Could CT Radiomic Analysis of Benign Adrenal Incidentalomas Suggest the Need for Further Endocrinological Evaluation?
Abstract
1. Introduction
2. Materials and Methods
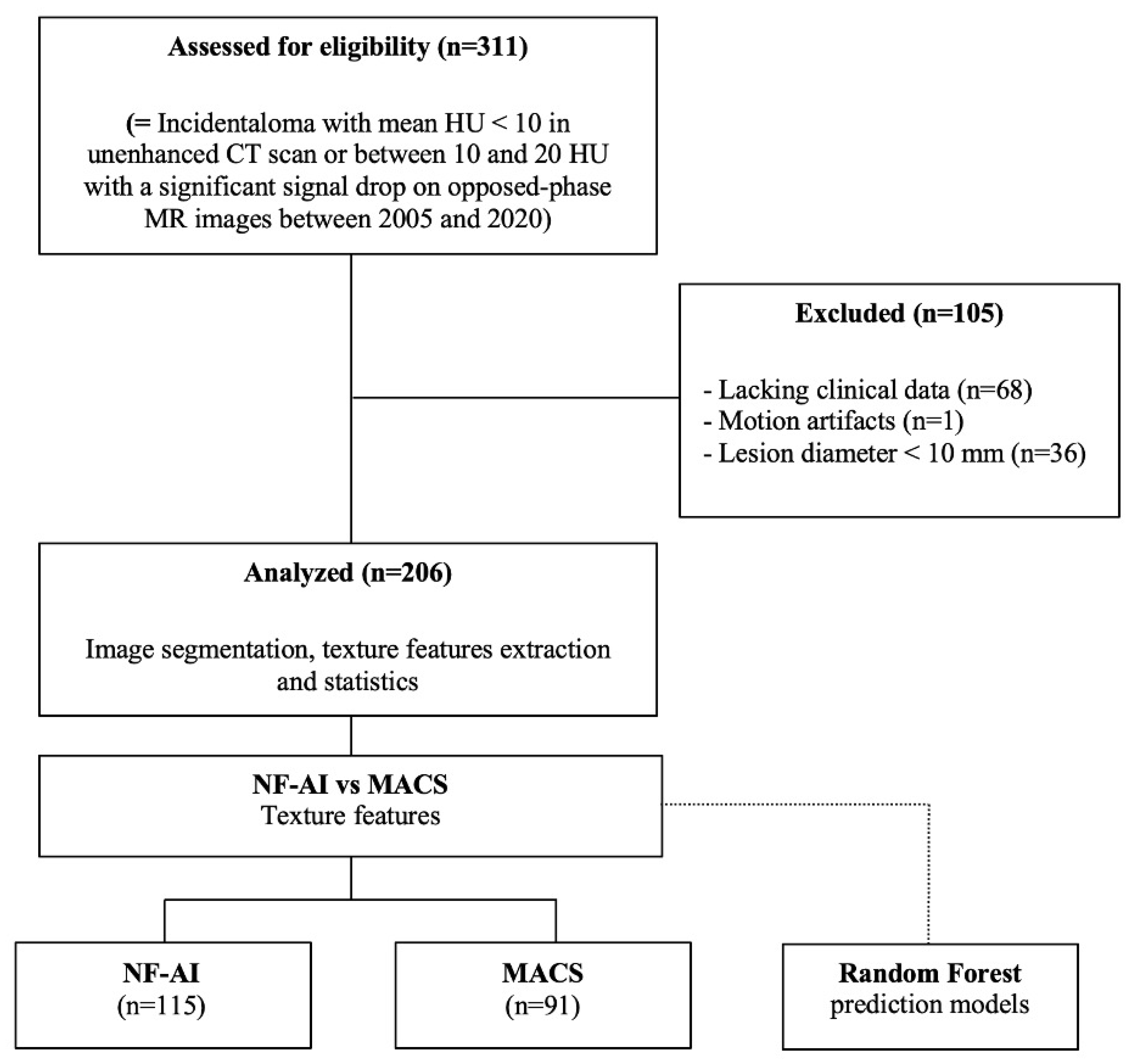
2.1. Patients
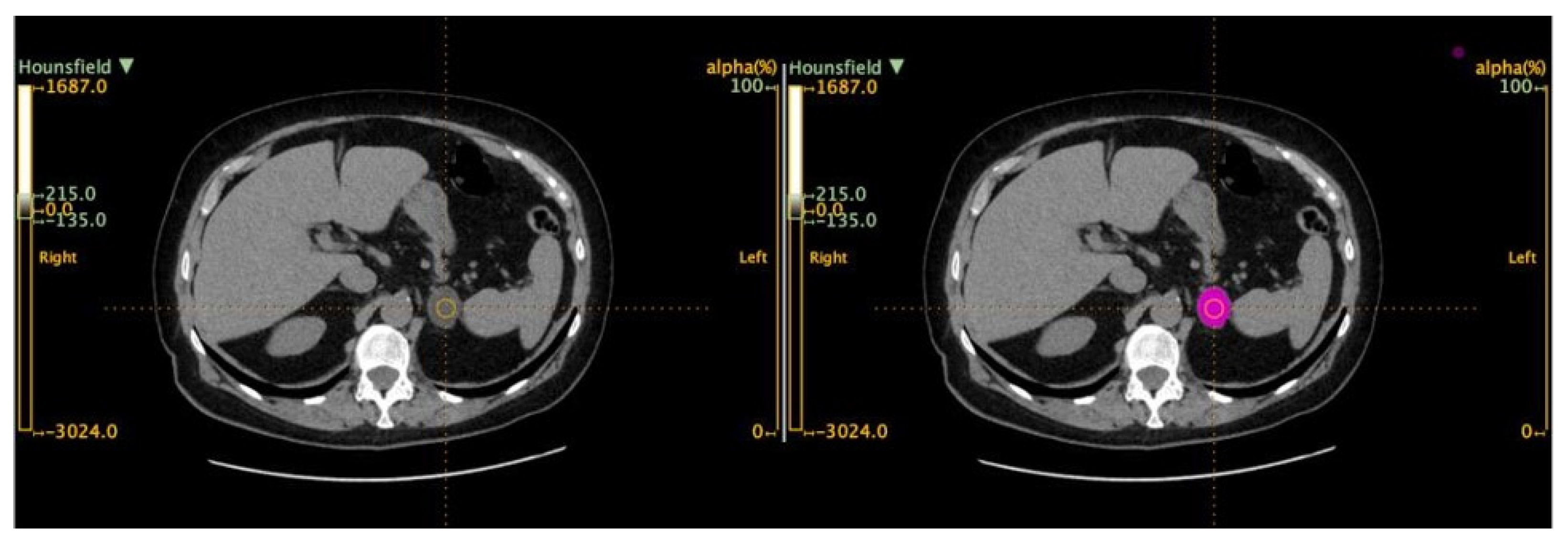
2.2. Imaging Protocol
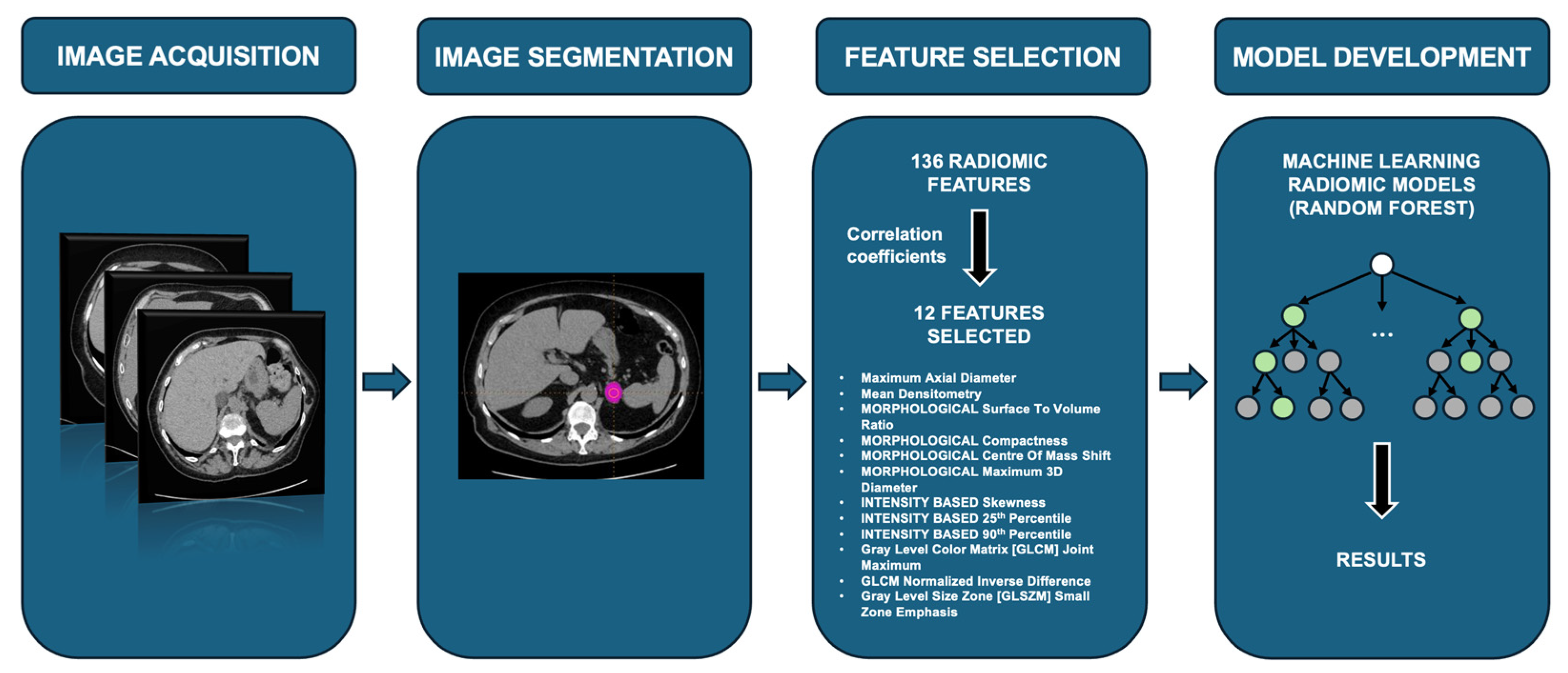
2.3. Radiomic-Based Machine Learning Modeling
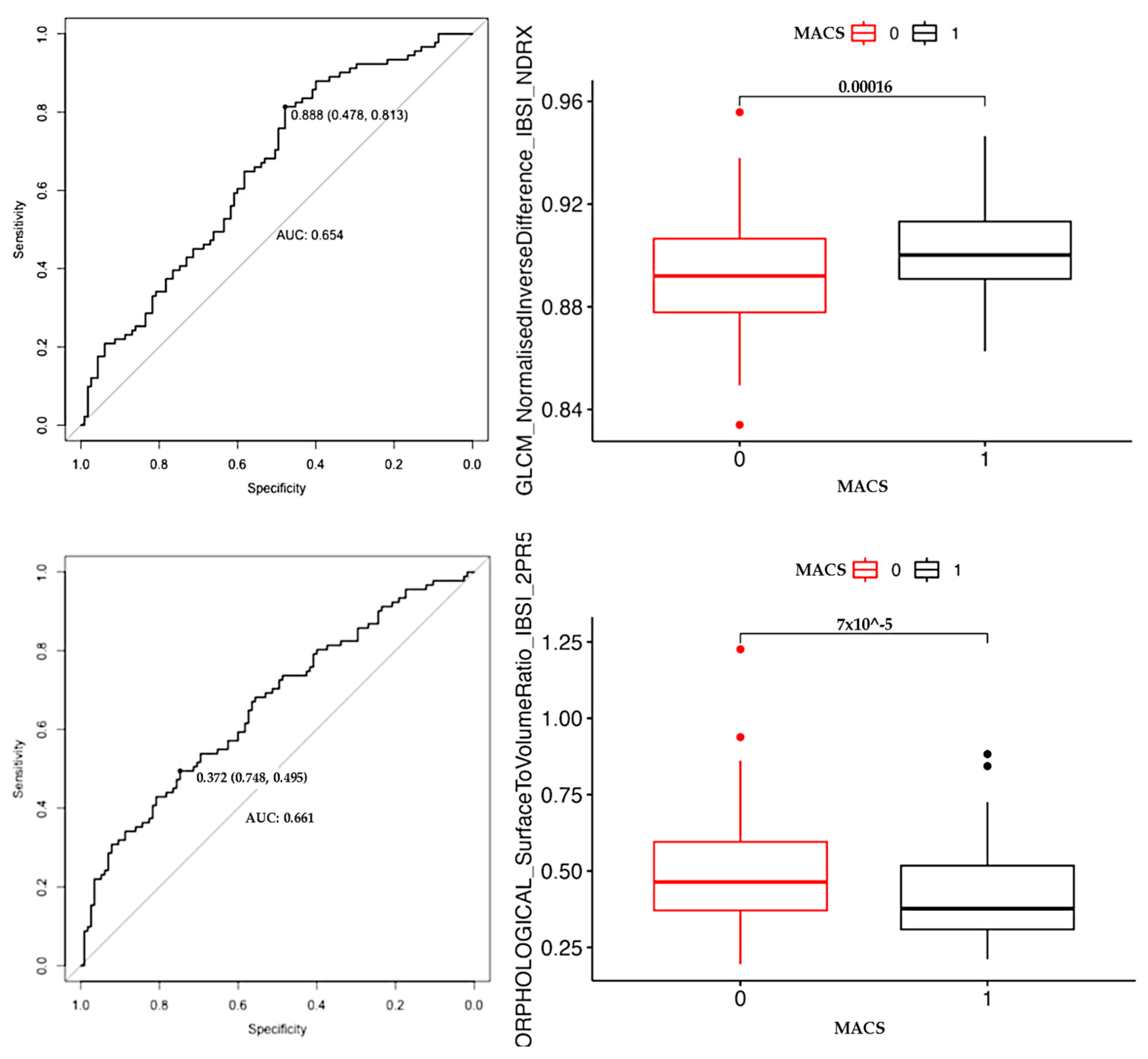
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reincke, M.; Fleseriu, M. Cushing Syndrome: A Review. JAMA 2023, 330, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, F.; Barbot, M.; Scaroni, C.; Boscaro, M. Frequently asked questions and answers (if any) in patients with adrenal incidentaloma. J. Endocrinol. Investig. 2021, 44, 2749–2763. [Google Scholar] [CrossRef] [PubMed]
- Pelsma, I.C.M.; Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Marina, L.; Lorenz, K.; Bancos, I.; et al. Comorbidities in mild autonomous cortisol secretion and the effect of treatment: Systematic review and meta-analysis. Eur. J. Endocrinol. 2023, 189, S88–S101. [Google Scholar] [CrossRef] [PubMed]
- Käyser, S.C.; Dekkers, T.; Groenewoud, H.J.; Van der Wilt, G.J.; Bakx, J.C.; Van der Wel, M.C.; Hermus, A.R.; Lenders, J.W.; Deinum, J. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. J. Clin. Endocrinol. Metab. 2016, 101, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Sandler, C.M.; Fishman, E.K.; Charnsangavej, C.; Yasumori, K.; Honda, H.; Ernst, R.D.; Takahashi, N.; Raval, B.K.; Masuda, K.; et al. Spectrum of CT findings in nonmalignant disease of the adrenal gland. Radiographics 1998, 18, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Korobkin, M.; Brodeur, F.J.; Yutzy, G.G.; Francis, I.R.; Quint, L.E.; Dunnick, N.R.; Kazerooni, E.A. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR Am. J. Roentgenol. 1996, 166, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Vicennati, V.; Garelli, S.; Casadio, E.; Rinaldi, E.; Giampalma, E.; Mosconi, C.; Golfieri, R.; Paccapelo, A.; Pagotto, U.; et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: A 15-year retrospective study. Lancet Diabetes Endocrinol. 2014, 2, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, F.; Tizianel, I.; Voltan, G.; Maggetto, G.; Merante Boschin, I.; Quaia, E.; Crimì, F.; Scaroni, C. Attenuation Value in Adrenal Incidentalomas: A Longitudinal Study. Front. Endocrinol. 2021, 12, 794197. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, Z.T.; Wang, Z.H.; Wang, Y.; Wang, Y.X.; Zhang, H.Z.; Dong, Y.F.; Xiao, X.L. A Clinical-Radiomic Nomogram Based on Unenhanced Computed Tomography for Predicting the Risk of Aldosterone-Producing Adenoma. Front. Oncol. 2021, 11, 634879. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.T.; Chang, D.; Liu, K.L.; Liao, W.C.; Wang, W.; Chang, C.C.; Wu, V.C.; Lin, Y.H. Radiomics utilization to differentiate nonfunctional adenoma in essential hypertension and functional adenoma in primary aldosteronism. Sci. Rep. 2022, 12, 8892. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zuo, Y.; Chang, R.; Huang, K.; Liu, J.; Zhang, Z. Using CT radiomic features based on machine learning models to subtype adrenal adenoma. BMC Cancer 2023, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 23 July 2024).
- LIFEx (Local Image Features Extraction)—User Guide–Texture. Available online: https://www.lifexsoft.org/images/phocagallery/documentation/ProtocolTexture/UserGuide/TextureUserGuide.pdf (accessed on 23 July 2024).
- Song, J.H.; Chaudhry, F.S.; Mayo-Smith, W.W. The incidental adrenal mass on CT: Prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am. J. Roentgenol. 2008, 190, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Mantero, F.; Terzolo, M.; Arnaldi, G.; Osella, G.; Masini, A.M.; Alì, A.; Giovagnetti, M.; Opocher, G.; Angeli, A. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 2000, 85, 637–644. [Google Scholar] [PubMed]
- Šikić, Z. Rules of thumb for positive and negative test results. J. Eval. Clin. Pract. 2021, 27, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Power, M.; Fell, G.; Wright, M. Principles for high-quality, high-value testing. BMJ Evid. Based Med. 2013, 18, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.J.; Hsu, K.T.; Lee, M.H.; Pickhardt, P.J.; Kim, D.H.; Sippel, R.S.; Dedhia, P.H. Hormonal Evaluation of Incidental Adrenal Masses: The Exception, Not the Rule. World J. Surg. 2020, 44, 3778–3785. [Google Scholar] [CrossRef] [PubMed]
- Khayyam, H.; Madani, A.; Kafieh, R.; Hekmatnia, A. Artificial Intelligence in Cancer Diagnosis and Therapy; MDPI-Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2023; ISBN 978-3-0365-6673-3. [Google Scholar] [CrossRef]
- Veiga-Canuto, D.; Cerdà-Alberich, L.; Sangüesa Nebot, C.; Martínez de las Heras, B.; Pötschger, U.; Gabelloni, M.; Carot Sierra, J.M.; Taschner-Mandl, S.; Düster, V.; Cañete, A.; et al. Comparative Multicentric Evaluation of Inter-Observer Variability in Manual and Automatic Segmentation of Neuroblastic Tumors in Magnetic Resonance Images. Cancers. 2022, 14, 3648. [Google Scholar] [CrossRef] [PubMed]
| NFAI (n = 115) | MACS (n = 91) | p-Value | |
|---|---|---|---|
| Age at diagnosis (years) | 64.0 ± 9.8 | 67.2 ± 8.7 | 0.009 * |
| Gender, female/male (% female) | 59/56 (51%) | 54/37 (60%) | 0.232 |
| BMI (kg/m2) | 30.4 ± 5.0 | 28.0 ± 4.5 | 0.578 |
| Basal ACTH (ng/L) | 18.6 ± 11.2 | 14.1 ± 9.9 | 0.003 * |
| HbA1c (mmol/mol) | 41.4 ± 8.0 | 42.5 ± 9.6 | 0.422 |
| Mean diameter (mm) | 18.1 ± 6.1 | 22.7 ± 7.3 | <0.001 * |
| Mean attenuation value (HUm) | −1.0 ± 10.1 | 1.8 ± 11.6 | 0.103 |
| Hypertension (%) | 70 (60%) | 63 (68%) | 0.036 * |
| Diabetes mellitus (%) | 20 (17%) | 18 (20%) | 0.191 |
| Dyslipidemia (%) | 47 (40%) | 37 (40%) | 0.943 |
| Osteoporosis (%) | 7 (6%) | 15 (16%) | 0.015 * |
| Training Set (n = 143) | Test Set (n = 63) | p-Value | |
|---|---|---|---|
| Age at diagnosis (years) | 64.5 ± 9.8 | 67.7 ± 8.1 | 0.238 |
| Gender, female/male (% female) | 73/70 (51%) | 41/22 (65%) | 0.068 |
| Number of incidentalomas | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.881 |
| Fasting blood glucose (mg/dL) | 107 ± 22.0 | 104.3 ± 23.2 | 0.704 |
| Oncologic history (%) | 33 (23%) | 11 (17%) | 0.436 |
| Hypertension (%) | 110 (77%) | 51 (81%) | 0.467 |
| Diabetes mellitus (%) | 25 (17%) | 13 (21%) | 0.576 |
| Dyslipidemia (%) | 69 (48%) | 32 (51%) | 0.703 |
| Osteoporosis (%) | 12 (8%) | 7 (11%) | 0.766 |
| Parameter Name | Meaning |
|---|---|
| Maximum_axial_diameter | Maximum 2D dimension in the axial plane of the incidentaloma. |
| Mean_densitometry | Mean densitometry of the adenoma in the HU. |
| MORPHOLOGICAL Surface_to_Volume_Ratio | The ratio between the surface area and volume of an object; lower values mean that the incidentaloma tends toward a spherical shape, in contrast to elongated or heterogeneous shapes. |
| MORPHOLOGICAL Compactness 1 | The compacity feature reflects how compact the volume of interest is. Compacity = A3/2V, where V and A correspond to the volume and the surface of the volume of interest based on the Delaunay triangulation. |
| MORPHOLOGICAL Centre_Of_Mass_Shift | Distance in millimeters between the normalized sphere radius of the activity hotspot with a weighted center of mass. |
| MORPHOLOGICAL Maximum_3D_Diameter | Maximum dimension in every plane of the incidentaloma. |
| INTENSITY_BASED Skewness | Measures the asymmetry of the distribution of values about the mean value. Depending on where the tail is elongated and the mass of the distribution is concentrated, this value can be positive or negative. |
| INTENSITY_BASED 25%_Percentile | Density value below which 25% of the image pixel density values are located (first quartile). |
| INTENSITY_BASED 90th_Percentile | Density value below which 90% of the image pixel density values are located. |
| GLCM Joint_Maximum | Gray Level Co-Occurrence Matrix (second order feature) Joint Maximum—in other software called “maximum probability”—measures the largest probability of occurrence of a specific gray-level value in the GLCM matrix. It is calculated by finding the maximum value in the GLCM matrix. |
| GLCM Normalised_Inverse_Difference | Measure of the local homogeneity of an image. |
| GLSZM Small_Zone_Emphasis | Gray Level Size Zone Matrix (second order feature) Small Zone Emphasis—measures the distribution of small size zones. |
| Training Cohort | Biochemically Confirmed MACS | Biochemically Confirmed NF-AI | Total |
|---|---|---|---|
| Radiomics model classification as MACS | 55 | 0 | 55 |
| Radiomics model classification as NF-AI | 0 | 88 | 88 |
| 55 | 88 | 143 | |
| Apparent prevalence | 38% | ||
| True prevalence | 38% | ||
| Sensitivity | 100% | ||
| Specificity | 100% | ||
| Positive predictive value | 100% | ||
| Negative predictive value | 100% | ||
| Validation cohort | Biochemically confirmed MACS | Biochemically confirmed NF-AI | Total |
| Radiomics model classification as MACS | 14 | 5 | 19 |
| Radiomics model classification as NF-AI | 22 | 22 | 44 |
| 36 | 27 | 63 | |
| Apparent prevalence | 30% | ||
| True prevalence | 57% | ||
| Sensitivity | 39% | ||
| Specificity | 81% | ||
| Positive predictive value | 74% | ||
| Negative predictive value | 50% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toniolo, A.; Agostini, E.; Ceccato, F.; Tizianel, I.; Cabrelle, G.; Lupi, A.; Pepe, A.; Campi, C.; Quaia, E.; Crimì, F. Could CT Radiomic Analysis of Benign Adrenal Incidentalomas Suggest the Need for Further Endocrinological Evaluation? Curr. Oncol. 2024, 31, 4917-4926. https://doi.org/10.3390/curroncol31090364
Toniolo A, Agostini E, Ceccato F, Tizianel I, Cabrelle G, Lupi A, Pepe A, Campi C, Quaia E, Crimì F. Could CT Radiomic Analysis of Benign Adrenal Incidentalomas Suggest the Need for Further Endocrinological Evaluation? Current Oncology. 2024; 31(9):4917-4926. https://doi.org/10.3390/curroncol31090364
Chicago/Turabian StyleToniolo, Alessandro, Elena Agostini, Filippo Ceccato, Irene Tizianel, Giulio Cabrelle, Amalia Lupi, Alessia Pepe, Cristina Campi, Emilio Quaia, and Filippo Crimì. 2024. "Could CT Radiomic Analysis of Benign Adrenal Incidentalomas Suggest the Need for Further Endocrinological Evaluation?" Current Oncology 31, no. 9: 4917-4926. https://doi.org/10.3390/curroncol31090364
APA StyleToniolo, A., Agostini, E., Ceccato, F., Tizianel, I., Cabrelle, G., Lupi, A., Pepe, A., Campi, C., Quaia, E., & Crimì, F. (2024). Could CT Radiomic Analysis of Benign Adrenal Incidentalomas Suggest the Need for Further Endocrinological Evaluation? Current Oncology, 31(9), 4917-4926. https://doi.org/10.3390/curroncol31090364








