A Rare Case of Breast Metastasis from a Primary Lung Tumor: Case Report
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirrielees, J.A.; Kapur, J.H.; Szalkucki, L.M.; Harter, J.M.; Salkowski, L.R.; Strigel, R.M.; Traynor, A.M.; Wilke, L.G. Metastasis of primary lung carcinoma to the breast: A systematic review of the literature. J. Surg. Res. 2014, 188, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Long, D.S.; Sharma, S. Metastatic melanoma presenting as a breast mass—Role of radiologist as a clinician. Radiol. Case Rep. 2020, 15, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Cuniolo, L.; Gipponi, M.; Murelli, F.; Depaoli, F.; Cornacchia, C.; Franchelli, S.; Pesce, M.; Ronda, E.; Picardi, S.; Diaz, R.; et al. Multidisciplinary and Tailored Treatment of Locally Advanced Breast Cancer in Progression during Neoadjuvant Chemotherapy: Case Report. Curr. Oncol. 2024, 31, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Roberts, F.; Bryden, F.; McCafferty, A.; Downer, P.; Hansell, D.T.; Jones, R.; Milroy, R. Metastases to breast from primary lung cancer. J. Thorac. Oncol. 2009, 4, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Branica, B.V.; Meniga, I.N.; Puljić, I.; Marusić, A.; Chalfe, N.; Ivicević, A. Breast metastasis from lung adenocarcinoma diagnosed with fine needle aspiration cytology: A case report. Coll. Antropol. 2012, 36, 1461–1465. [Google Scholar] [PubMed]
- Chattopadhyay, S.; Aich, R.K.; Sengupta, A.; Kumari, P. Squamous cell carcinoma of lung metastasizinig in breast. J. Cancer Res. Ther. 2012, 8, 630–632. [Google Scholar] [PubMed]
- Choi, J.J.; Buch, K.E.; Warner, R.R.; Divino, C.M. Atypical lung carcinoid metastasis to breast: A case report. Pancreas 2011, 40, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Usami, N.; Okasaka, T.; Kawaguchi, K.; Okagawa, T.; Suzuki, H.; Yokoi, K. Late breast metastasis from resected lung cancer diagnosed by epidermal growth factor receptor gene mutation. Lung Cancer 2011, 74, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caro, A.; Piñero, A.; Roca, M.J.; Torres, J.; Ferri, B.; Galindo, P.J.; Parrilla, P. Surgical treatment of solitary metastasis in the male breast from non-small cell lung cancer. Breast J. 2006, 12, 366–367. [Google Scholar] [CrossRef]
- Hsu, W.; Sheen-Chen, S.M.; Wang, J.L.; Huang, C.C.; Ko, S.F. Squamous cell lung carcinoma metastatic to the breast. Anticancer Res. 2008, 28, 1299–1301. [Google Scholar]
- Hunter, G.J.; Choi, N.C.; McLoud, T.C.; Fischman, A.J. Lung tumor metastasis to breast detected by fluorine-18-fluorodeoxyglucose PET. J. Nucl. Med. 1993, 34, 1571–1573. [Google Scholar]
- Ji, F.F.; Gao, P.; Wang, J.G.; Zhao, J.; Zhao, P. Contralateral breast metastasis from pulmonary adenocarcinoma: Two cases report and literature review. J. Thorac. Dis. 2012, 4, 384–389. [Google Scholar] [PubMed]
- Jitendra, N.G.; Parikh, B.; Shah, M. Bilateral breast metastasis from an adenocarcinoma of lung: A case report. Natl. J. Med. Res. 2011, 1, 83. [Google Scholar]
- Klingen, T.A.; Klaasen, H.; Aas HA, N.S.; Chen, Y.; Akslen, L.A. Secondary breast cancer: A 5-year population-based study with review of the literature. APMIS 2009, 117, 762. [Google Scholar] [CrossRef]
- Ko, K.; Ro, J.Y.; Hong, E.K.; Lee, S. Micropapillary lung cancer with breast metastasis simulating primary breast cancer due to architectural distortion on images. Korean J. Radiol. 2012, 13, 249–253. [Google Scholar] [CrossRef]
- Maounis, N.; Chorti, M.; Legaki, S.; Ellina, E.; Emmanouilidou, A.; Demonakou, M.; Tsiafaki, X. Metastasis to the breast from an adenocarcinoma of the lung with extensive micropapillary component: A case report and review of the literature. Diagn. Pathol. 2010, 5, 82. [Google Scholar] [CrossRef]
- Noguera, J.J.; Martínez-Miravete, P.; Idoate, F.; Diaz, L.; Pina, L.; Zornoza, G.; Martínez-Regueira, F. Metastases to the breast: A review of 33 cases. Australas Radiol. 2007, 51, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Rimner, A.; Rosenzweig, K.E. Palliative radiation for lung cancer metastases to the breast: Two case reports. J. Thorac. Oncol. 2007, 2, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Sadikot, R.T.; Renwick, D.S.; DaCosta, P.; Chalmers, A.G.; Pearson, S.B. Breast metastasis from non-small cell lung cancer. S. Med. J. 1997, 90, 1063. [Google Scholar] [CrossRef]
- Sato, K.; Takeyama, Y.; Yoshihara, M.; Kato, T.; Hashimoto, H.; Fukui, Y.; Gonda, H.; Suzuki, R. CBDCA + Pemetrexed + Bevacizumab and Its Maintenance Chemotherapy in a Case of Solitary Breast Metastasis from a Lung Adenocarcinoma Resistant to Gefitinib. Case Rep. Oncol. 2012, 5, 546–553. [Google Scholar] [CrossRef]
- Sengupta, A.; Saha, K.; Jash, D.; Banerjee, S. Metastatic breast lump: A rare presentation of squamous cell lung cancer. CCIJ 2012, 1, 97. [Google Scholar]
- Ucar, N.; Kurt, O.K.; Alpar, S.; Orsel, O.; Demirag, F.; Kurt, B. Breast metastasis in a male patient with nonsmall cell lung carcinoma. S. Med. J. 2007, 100, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Dietz, J.R.; Moley, J.F.; DeBenedetti, M.K.; Aft, R.L.; Gillanders, W.E.; Eberlein, T.J.; Ritter, J.; Margenthaler, J.A. Metastatic disease to the breast: The Washington University experience. World J. Surg. Oncol. 2007, 5, 74. [Google Scholar] [CrossRef]
- Verger, E.; Conill, C.; Velasco, M.; Sole, M. Metastasis in the male breast from a lung adenocarcinoma. Acta Oncol. 1992, 31, 479. [Google Scholar] [CrossRef]
- Yeh, C.N.; Lin, C.H.; Chen, M.F. Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am. Surg. 2004, 70, 287–290. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Song, C.S.; Seo, M.H.; Kim, M.J.; Oh, T.Y.; Jang, U.H.; Kwag, H.J.; Kim, H.S.; Lim, S.Y.; Lim, S.Y.; et al. A case of metachronous metastasis to the breast from non-small cell lung carcinoma. Cancer Res. Treat. 2010, 42, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.W.; Sui, Y.X.; Zhang, X.M.; Lv, M.; Zhang, X.; Liu, P.J.; Yang, J. Ipsilateral breast metastasis from a pulmonary adenocarcinoma: A case report and a focused review of the literature. Int. J. Clin. Exp. Pathol. 2015, 8, 9647–9654. [Google Scholar]
- Cao, L.; Lv, L. Breast metastasis from EGFR/ALK negative lung adenocarcinoma: A case report. Medicine 2020, 99, e23503. [Google Scholar] [CrossRef] [PubMed]
- Ramwani, R.; Wernberg, J. Three Cases of Atypical Breast Metastasis from Lung Adenocarcinoma. Clin. Med. Res. 2022, 20, 231–235. [Google Scholar] [CrossRef]
- Cserni, G. Solitary breast metastasis from oestrogen receptor-positive pulmonary adenocarcinoma: Report of a case with a potential pitfall. Pol. J. Pathol. 2017, 68, 168–172. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.S.; Liu, D.D.; Li, X.L. Metastasis to breast from primary lung Cancer: A rare case report. Asian J. Surg. 2022, 45, 2562–2563. [Google Scholar] [CrossRef]
- Valenza, C.; Porta, F.M.; Rappa, A.; Guerini-Rocco, E.; Viale, G.; Barberis, M.; de Marinis, F.; Curigliano, G.; Catania, C. Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review. Curr. Oncol. 2021, 28, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- Chuang, X.; Chen, Y.; Yu, P.; Qiu, X.; Wang, J.; Qu, X.; Teng, Y.; Liu, Y.; Jin, B. ALK rearrangement in lung adenocarcinoma with concurrent cervix and breast metastases: A case report. Thorac. Cancer 2018, 9, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Altintoprak, F.; Baytekin, H.F.; Tasdemir, C. Primary small cell carcinoma of the lung presenting with breast and skin metastases. Korean J. Intern. Med. 2011, 26, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Courtney, S.P.; Polacarz, S.; Raftery, A.T. Mondor’s disease associated with metastatic lung cancer in the breast. Postgrad Med. J. 1989, 65, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, B.; Stevanović, O.; Bacić, G. Metastases to the breast from small-cell lung cancer: MR findings. A case report. Acta Radiol. 2003, 44, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Henderson, D.; Corris, P. Breast lumps: Rare presentation of oat cell carcinoma of lung. J. Clin. Pathol. 1988, 41, 171–172. [Google Scholar] [CrossRef]
- Liu, W.; Palma-Diaz, F.; Alasio, T.M. Primary small cell carcinoma of the lung initially presenting as a breast mass: A fine-needle aspiration diagnosis. Diagn. Cytopathol. 2009, 37, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Luh, S.P.; Kuo, C.; Tsao, T.C. Breast metastasis from small cell lung carcinoma. J. Zhejiang Univ. Sci. B 2008, 9, 39–43. [Google Scholar] [CrossRef]
- Sharma, S.; Kotru, M. Metastatic small cell carcinoma lung presenting as a breast mass: Cytologic findings. Indian J. Cancer 2010, 47, 72–73. [Google Scholar] [CrossRef]
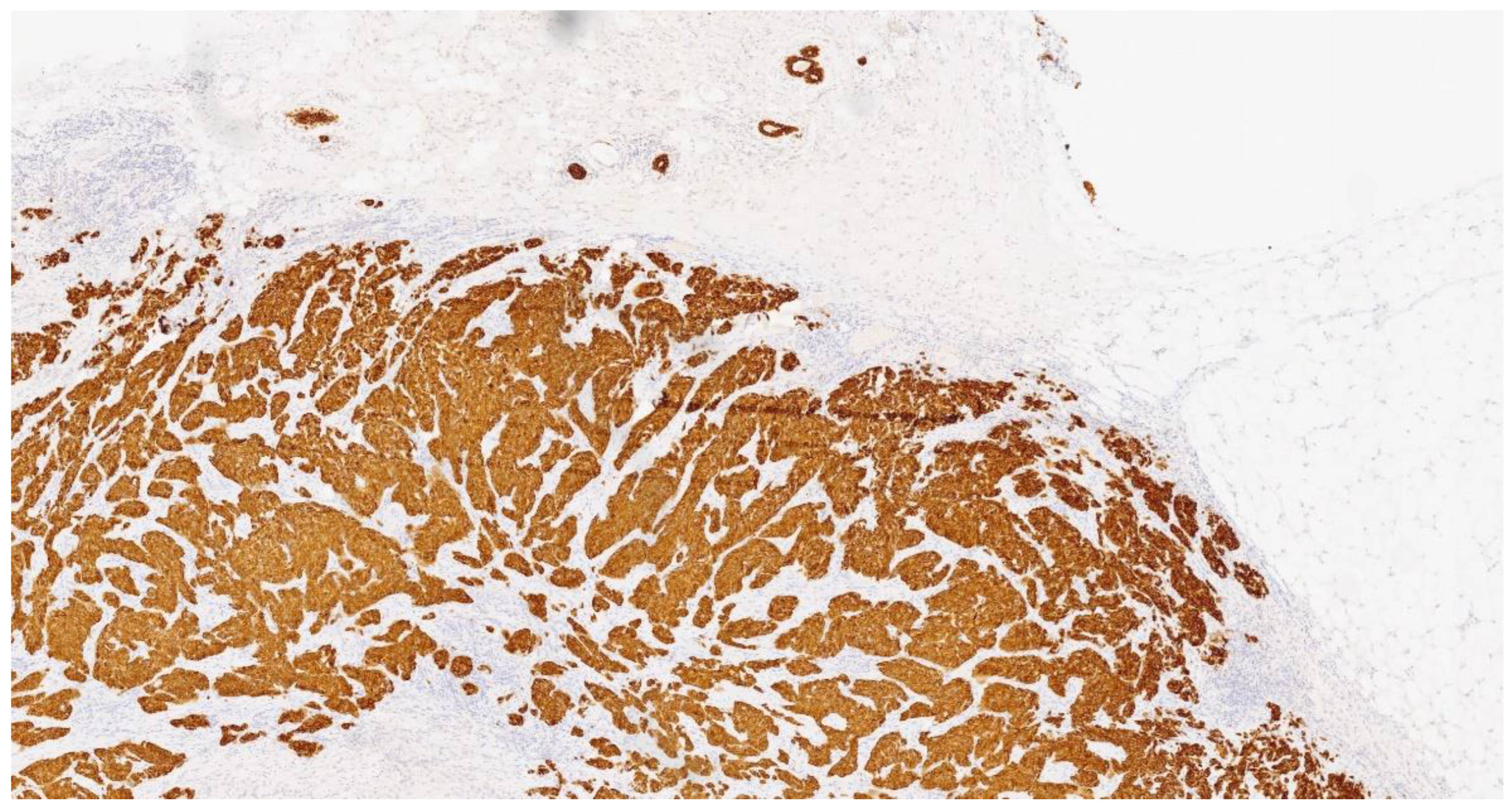


| Molecule | Results |
|---|---|
| Cytokeratin 7 | + |
| TTF1 | − |
| GATA3 | − |
| Napsin A | − |
| P40 | − |
| PD-L1 | absent |
| ALK | wt |
| EGFR | wt |
| ROS1 | wt |
| RET | wt |
| METex14 | wt |
| NTRK1/2/3 | wt |
| BRAF | wt |
| Source | Primary Lung Carcinoma | Gender | Age | Stage | TTF1 | ER | PR | HER2 |
|---|---|---|---|---|---|---|---|---|
| Non-small-cell lung carcinomas | ||||||||
| Babu 2009 [4] | ADCA LCC | Female Female | 51 82 | IV IV | + + | − − | − − | − − |
| Branica 2012 [5] | ADCA | Female | 55 | NR | + | NR | NR | NR |
| Chattopadhyay 2012 [6] | SQCC | Female | 55 | IV | NR | − | − | NR |
| Choi 2011 [7] | NEC | Female | 62 | IIA | + | − | − | − |
| Fukumoto 2011 [8] | ADCA | Female | 65 | IIIA | + | − | NR | NR |
| Gomez-Caro 2006 [9] | ADCA | Male | 65 | IB | − | NR | NR | NR |
| Hsu 2008 [10] | SQCC | Female | 48 | IV | − | − | − | NR |
| Hunter 1993 [11] | LCC | Female | 57 | III | − | − | − | − |
| Ji 2012 [12] | ADCA ADCA | Female Female | 49 40 | IV IV | + + | − − | − − | − − |
| Jitendra 2011 [13] | ADCA | Female | 42 | NR | + | − | − | − |
| Klingen 2009 [14] | ADCA ADCA | Female Male | 79 70 | NR NR | + + | NR NR | NR NR | NR NR |
| Ko 2012 [15] | ADCA | Female | 47 | IV | + | − | − | − |
| Maounis 2010 [16] | ADCA | Female | 73 | IV | + | − | NR | NR |
| Noguera 2007 [17] | SC NS | Female Female | 41 53 | NR NR | NR NR | NR NR | NR NR | NR NR |
| Rimner 2007 [18] | LCC ADCA | Female Female | 49 81 | IV NR | NR + | NR − | NR − | NR − |
| Sadikot 1997 [19] | SPCC | Female | 47 | IV | NR | NR | NR | NR |
| Sato 2012 [20] | ADCA | Female | 57 | IV | + | − | − | − |
| Sengupta 2012 [21] | SQCC | Female | 60 | IV | − | − | − | NR |
| Ucar 2007 [22] | ADCA | Male | 63 | IV | + | NR | NR | NR |
| Vaughan 2007 [23] | NEC NEC NEC | Female Female Female | 30 35 28 | NR IV NR | NR NR NR | NR − NR | NR − NR | NR − NR |
| Verger 1999 [24] | ADCA | Male | 63 | IIA | NR | NR | NR | NR |
| Yeh 2004 [25] | ADCA | Female | 44 | NR | NR | NR | NR | NR |
| Yoon 2010 [26] | ADCA | Female | 42 | IIB | + | − | − | − |
| Mirrielees 2014 [1] | LCC ADCA | Female Female | 67 58 | IV IIIA | + + | NR − | NR − | NR − |
| Yan-Wei Shen 2015 [27] | ADCA | Female | 54 | NR | NR | NR | NR | NR |
| Liyu Cao 2020 [28] | ADCA | Female | 55 | NR | + | − | − | − |
| Roshini Ramwani 2022 [29] | ADCA ADCA ADCA | Female Female Female | 65 74 65 | NR NR NR | NR + + | − NR + | − NR − | − NR − |
| Gábor Cserni 2017 [30] | ADCA | Female | 60 | NR | + | + | − | − |
| Juan Li 2022 [31] | ADCA | Female | 44 | NR | + | − | + | − |
| Carmine Valenza 2022 [32] | ADCA | Female | 63 | IIB | + | + | + | + |
| Xin Chuang 2018 [33] | ADCA | Female | 44 | NR | + | − | − | − |
| Small-cell lung carcinomas | ||||||||
| Altintoprak 2011 [34] | SCC | Male | 47 | IV | + | − | − | NR |
| Babu 2009 [4] | SCC | Female | 69 | IV | + | NR | NR | NR |
| Courtney 1989 [35] | OCC | Female | 59 | IV | NR | NR | NR | NR |
| Jakovijevic 2003 [36] | SCC | Female | 44 | IV | NR | NR | NR | NR |
| Kelly 1998 [37] | SCC SCC | Female Female | 64 39 | IV IV | NR NR | NR NR | NR NR | NR NR |
| Liu 2009 [38] | SCC | Female | 45 | IV | NR | NR | NR | NR |
| Luh 2008 [39] | SCC | Female | 50 | IV | + | + | + | + |
| Sharma 2010 [40] | SCC | Female | 66 | NR | NR | − | − | − |
| Vaughan 2007 [23] | SCC | Female | 83 | IV | NR | NR | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, R.; Murelli, F.; Cuniolo, L.; Cornacchia, C.; Depaoli, F.; Margarino, C.; Boccardo, C.; Gipponi, M.; Franchelli, S.; Pesce, M.; et al. A Rare Case of Breast Metastasis from a Primary Lung Tumor: Case Report. Curr. Oncol. 2024, 31, 4695-4703. https://doi.org/10.3390/curroncol31080350
Diaz R, Murelli F, Cuniolo L, Cornacchia C, Depaoli F, Margarino C, Boccardo C, Gipponi M, Franchelli S, Pesce M, et al. A Rare Case of Breast Metastasis from a Primary Lung Tumor: Case Report. Current Oncology. 2024; 31(8):4695-4703. https://doi.org/10.3390/curroncol31080350
Chicago/Turabian StyleDiaz, Raquel, Federica Murelli, Letizia Cuniolo, Chiara Cornacchia, Francesca Depaoli, Cecilia Margarino, Chiara Boccardo, Marco Gipponi, Simonetta Franchelli, Marianna Pesce, and et al. 2024. "A Rare Case of Breast Metastasis from a Primary Lung Tumor: Case Report" Current Oncology 31, no. 8: 4695-4703. https://doi.org/10.3390/curroncol31080350





