Abstract
Breast cancer is the leading cause of cancer-related death in women worldwide and the fifth most common cause of cancer death overall. Most women with breast cancer have a good prognosis if the cancer is detected at an early stage and the patients have access to the appropriate treatment and disease management. This study aims to evaluate the impact of pharmacist-led interventions on breast cancer management and health outcomes. A literature review was carried out through the scientific databases PubMed, Scopus, and Web of Science using predefined keywords. Only full-text original articles written in English that investigated the role of the pharmacist in the management of breast cancer were included in the final analysis. No publication date limits were set. A total of 1625 articles were retrieved from the electronic databases, of which 14 met the inclusion criteria. The current scoping review consists of different study types, including randomized controlled trials, cross-sectional studies, pre-post studies, retrospective cohort studies, quality improvement projects, case-control studies, and one pharmacoeconomic study. Pharmacists commonly provided the following interventions: consultations regarding chemotherapy treatment, risk assessment and patient education, adverse drug reactions and drug-drug interactions detection, and adherence assessment. This scoping review highlights the beneficial effects of the involvement of pharmacists in breast cancer management, such as better quality of life, reduced drug interaction risk, greater adherence rates, and improved patient knowledge. This confirms the importance of including the pharmacist in the oncology team caring for patients with breast cancer.
1. Introduction
Women’s health is a critical aspect of overall well-being, yet there is often a tendency to neglect it due to societal expectations and roles [1]. Breast cancer poses a significant challenge and is the leading cause of cancer-related death among women [2]. According to data from the World Agency for Research on Cancer (IARC) in 2020, about 2.26 million women were diagnosed with breast cancer, and almost 685,000 deaths were caused by the disease globally [2]. Despite the multiple effective therapeutic options, breast cancer is the leading cause of cancer-related death in women worldwide and the fifth most common cause of cancer death overall [3]. Late diagnosis is a prevailing issue, resulting in reduced treatment efficacy and higher mortality rates [4]. Most women with breast cancer have a good prognosis if the cancer is detected at an early stage and the patients have access to the appropriate treatment. Chemotherapy remains the standard of care for this type of disease, although complementary and alternative treatment methods are also described in the literature [5,6]. Despite remarkable progress in this setting to date, some patients will see their disease return in the long term, which is why there is a critical need to continue to optimize early breast cancer care and to take additional measures to prevent the disease from evolving into an advanced, incurable stage [7].
Breast cancer treatment is a complex and multi-step process, the outcome of which depends on the cooperation and coordination between different healthcare providers. After diagnosis, patients need to be provided with detailed chemotherapy education, including the mechanism of action of chemotherapy agents, treatment goals, possible side effects, and symptom management [8]. Therefore, according to the current recommendations of the European Association of Medical Oncology (ESMO), the treatment should be carried out by a multidisciplinary team consisting of a medical oncologist, a surgeon, a radiation therapist, a pathologist, and a specialized (best in breast cancer) oncology nurse [9]. Patients should be actively involved in all management decisions during the therapeutic process. The result of the healthcare team’s efforts depends to a large extent on the involvement of the patients and their clear understanding and support of the jointly selected therapeutic decisions [10].
For more than 50 years, oncology pharmacists have proved to be core multidisciplinary team members in the care of patients with cancer [11]. Recent studies confirm oncology pharmacists’ contribution to better patient outcomes, including a reduced number of drug-related problems (DRPs), improved quality of life (QoL), and increased adherence rates [12,13,14]. Additionally, pharmacist-led interventions in oncology care have been reported to be cost-effective and cost-saving [15,16]. The role and activities of oncology pharmacists are continuously expanding, and now this role is focused on patient-centered care [8,11]. Common roles for oncology pharmacists, their clinical functions, and different settings for professional development, have been comprehensively discussed by Holle et al. [11]. One of the key activities provided by oncology pharmacists is patient counseling and education [17].
Oncology pharmacists meet and monitor the patient throughout the course of treatment to assess and reduce the potential for adverse drug reactions (ADRs). This helps build a relationship between pharmacists and cancer patients and can be a key element in identifying ADRs as well as ensuring medication adherence [18]. The results of a recent interventional study show a significant improvement in medication adherence rates after education and counseling provided by pharmacists [19].
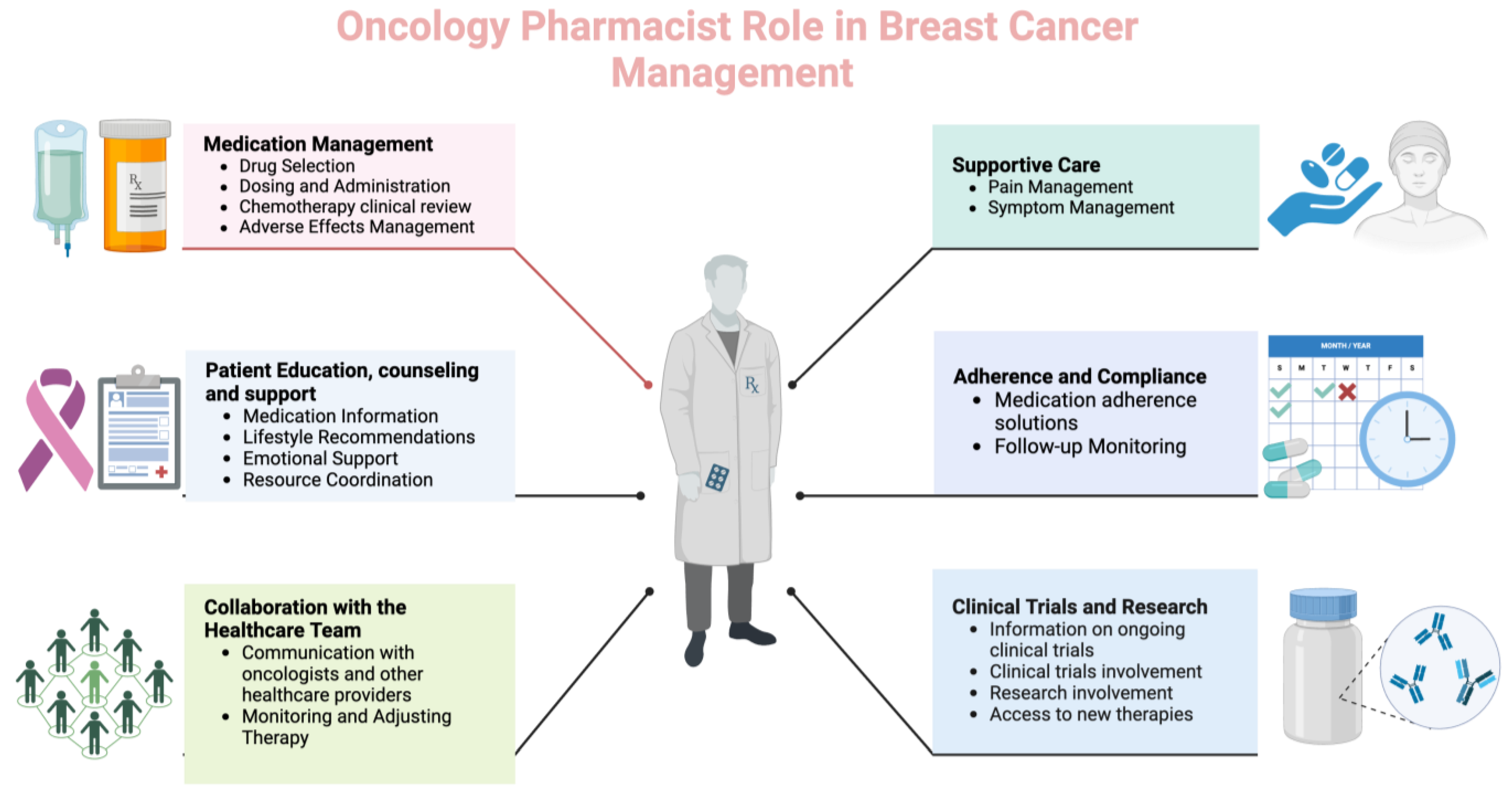
Pharmacists can play a crucial role in managing patients with breast cancer as part of the healthcare team. Their responsibilities encompass various aspects of pharmaceutical care, including medication management, education, and support (Figure 1).
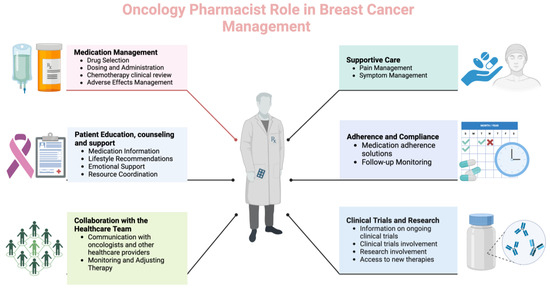
Figure 1.
Different roles and functions of oncology pharmacists in breast cancer care (Created with BioRender.com, accessed on 23 April 2024).
Over the years, in many countries, clinical pharmacists have become an integral part of the oncology team involved in the treatment of breast cancer. Oncology pharmacists have expert knowledge of medications used in the treatment of breast cancer, including their pharmacokinetic properties. As essential multidisciplinary team members, oncology pharmacists help optimize the benefits of drug therapy and help reduce adverse and toxic effects [18]. Integrating oncology pharmacists into healthcare teams adds value through their expertise in medication management and enhancing adherence, allowing physicians to optimize time during clinic appointments and potentially see more patients [20].
To the best of our knowledge, no systematic review has assessed the clinical pharmacist’s role in caring for breast cancer patients. Therefore, this study aims to evaluate the impact of pharmacist-led interventions on breast cancer management and health outcomes.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
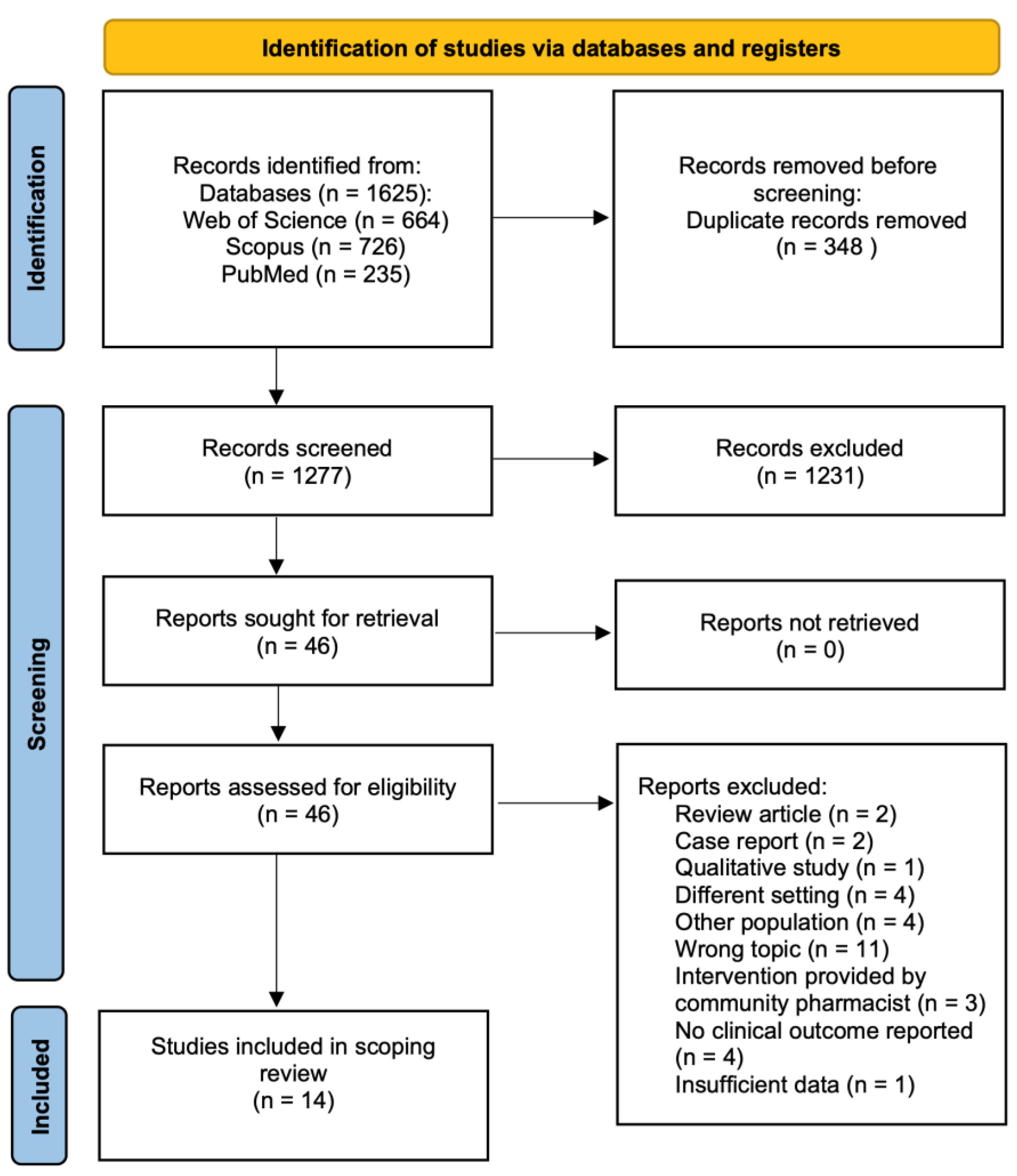
A systematic search was conducted on the PubMed, Web of Science, and Scopus databases. The following keywords were used: (“pharmacist” OR “clinical pharmacist” OR “oncology pharmacist”) AND (“breast cancer”). The initial search for relevant articles started in December 2023 and was finalized in March 2024. No limitations on the date of published articles were set. The current scoping review was carried out in accordance with the Preferred Reporting for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 2) [21].
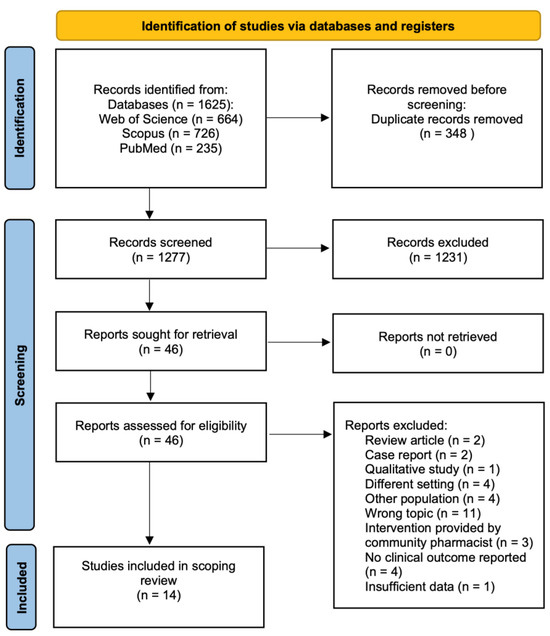
Figure 2.
PRISMA flowchart for study selection.
2.2. Eligibility Criteria
Inclusion criteria were full-text research articles written in English that investigate the impact of pharmacist-led interventions on health outcomes in breast cancer patients (e.g., quality of life, drug interaction risk, adherence rate, patient knowledge, etc.). Non-English-language articles and those that did not report on the primary outcome of the current study were excluded from the analysis. Systematic reviews, meta-analyses, narrative reviews, case reports, qualitative studies, editorials, letters to the editor, commentaries, and abstracts from conferences were not considered for inclusion in this review. The eligibility criteria of articles based on the PICO framework (population, intervention, comparison, and outcome) are presented in Table 1.

Table 1.
PICOS criteria for study selection.
2.3. Data Extraction
One author (R.S.) performed the initial search in the selected databases. In the next stage, duplicates were removed using Zotero software v. 6.0.37. Two authors (R.S. and E.G.) independently screened the titles and abstracts of identified articles to exclude ineligible studies. The full text of papers that met the inclusion criteria was retrieved and assessed by one author (R.S.) and rechecked by a second author (E.G.). In case of any discrepancies, they were addressed following a discussion with a third author (D.K.).
The following relevant data from each publication that met the inclusion criteria were extracted:
- (1)
- Primary author and year of publication
- (2)
- Country
- (3)
- Study design
- (4)
- Sample size
- (5)
- Objective
- (6)
- Study outcomes and main results
3. Results
A total of 1625 articles were retrieved from the electronic databases in the initial search, of which 14 met the inclusion criteria and assessed pharmacist interventions on health outcomes in breast cancer patients (Table 2) [13,22,23,24,25,26,27,28,29,30,31,32,33,34]. After the removal of duplicates, the remaining 1277 papers were screened by title and abstract. Following the screening process, 1231 records were excluded due to their irrelevance to the main topic of this review. The full texts of the remaining articles were assessed for eligibility. Of those, 32 were removed for several reasons, as illustrated in Figure 2.

Table 2.
Characteristics of included studies.
3.1. Characteristics of Studies Included
Of the 14 studies included in the review, the majority were interventional, including pre-post studies (n = 5, 35.7%) [13,22,23,24,25], randomized controlled studies (n = 1, 7.1%) [26] and prospective clinical trials (n = 1, 7.1%) [27], followed by observational studies, including case-control studies (n = 2, 14.3%) [30,34], cross-sectional studies (n = 2, 14.3%) [28,33] and retrospective cohort studies (n = 1, 7.1%) [32], and the remaining were quality improvement projects (n = 1, 7.1%) [31] and economic evaluations (n = 1, 7.1%) [29]. Regarding country of origin, five of the included studies were conducted in the USA [13,23,26,31,32], three in Japan [25,28,29], one in Egypt [24], India [22], France [27], Brazil [33], Malaysia [30], and Iraq [34]. The studies were published between 2012 and 2023. The pharmacist’s intervention involved mainly one or more of the following: pharmacist-led counselling or education [24,25,28,29,30] and management of medication adherence, ADRs and/or drug-interactions assessment [13,23,25,26,27,31,32,33,34]. The sample size of breast cancer patients in each study ranged from eighteen [23] to one-hundred forty-five [32]. Three of the studies reported health outcomes in patients diagnosed with different types of malignancies, including breast cancer [13,26,33]. For the purpose of our study, only the results related to breast cancer patients were extracted and analyzed.
3.2. Patient Education and Counselling
Six studies reported pharmacist engagement in patient education and counselling [22,24,25,28,29,30]. Of those, four studies explored the effect of pharmacist counselling on quality of life (QoL) in patients with breast cancer [24,28,29,30]. These studies are analyzed in the ‘Improving Quality of Life (QoL)’ section presented below.
The results from a pre-post study conducted in Japan revealed that pharmacists spent 75 h per month in patient education and ADRs monitoring, which led to the reduction of ADRs (nausea and vomiting) and facilitated the rational use of anti-emetic drugs [25].
The beneficial effect of pharmacist-led intervention on patient knowledge was also demonstrated by Dang et al. [30]. Pharmacist-led pre-chemotherapy counselling led to improvements in patient knowledge scores and a better understanding of the chemotherapy regimen and side effects [30].
3.3. Adherence Assessment
Four of the included studies assessed the change in adherence rate after pharmacist-led interventions [13,23,31,34]. A study conducted in the USA by Muluneh et al. described an innovative model involving the integration of a closed-loop, pharmacy-led oral chemotherapy management program at ambulatory oncology clinics [13]. In this closed-loop model, clinical pharmacists specializing in oncology were integrated into breast oncology clinics to manage patients receiving oral chemotherapy. One of the activities of clinical pharmacists was to assess and enhance the adherence to oral chemotherapy, which was performed at every patient encounter. For measuring adherence, patient self-reports and medication possession ratio (MPR) were used. After pharmacist-led interventions, patients achieved a self-reported adherence rate of 86%, which was verified by the MPR calculation of 85% (the goal adherence rate was >80%) [13].
Another study conducted in the USA aimed to assess the feasibility of an intervention for symptom monitoring and management, which clinical pharmacists facilitated to improve adherence to breast cancer adjuvant endocrine therapy (AET) [23]. In this pilot study, clinical pharmacists used guideline-based symptom management and adherence-supporting tools with nonadherent breast cancer patients. In the six-month study period, 44% of patients became adherent. Despite its small sample size (only 18 patients), this intervention has promising potential to enhance support and self-efficacy and improve patient symptoms and adherence rates [23].
A recent quality improvement project describes different interventions provided by outpatient clinical pharmacists for improving management and adherence to oral cancer therapy [31]. A total of 31 adherence counseling interventions were documented, including different methods such as medication calendars and increased monitoring frequency. Other pharmacist-led interventions included in this project consist of medication reconciliations and clinical recommendations (e.g., therapy modification, dose adjustments, toxicity management, and monitoring). The findings from this project indicate that the incorporation of a clinical pharmacist in a multidisciplinary team was associated with decreased treatment day delays (from 7.7 days in the preintervention assessment to 2.1 days after the implementation of the program, p < 0.001) [31].
An Iraqi study measured the adherence rate after pharmacist-led intervention in women with breast cancer on adjuvant oral hormonal therapy [34]. The researchers used the Morisky Medication Adherence Scale eight (8) items (MMAS-8) and the Beliefs about Medication Questionnaire (BMQ) for measuring adherence to medication therapy and assessing women’s beliefs. Two months after the initiation of the intervention, 65.4% of the patients in the pharmacist-led group demonstrated apparent adherence to adjuvant oral hormonal therapy. In addition, the pharmacist intervention significantly improved the necessity beliefs and necessity-concern differential when compared with the control group [34].
3.4. Management of Adverse Side Effects and Drug Interactions
Five studies reported the impact of pharmacist involvement on the detection and management of ADRs, drug-drug interactions (DDIs), and other DRPs [25,26,27,32,33]. One study reported that clinical pharmacist interventions facilitated a decrease in chemotherapy-induced nausea and vomiting. Additionally, pharmacist activities were cost-effective and reduced anti-emesis costs by 16% [25]. In a randomized clinical trial, older cancer patients received a multidisciplinary geriatric assessment-driven intervention that generated a significant reduction in chemotherapy-related toxic effects. In this RCT, pharmacists were involved in the detection of inappropriate polypharmacy and its rectification (e.g., providing recommendations for DDIs, potentially inappropriate medications, therapeutic duplication, etc.) [26].
In a recent prospective study, researchers from France presented the results of a comprehensive medication history carried out by a hospital pharmacist who participated in a clinical trial to determine the risk of DDIs. Pharmacist-led interventions have reduced the risk of DDIs in almost one-third of patients [27].
A pharmacist-led anticoagulation management service in breast cancer clinics has shown that, compared to other studies conducted on cancer patients receiving regular medical care, the pharmacy service reduced the frequency of recurrent venous thromboembolism and increased the time spent on therapeutic International Normalized Ratios (INRs) [32].
Ferracini et al. reported that pharmacist-led interventions had a significant impact on avoiding prescribing errors. In this cross-sectional study, clinical pharmacists monitored drug therapy and analyzed 1874 prescriptions from 248 hospitalized patients with gynecological and breast cancer. Of the 1874 evaluated prescriptions, 215 (11.5%) contained at least one error. Pharmacists provided 294 pharmaceutical interventions, including drug interaction assessment and dose adjustment, of which 73.5% were accepted by oncologists [33].
3.5. Improving Quality of Life (QoL)
Four of the analyzed articles evaluated the effect of pharmacist consultations and interventions on the QoL in patients with breast cancer [22,24,28,29].
Tanaka et al. applied the Quality-of-Life Questionnaire for Cancer Patients Treated with Anticancer Drugs (QOL-ACD) to evaluate the impact of pharmacist counseling on breast cancer patients who underwent their initial course of outpatient cancer chemotherapy [28]. The QOL-ACD instrument is a generic questionnaire that was developed in Japan by Kurihara et al. for measuring QoL in patients diagnosed with different types of cancer who are treated with antineoplastic drugs [35]. Findings from this study revealed that pharmacists can improve patients’ QoL regarding malaise and nausea by providing personal counseling before medical examinations [28].
The same authors performed a pharmacoeconomic analysis to assess the utility of pharmacist counseling care for breast cancer chemotherapy outpatients compared with standard care [29]. The researchers used the EQ-5D instrument to evaluate the QoL. Results from this study revealed that pharmacists’ counseling in patients with breast cancer led to improved patient QoL with an acceptable incremental cost-effectiveness ratio. As a limitation of this study, it can be mentioned that its small sample size consisted of 38 patients, of whom 19 received pharmacist consultation [29].
Another study also applied the EQ-5D-5L instrument to determine quality-adjusted life-years (QALYs) for measuring humanistic outcomes after the provision of oncology pharmacist services to patients with breast cancer [22]. Services delivered by oncology pharmacists in this study included drug-related information provided to oncologists, medication chart reviews for verifying the appropriateness of anti-neoplastic medications, counseling on the usage of medications, etc. Pharmacists’ involvement after the administration of three chemotherapy cycles led to significantly improved QALY in the intervention group compared to the control group [22].
The fourth study was conducted in Egypt, and the effect of the pharmacists’ interventions on QoL was assessed using the EORTC QLQ-BR23 questionnaire [24]. The results obtained by Farrag et al. demonstrated a significant overall improvement in the QOL regarding functional and symptom scales (p < 0.001) of the EORTC QLQ-BR23 instrument after 6 months of follow-up and the involvement of a clinical pharmacist [24].
4. Discussion
This scoping review highlighted four key themes related to clinical pharmacist-led interventions for improving breast cancer management: patient education and counseling, adherence assessment, management of adverse side effects and drug interactions, and improving QoL.
Patient education is an essential element in the treatment of cancer patients and is vital to the success of oral treatment [36]. The transition from intravenous delivery of medications to oral therapy allows for more opportunities to improve the way of prescribing, dispensing, and monitoring the therapeutic process [18]. Oncology pharmacists are uniquely positioned to improve patient care in each of these areas [37]. They can play an important role in the education process of breast cancer patients by providing information on prescribed medications, potential side effects, drug interactions, and the importance of adherence [38]. Moreover, pharmacists may offer recommendations on lifestyle modifications, such as dietary considerations, exercise, and smoking cessation [39]. In addition to their undeniable role in patient education, interventions provided by oncology pharmacists have been reported to be associated with improved patient knowledge and higher satisfaction levels [13,14,40,41]. A systematic review conducted by Segal et al. confirms the impact of the oncology pharmacist on patient satisfaction [14]. Furthermore, this is demonstrated in a study by Mulunesh et al. in which patient satisfaction with pharmacist education is rated “excellent” [13].
Adherence is another key component in the management of cancer patients undergoing oral chemotherapy. According to the WHO, poor adherence to long-term treatment is a major global problem, resulting in poor health outcomes and higher health costs [42]. Suboptimal medication adherence may lead to the recurrence of cancer, elevated rates of hospital admissions, and an increased risk of mortality [43]. Factors that hinder adherence include complex treatment regimes, side effects, higher medication costs, low health literacy, and limited patient knowledge [37]. Since 80% of patients with breast cancer have hormone receptor-positive tumors, AET is a common approach [44]. Nonadherence is a significant problem of AET in women with breast cancer [45,46,47]. Although AET offers positive health benefits, this therapy is associated with significant side effects that lead to poor medication adherence [42,44]. Studies have shown that treating and managing breast cancer in older women can be challenging. This is largely due to polypharmacy, which in turn causes DRPs and leads to poor adherence [48]. A recent systematic review has shown that between 30 and 60% of women take less of the prescribed AET than recommended, while between 30 and 70% of women prematurely stop their treatment at the end of the fifth year [49]. As medication experts, pharmacists can help fill the gaps in medication adherence in breast cancer patients by providing education and counselling, social support, and developing ongoing adherence assessments [50]. The pharmaceutical interventions described in the studies included in our scoping review demonstrated a measurable impact on adherence levels following pharmacist involvement. Moreover, clinical pharmacists used various aids to enhance medication adherence, such as calendars and schedules [31]. One of the included studies applied two validated questionnaires, MMAS-8 and BMQ, to measure medication adherence [34]. Several other studies evaluating the role of pharmacists in improving adherence among cancer patients have also reported using these tools [19,51,52]. Despite the limited number of studies evaluating the pharmacist’s role in improving adherence in breast cancer patients, this is an ongoing and promising area of research.
Oncology pharmacists are key specialists in the provision of up-to-date information to oncologists and other healthcare providers regarding antineoplastic medications, potential drug interactions, and adverse side effects [36]. Oncology pharmacists meet with patients shortly after a cancer diagnosis to gain a better understanding of their habits and current medications. Based on this information, pharmacists determine the risk of drug interactions and develop a patient-specific treatment plan [18]. Many published studies confirm the beneficial role of clinical pharmacists in the identification of DRPs in cancer patients [12,53,54,55]. Different tools, like DDI checkers, websites, and mobile applications, are reported to be used [56]. DRPs observed in studies involving patients with cancer include ADRs, DDIs, inappropriate medications, overdosing, untreated indications, contraindications, administered omissions, etc. [33,53,54,57]. In addition to the detection of DRPs, interventions delivered by pharmacists resulted in a significant reduction of ADRs [58]. The most commonly performed pharmaceutical interventions reported in the studies identified by our review include medication review, management of ADRs, patient education, dosage adjustment, treatment discontinuation, and therapeutic drug monitoring [25,26,27,32,33].
In addition to breast cancer survival, QoL is another important determinant in the overall process of disease management [59]. In the scientific literature, there are many research articles that assess the role of the pharmacist in improving the QoL of patients suffering from different chronic diseases, including cancer [60]. There are also a variety of studies measuring QoL in breast cancer patients [59,61,62]. However, only these four articles included in our scoping review evaluate both aspects. Various validated tools are available for measuring QoL, including those specifically developed for cancer patients. One of the studies included in our review assessed the effect of the pharmacist’s interventions on QoL using the EORTC QLQ-BR23 questionnaire [24]. The latter is a specific breast cancer instrument designed by the European Organization for Research and Treatment of Cancer (EORTC) [63]. This questionnaire was widely used in many studies for measuring QoL in patients with breast cancer [64,65,66,67]. Despite the encouraging findings demonstrated in the scoping review, future studies involving a larger sample size would confirm the benefits of pharmacist-led interventions on QoL in breast cancer patients. The availability of generic (e.g., EQ-5D or EQ-5D-5L) and specific (EORTC QLQ-BR23) instruments to assess the QoL of breast cancer patients would facilitate this process.
Strengths and Limitations
We emphasize that this is the first scoping review that comprehensively synthesizes the current data on the role of clinical pharmacists in breast cancer management. Moreover, the search period was broad enough, without limitations on the date of published papers, and three major databases were investigated to find every relevant record.
It is important to highlight a few of this study’s limitations. Firstly, some of the studies reviewed had small sample sizes, which increased the risk of error (sampling bias). Secondly, only articles published in English were selected for inclusion. As a result, we may have missed important findings from studies published in other languages. Thirdly, the review only considered research reports with defined, measurable outcomes. Furthermore, a meta-analysis was not conducted due to the inclusion of many different study designs and the heterogeneity of the data.
5. Conclusions
This scoping review highlights the beneficial effects of the involvement of pharmacists in breast cancer management, such as better QoL, reduced drug interaction risk, greater adherence rates, and improved patient knowledge. Additionally, some pharmacist-led interventions were reported to be cost-effective or associated with a high level of patient satisfaction. This underscores the importance of incorporating a clinical pharmacist into the oncology team caring for patients with breast cancer. The findings summarized in the review could serve as a strong foundation for future research. More randomized controlled studies involving a larger sample of breast cancer patients are needed to confirm the clinical and economic benefits of pharmacist-led interventions.
Author Contributions
Conceptualization, R.S.; methodology, R.S.; software, R.S.; investigation, R.S. and E.G.; writing—original draft preparation, R.S.; writing—review and editing, R.S., E.G. and D.K; visualization, R.S.; supervision, R.S. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siristatidis, C.; Karageorgiou, V.; Vogiatzi, P. Current Issues on Research Conducted to Improve Women’s Health. Healthcare 2021, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Ades, F.; Tryfonidis, K.; Zardavas, D. The Past and Future of Breast Cancer Treatment—From the Papyrus to Individualised Treatment Approaches. Ecancermedicalscience 2017, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.; Ashraf, M.A.; Sarfraz, M. Natural Cures for Breast Cancer Treatment. Saudi Pharm. J. 2016, 24, 233–240. [Google Scholar] [CrossRef]
- Lechkova, B.; Karcheva-Bahchevanska, D.; Ivanov, K.; Todorova, V.; Benbassat, N.; Penkova, N.; Atanassova, P.; Peychev, L.; Hrischev, P.; Peychev, Z.; et al. A Study of the Chemical Composition, Acute and Subacute Toxicity of Bulgarian Tanacetum Parthenium Essential Oil. Molecules 2023, 28, 4906. [Google Scholar] [CrossRef]
- Morganella, S.; Alexandrov, L.B.; Glodzik, D.; Zou, X.; Davies, H.; Staaf, J.; Sieuwerts, A.M.; Brinkman, A.B.; Martin, S.; Ramakrishna, M.; et al. The Topography of Mutational Processes in Breast Cancer Genomes. Nat. Commun. 2016, 7, 11383. [Google Scholar] [CrossRef] [PubMed]
- Avery, M.; Williams, F. The Importance of Pharmacist Providing Patient Education in Oncology. J. Pharm. Pract. 2015, 28, 26–30. [Google Scholar] [CrossRef]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26, v8–v30. [Google Scholar] [CrossRef]
- Yeo, H.Y.; Liew, A.C.; Chan, S.J.; Anwar, M.; Han, C.; Marra, C. Understanding Patient Preferences Regarding the Important Determinants of Breast Cancer Treatment: A Narrative Scoping Review. Patient Prefer. Adherence 2023, 17, 2679–2706. [Google Scholar] [CrossRef]
- Holle, L.M.; Segal, E.M.; Jeffers, K.D. The Expanding Role of the Oncology Pharmacist. Pharmacy 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Umar, R.M.; Apikoglu-Rabus, S.; Yumuk, P.F. Significance of a Clinical Pharmacist-Led Comprehensive Medication Management Program for Hospitalized Oncology Patients. Int. J. Clin. Pharm. 2020, 42, 652–661. [Google Scholar] [CrossRef]
- Muluneh, B.; Schneider, M.; Faso, A.; Amerine, L.; Daniels, R.; Crisp, B.; Valgus, J.; Savage, S. Improved Adherence Rates and Clinical Outcomes of an Integrated, Closed-Loop, Pharmacist-Led Oral Chemotherapy Management Program. J. Oncol. Pract. 2018, 14, e324–e334. [Google Scholar] [CrossRef] [PubMed]
- M Segal, E.; Bates, J.; Fleszar, S.L.; Holle, L.M.; Kennerly-Shah, J.; Rockey, M.; Jeffers, K.D. Demonstrating the Value of the Oncology Pharmacist within the Healthcare Team. J. Oncol. Pharm. Pract. 2019, 25, 1945–1967. [Google Scholar] [CrossRef] [PubMed]
- Randolph, L.A.; Walker, C.K.; Nguyen, A.T.; Zachariah, S.R. Impact of Pharmacist Interventions on Cost Avoidance in an Ambulatory Cancer Center. J. Oncol. Pharm. Pract. 2018, 24, 3–8. [Google Scholar] [CrossRef]
- Walko, C.; Kiel, P.J.; Kolesar, J. Precision Medicine in Oncology: New Practice Models and Roles for Oncology Pharmacists. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2016, 73, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Kinnaer, L.-M.; De Coster, S.; Coolbrandt, A.; Decoene, E.; Van Hecke, A.; Foulon, V. Key Elements for the Education and Counselling of Patients Treated with Oral Anticancer Drugs. Eur. J. Oncol. Nurs. 2019, 41, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, T. The Dual Role of Oncology Pharmacists in Multidisciplinary Cancer Care Teams. Available online: https://www.oncologynurseadvisor.com/home/departments/navigation/the-dual-role-of-oncology-pharmacists-in-multidisciplinary-cancer-care-teams/ (accessed on 21 March 2024).
- Joy, A.M.; Up, N.; Chand, S.; Shetty, K.J.; George, S.M.; Chacko, C.S.; Joel, J.J. Role of Clinical Pharmacist in the Medication Adherence Behaviour of Cancer Patients: An Interventional Study. Pharm. Hosp. Clin. 2021, 56, 291–297. [Google Scholar] [CrossRef]
- Goh, E.; Labelle, S.; Chan, A. Implementation and Evaluation of a Shared Care Model between Oncologists and Pharmacists for Breast Cancer Patients at a Canadian Regional Ambulatory Cancer Centre. J. Oncol. Pharm. Pract. 2024, 30, 622–627. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Khadela, A.; Bhikadiya, V.; Vyas, B. Impact of Oncology Pharmacist Services on Humanistic Outcome in Patients with Breast Cancer. J. Oncol. Pharm. Pract. 2022, 28, 302–309. [Google Scholar] [CrossRef]
- Neuner, J.; Weil, E.; Fergestrom, N.; Stolley, M.; Kamaraju, S.; Oxencis, C.; Winn, A.; Laud, P.W.; Flynn, K.E. Feasibility of a Pharmacist-Led Symptom Monitoring and Management Intervention to Improve Breast Cancer Endocrine Therapy Adherence. J. Am. Pharm. Assoc. 2022, 62, 1321–1328.e3. [Google Scholar] [CrossRef] [PubMed]
- Farrag, D.K.; Sabri, N.A.; Tawfik, A.S.; Shaheen, S.M. Evaluation of the Clinical Effect of Pharmacist Intervention: Results of Patient Education about Breast Cancer. Eur. J. Oncol. Pharm. 2020, 3, e23. [Google Scholar] [CrossRef]
- Iihara, H.; Ishihara, M.; Matsuura, K.; Kurahashi, S.; Takahashi, T.; Kawaguchi, Y.; Yoshida, K.; Itoh, Y. Pharmacists Contribute to the Improved Efficiency of Medical Practices in the Outpatient Cancer Chemotherapy Clinic. J. Eval. Clin. Pract. 2012, 18, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sun, C.-L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric Assessment–Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults With Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, F.; Alexandre, M.; Guiu, S.; Pouderoux, S.; Beaujouin, M.; Lossaint, G.; Philibert, L.; Evrard, A.; Jacot, W. Impact of Pharmacist Consultation at Clinical Trial Inclusion: An Effective Way to Reduce Drug–Drug Interactions with Oral Targeted Therapy. Cancer Chemother. Pharmacol. 2021, 88, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hori, A.; Tachi, T.; Osawa, T.; Nagaya, K.; Makino, T.; Inoue, S.; Yasuda, M.; Mizui, T.; Nakada, T.; et al. Impact of Pharmacist Counseling on Reducing Instances of Adverse Events That Can Affect the Quality of Life of Chemotherapy Outpatients with Breast Cancer. J. Pharm. Health Care Sci. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tachi, T.; Hori, A.; Osawa, T.; Nagaya, K.; Makino, T.; Inoue, S.; Yasuda, M.; Mizui, T.; Nakada, T.; et al. Cost Utility Analysis of Pharmacist Counseling Care for Breast Cancer Chemotherapy Outpatients. Pharmazie 2019, 74, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.C.; Amiruddin, M.; Lai, S.S.; Low, C.F. An Emerging Role of Pharmacist in Pre-Chemotherapy Counseling Among Breast Cancer Patients. Indian J. Pharm. Sci. 2017, 79, 294–302. [Google Scholar] [CrossRef]
- Patel, J.V.; Hughes, D.M.; Ko, N.Y. OPTIMAL Breast Cancer Care: Effect of an Outpatient Pharmacy Team to Improve Management and Adherence to Oral Cancer Treatment. JCO Oncol. Pract. 2023, 19, e306–e314. [Google Scholar] [CrossRef]
- Jones, K.L.; Barnett, C.; Gauthier, M.; Boster, B.; Espirito, J.L.; Michaud, L.B. Clinical Outcomes of a Pharmacist-Managed Anticoagulation Service for Breast Cancer Patients. J. Oncol. Pharm. Pract. 2012, 18, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, A.C.; Rodrigues, A.T.; De Barros, A.A.; Derchain, S.F.; Mazzola, P.G. Prescribing Errors Intercepted by Pharmacist Intervention in Care of Patients Hospitalised with Breast and Gynaecological Cancer at a Brazilian Teaching Hospital. Eur. J. Cancer Care 2018, 27, e12767. [Google Scholar] [CrossRef] [PubMed]
- Rabeea, I.; Saad, A.; Waleed, S.M.; Al-Jalehawi, A.; Kermasha, Z. The Impact of Pharmacist Intervention in Augmenting the Adherence of Breast Cancer Women to Oral Hormonal Therapy. Lat. Am. J. Pharm. 2023, 42, 99–107. [Google Scholar]
- Kurihara, M.; Shimizu, H.; Tsuboi, K.; Kobayashi, K.; Murakami, M.; Eguchi, K.; Shimozuma, K. Development of Quality of Life Questionnaire in Japan: Quality of Life Assessment of Cancer Patients Receiving Chemotherapy. Psychooncology 1999, 8, 355–363. [Google Scholar] [CrossRef]
- Ma, C. Role of Pharmacists in Optimizing the Use of Anticancer Drugs in the Clinical Setting. Integr. Pharm. Res. Pract. 2014, 3, 11–24. [Google Scholar] [CrossRef]
- Mackler, E.; Segal, E.M.; Muluneh, B.; Jeffers, K.; Carmichael, J. 2018 Hematology/Oncology Pharmacist Association Best Practices for the Management of Oral Oncolytic Therapy: Pharmacy Practice Standard. J. Oncol. Pract. 2019, 15, e346–e355. [Google Scholar] [CrossRef] [PubMed]
- Lively, A.; Minard, L.V.; Scott, S.; Deal, H.; Lambourne, T.; Giffin, J. Exploring the Perspectives of Healthcare Professionals in Delivering Optimal Oncology Medication Education. PLoS ONE 2020, 15, e0228571. [Google Scholar] [CrossRef]
- Hamer, J.; Warner, E. Lifestyle Modifications for Patients with Breast Cancer to Improve Prognosis and Optimize Overall Health. Can. Med. Assoc. J. 2017, 189, E268–E274. [Google Scholar] [CrossRef]
- Munro, L.; Myers, G.; Gould, O.; LeBlanc, M. Clinical Pharmacy Services in an Ambulatory Oncology Clinic: Patient Perception and Satisfaction. J. Oncol. Pharm. Pract. 2021, 27, 1086–1093. [Google Scholar] [CrossRef]
- Crespo, A.; Tyszka, M. Evaluating the Patient-Perceived Impact of Clinical Pharmacy Services and Proactive Follow-up Care in an Ambulatory Chemotherapy Unit. J. Oncol. Pharm. Pract. 2017, 23, 243–248. [Google Scholar] [CrossRef]
- Yussof, I.; Mohd Tahir, N.A.; Hatah, E.; Mohamed Shah, N. Factors Influencing Five-Year Adherence to Adjuvant Endocrine Therapy in Breast Cancer Patients: A Systematic Review. Breast 2022, 62, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Pensak, N.A.; Sporn, N.J.; MacDonald, J.J.; Lennes, I.T.; Safren, S.A.; Pirl, W.F.; Temel, J.S.; Greer, J.A. Treatment Satisfaction and Adherence to Oral Chemotherapy in Patients with Cancer. J. Oncol. Pract. 2017, 13, e474–e485. [Google Scholar] [CrossRef] [PubMed]
- Rosso, R.; D’Alonzo, M.; Bounous, V.E.; Actis, S.; Cipullo, I.; Salerno, E.; Biglia, N. Adherence to Adjuvant Endocrine Therapy in Breast Cancer Patients. Curr. Oncol. 2023, 30, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Moon, Z.; Moss-Morris, R.; Hunter, M.S.; Carlisle, S.; Hughes, L.D. Barriers and Facilitators of Adjuvant Hormone Therapy Adherence and Persistence in Women with Breast Cancer: A Systematic Review. Patient Prefer. Adherence 2017, 11, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Moon, Z.; Moss-Morris, R.; Hunter, M.S.; Norton, S.; Hughes, L.D. Nonadherence to Tamoxifen in Breast Cancer Survivors: A 12 Month Longitudinal Analysis. Health Psychol. 2019, 38, 888–899. [Google Scholar] [CrossRef]
- Lambert, L.K.; Balneaves, L.G.; Howard, A.F.; Gotay, C.C. Patient-Reported Factors Associated with Adherence to Adjuvant Endocrine Therapy after Breast Cancer: An Integrative Review. Breast Cancer Res. Treat. 2018, 167, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Pieters, H.C.; Green, E.; Khakshooy, S.; Sleven, M.; Stanton, A.L. A Qualitative Comparison of How Older Breast Cancer Survivors Process Treatment Information Regarding Endocrine Therapy. PLoS ONE 2019, 14, e0210972. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.; Waldron, C.; McGeagh, L.; Norris, R.; Bolnykh, I.; Stewart, S.J.; Slodkowska-Barabasz, J.; Moon, Z.; Cahir, C.; Thompson, S.; et al. Identifying Determinants of Adherence to Adjuvant Endocrine Therapy Following Breast Cancer: A Systematic Review of Reviews. Cancer Med. 2024, 13, e6937. [Google Scholar] [CrossRef] [PubMed]
- Gershman, J. Pharmacists Can Improve Adherence to Oral Oncolytics. Pharmacy Times 2022, 4, 8–10. [Google Scholar]
- Birand, N.; Boşnak, A.S.; Diker, Ö.; Abdikarim, A.; Başgut, B. The Role of the Pharmacist in Improving Medication Beliefs and Adherence in Cancer Patients. J. Oncol. Pharm. Pract. 2019, 25, 1916–1926. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, H.; Xu, S.; Xie, R.; Liu, Y.; Zhai, Q.; Fang, L.; Tong, Y.; Sun, J.; Xin, W.; et al. Pharmacist-Led Management Improves Treatment Adherence and Quality of Life in Opioid-Tolerant Patients with Cancer Pain: A Randomized Controlled Trial. Pain Ther. 2022, 11, 241–252. [Google Scholar] [CrossRef]
- Moukafih, B.; Abahssain, H.; Mrabti, H.; Errihani, H.; Rahali, Y.; Taoufik, J.; Chaibi, A. Impact of Clinical Pharmacy Services in a Hematology/Oncology Ward in Morocco. J. Oncol. Pharm. Pract. 2021, 27, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Delpeuch, A.; Leveque, D.; Gourieux, B.; Herbrecht, R. Impact of Clinical Pharmacy Services in a Hematology/Oncology Inpatient Setting. Anticancer Res. 2015, 35, 457. [Google Scholar] [PubMed]
- Albayrak, A.; Düzenli, T.; Kayıkçıoğlu, E. Potential Drug–Drug Interactions in Patients with Non-Small Cell Lung Cancer at a University Hospital in Turkey. J. Cancer Res. Clin. Oncol. 2023, 149, 9621–9627. [Google Scholar] [CrossRef] [PubMed]
- Hammar, T.; Hamqvist, S.; Zetterholm, M.; Jokela, P.; Ferati, M. Current Knowledge about Providing Drug–Drug Interaction Services for Patients—A Scoping Review. Pharmacy 2021, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kc, B.; Blebil, A.Q.; Teoh, S.L. Pharmacist Involvement in Cancer Pain Management: A Systematic Review and Meta-Analysis. J. Pain 2022, 23, 1123–1142. [Google Scholar] [CrossRef] [PubMed]
- Edwards, Z.; Ziegler, L.; Craigs, C.; Blenkinsopp, A.; Bennett, M.I. Pharmacist Educational Interventions for Cancer Pain Manaaement: A Systematic Review and Meta-Analvsis. Int. J. Pharm. Pract. 2019, 27, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Fong, D.Y.T.; Zhou, J.; Chen, H.; Wan, C. Health-Related Quality of Life and Its Influencing Factors in Patients with Breast Cancer Based on the Scale QLICP-BR. Sci. Rep. 2023, 13, 15176. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, U.; Mohd Sidik, S.; Rampal, L.; Fadhilah, S.I.; Akhtari-Zavare, M.; Mahmud, R. Effect of Chemotherapy Counseling by Pharmacists on Quality of Life and Psychological Outcomes of Oncology Patients in Malaysia: A Randomized Control Trial. Health Qual. Life Outcomes 2017, 15, 104. [Google Scholar] [CrossRef]
- Alvarez-Pardo, S.; Romero-Pérez, E.M.; Camberos-Castañeda, N.; De Paz, J.A.; Horta-Gim, M.A.; González-Bernal, J.J.; Mielgo-Ayuso, J.; Simón-Vicente, L.; Fernández-Solana, J.; González-Santos, J. Quality of Life in Breast Cancer Survivors in Relation to Age, Type of Surgery and Length of Time since First Treatment. Int. J. Environ. Res. Public. Health 2022, 19, 16229. [Google Scholar] [CrossRef]
- Heidary, Z.; Ghaemi, M.; Hossein Rashidi, B.; Kohandel Gargari, O.; Montazeri, A. Quality of Life in Breast Cancer Patients: A Systematic Review of the Qualitative Studies. Cancer Control 2023, 30, 10732748231168318. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Idris, D.B.; Teo, L.W.; Loh, S.Y.; Seow, G.C.; Chia, Y.Y.; Tin, A.S. Validation of EORTC QLQ-C30 and QLQ-BR23 Questionnaires in the Measurement of Quality of Life of Breast Cancer Patients in Singapore. Asia-Pac. J. Oncol. Nurs. 2014, 1, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Popovic, M.; Chow, E.; Cella, D.; Beaumont, J.L.; Chu, D.; DiGiovanni, J.; Lam, H.; Pulenzas, N.; Bottomley, A. EORTC QLQ-BR23 and FACT-B for the Assessment of Quality of Life in Patients with Breast Cancer: A Literature Review. J. Comp. Eff. Res. 2015, 4, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, C.; Porciello, G.; Vitale, S.; Palumbo, E.; Crispo, A.; Grimaldi, M.; Calabrese, I.; Pica, R.; Prete, M.; Falzone, L.; et al. Quality of Life in Women Diagnosed with Breast Cancer after a 12-Month Treatment of Lifestyle Modifications. Nutrients 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Karsten, M.M.; Roehle, R.; Albers, S.; Pross, T.; Hage, A.M.; Weiler, K.; Fischer, F.; Rose, M.; Kühn, F.; Blohmer, J.-U. Real-World Reference Scores for EORTC QLQ-C30 and EORTC QLQ-BR23 in Early Breast Cancer Patients. Eur. J. Cancer 2022, 163, 128–139. [Google Scholar] [CrossRef]
- Miret, C.; Orive, M.; Sala, M.; García-Gutiérrez, S.; Sarasqueta, C.; Legarreta, M.J.; Redondo, M.; Rivero, A.; Castells, X.; Quintana, J.M.; et al. Reference Values of EORTC QLQ-C30, EORTC QLQ-BR23, and EQ-5D-5L for Women with Non-Metastatic Breast Cancer at Diagnosis and 2 Years After. Qual. Life Res. 2023, 32, 989–1003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).