, R. New Target for Precision Medicine Treatment of Giant-Cell Tumor of Bone: Sunitinib Is Effective in the Treatment of Neoplastic Stromal Cells with Activated PDGFRβ Signaling. Cancers 2021, 13, 3543. [Google Scholar] [CrossRef]
- De Vita, A.; Vanni, S.; Miserocchi, G.; Fausti, V.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Liverani, C.; Calabrese, C.; Casadei, R.; et al. A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines 2022, 10, 372. [Google Scholar] [CrossRef]
- Metovic, J.; Annaratone, L.; Linari, A.; Osella-Abate, S.; Musuraca, C.; Veneziano, F.; Vignale, C.; Bertero, L.; Cassoni, P.; Ratto, N.; et al. Prognostic Role of PD-L1 and Immune-Related Gene Expression Profiles in Giant Cell Tumors of Bone. Cancer Immunol. Immunother. 2020, 69, 1905–1916. [Google Scholar] [CrossRef]
- Zheng, B.-W.; Zheng, B.-Y.; Niu, H.-Q.; Yang, Y.-F.; Zhu, G.-Q.; Li, J.; Zhang, T.-L.; Zou, M.-X. Tumor Growth Rate in Spinal Giant Cell Tumors of Bone and Association with the Immune Microenvironment and Denosumab Treatment Responsiveness: A Multicenter Study. Neurosurgery 2023, 92, 524–537. [Google Scholar] [CrossRef]
- Liu, W.; Chan, C.M.; Gong, L.; Bui, M.M.; Han, G.; Letson, G.D.; Yang, Y.; Niu, X. Malignancy in Giant Cell Tumor of Bone in the Extremities. J. Bone Oncol. 2021, 26, 100334. [Google Scholar] [CrossRef]
- Domovitov, S.V.; Healey, J.H. Primary Malignant Giant-Cell Tumor of Bone Has High Survival Rate. Ann. Surg. Oncol. 2010, 17, 694–701. [Google Scholar] [CrossRef]
- Rock, M.G.; Sim, F.H.; Unni, K.K.; Witrak, G.A.; Frassica, F.J.; Schray, M.F.; Beabout, J.W.; Dahlin, D.C. Secondary Malignant Giant-Cell Tumor of Bone. Clinicopathological Assessment of Nineteen Patients. J. Bone Jt. Surg. Am. 1986, 68, 1073–1079. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Righi, A.; Mavrogenis, A.F.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; Errani, C. Late Local Recurrence of Bone Giant Cell Tumors Associated with an Increased Risk for Malignant Transformation. Cancers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Alaqaili, S.I.; Abduljabbar, A.M.; Altaho, A.J.; Khan, A.A.; Alherabi, J.A. Malignant Sarcomatous Transformation of Benign Giant Cell Tumor of Bone after Treatment with Denosumab Therapy: A Literature Review of Reported Cases. Cureus 2018, 10, e3792. [Google Scholar] [CrossRef]
- Aponte-Tinao, L.A.; Piuzzi, N.S.; Roitman, P.; Farfalli, G.L. A High-Grade Sarcoma Arising in a Patient with Recurrent Benign Giant Cell Tumor of the Proximal Tibia While Receiving Treatment with Denosumab. Clin. Orthop. Relat. Res. 2015, 473, 3050–3055. [Google Scholar] [CrossRef]
- Broehm, C.J.; Garbrecht, E.L.; Wood, J.; Bocklage, T. Two Cases of Sarcoma Arising in Giant Cell Tumor of Bone Treated with Denosumab. Case Rep. Med. 2015, 2015, 767198. [Google Scholar] [CrossRef]
- Park, A.; Cipriano, C.A.; Hill, K.; Kyriakos, M.; McDonald, D.J. Malignant Transformation of a Giant Cell Tumor of Bone Treated with Denosumab: A Case Report. JBJS Case Connect. 2016, 6, e78. [Google Scholar] [CrossRef]
- Thomas, D.; Carriere, P.; Jacobs, I. Safety of Denosumab in Giant-Cell Tumour of Bone. Lancet Oncol. 2010, 11, 815. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Righi, A.; Vanel, D.; Honoki, K.; Donati, D.M.; Errani, C. Development of High-Grade Osteosarcoma in a Patient with Recurrent Giant Cell Tumor of the Ischium While Receiving Treatment with Denosumab. Jpn. J. Clin. Oncol. 2017, 47, 1090–1096. [Google Scholar] [CrossRef]
- Palmerini, E.; Seeger, L.L.; Gambarotti, M.; Righi, A.; Reichardt, P.; Bukata, S.; Blay, J.-Y.; Dai, T.; Jandial, D.; Picci, P. Malignancy in Giant Cell Tumor of Bone: Analysis of an Open-Label Phase 2 Study of Denosumab. BMC Cancer 2021, 21, 89. [Google Scholar] [CrossRef]
- van Langevelde, K.; Cleven, A.H.G.; Navas Cañete, A.; van der Heijden, L.; van de Sande, M.A.J.; Gelderblom, H.; Bovée, J.V.M.G. Malignant Transformation of Giant Cell Tumor of Bone and the Association with Denosumab Treatment: A Radiology and Pathology Perspective. Sarcoma 2022, 2022, 3425221. [Google Scholar] [CrossRef]
- Anract, P.; De Pinieux, G.; Cottias, P.; Pouillart, P.; Forest, M.; Tomeno, B. Malignant Giant-Cell Tumours of Bone. Clinico-Pathological Types and Prognosis: A Review of 29 Cases. Int. Orthop. 1998, 22, 19–26. [Google Scholar] [CrossRef]
- Morii, R.; Tsukamoto, S.; Righi, A.; Honoki, K.; Tanaka, Y.; Kido, A.; Fujii, H.; Mavrogenis, A.F.; Tanaka, Y.; Errani, C. Effect of Adjuvant Chemotherapy on Localized Malignant Giant Cell Tumor of Bone: A Systematic Review. Cancers 2021, 13, 5410. [Google Scholar] [CrossRef]
- Bertoni, F.; Bacchini, P.; Staals, E.L. Malignancy in Giant Cell Tumor of Bone. Cancer 2003, 97, 2520–2529. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Presneau, N.; Scheipl, S.; Pillay, N.; Van Loo, P.; Wedge, D.C.; Cooke, S.L.; Gundem, G.; Davies, H.; et al. Distinct H3F3A and H3F3B Driver Mutations Define Chondroblastoma and Giant Cell Tumor of Bone. Nat. Genet. 2013, 45, 1479–1482. [Google Scholar] [CrossRef]
- Khazaei, S.; De Jay, N.; Deshmukh, S.; Hendrikse, L.D.; Jawhar, W.; Chen, C.C.L.; Mikael, L.G.; Faury, D.; Marchione, D.M.; Lanoix, J.; et al. H3.3 G34W Promotes Growth and Impedes Differentiation of Osteoblast-Like Mesenchymal Progenitors in Giant Cell Tumor of Bone. Cancer Discov. 2020, 10, 1968–1987. [Google Scholar] [CrossRef]
- Yoshida, K.-I.; Nakano, Y.; Honda-Kitahara, M.; Wakai, S.; Motoi, T.; Ogura, K.; Sano, N.; Shibata, T.; Okuma, T.; Iwata, S.; et al. Absence of H3F3A Mutation in a Subset of Malignant Giant Cell Tumor of Bone. Mod. Pathol. 2019, 32, 1751–1761. [Google Scholar] [CrossRef]
- Oda, Y.; Sakamoto, A.; Saito, T.; Matsuda, S.; Tanaka, K.; Iwamoto, Y.; Tsuneyoshi, M. Secondary Malignant Giant-Cell Tumour of Bone: Molecular Abnormalities of P53 and H-Ras Gene Correlated with Malignant Transformation. Histopathology 2001, 39, 629–637. [Google Scholar] [CrossRef]
- Ishihara, S.; Yamamoto, H.; Iwasaki, T.; Toda, Y.; Yamamoto, T.; Yoshimoto, M.; Ito, Y.; Susuki, Y.; Kawaguchi, K.; Kinoshita, I.; et al. Histological and Immunohistochemical Features and Genetic Alterations in the Malignant Progression of Giant Cell Tumor of Bone: A Possible Association with TP53 Mutation and Loss of H3K27 Trimethylation. Mod. Pathol. 2022, 35, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Donigian, S.; Whiteway, S.L.; Hipp, S.J.; Lybeck, D.; Clark, R.O. Malignant Giant Cell Tumor of Bone with a KRAS G12V Mutation. J. Pediatr. Hematol. Oncol. 2022, 44, e268–e271. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Okajima, K.; Ishibashi, Y.; Zhang, L.; Hirai, T.; Kage, H.; Shinozaki-Ushiku, A.; Oda, K.; Tanaka, S.; Kobayashi, H. Clinical Genomic Profiling of Malignant Giant Cell Tumor of Bone: A Retrospective Analysis Using a Real-world Database. Med. Int. 2024, 4, 17. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukamoto, S.; Mavrogenis, A.F.; Masunaga, T.; Honoki, K.; Fujii, H.; Kido, A.; Tanaka, Y.; Errani, C. Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update. Curr. Oncol. 2024, 31, 2112-2132. https://doi.org/10.3390/curroncol31040157
Tsukamoto S, Mavrogenis AF, Masunaga T, Honoki K, Fujii H, Kido A, Tanaka Y, Errani C. Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update. Current Oncology. 2024; 31(4):2112-2132. https://doi.org/10.3390/curroncol31040157
Chicago/Turabian StyleTsukamoto, Shinji, Andreas F. Mavrogenis, Tomoya Masunaga, Kanya Honoki, Hiromasa Fujii, Akira Kido, Yasuhito Tanaka, and Costantino Errani. 2024. "Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update" Current Oncology 31, no. 4: 2112-2132. https://doi.org/10.3390/curroncol31040157
APA StyleTsukamoto, S., Mavrogenis, A. F., Masunaga, T., Honoki, K., Fujii, H., Kido, A., Tanaka, Y., & Errani, C. (2024). Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update. Current Oncology, 31(4), 2112-2132. https://doi.org/10.3390/curroncol31040157







