Complete Blood Count-Based Biomarkers as Predictors of Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients with PD-L1 < 50% Treated with First-Line Chemoimmunotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Data
2.2. Laboratory Tests
2.3. Statistical Analysis
3. Results
3.1. Blood-Based Biomarkers and Treatment Response
3.2. Blood-Based Biomarkers and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today. Lyon, France. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/15-trachea-bronchus-and-lung-fact-sheet.pdf (accessed on 8 May 2024).
- Colombino, M.; Paliogiannis, P.; Cossu, A.; Santeufemia, D.A.; Sardinian Lung Cancer (SLC) Study Group; Sini, M.C.; Casula, M.; Palomba, G.; Manca, A.; Pisano, M.; et al. EGFR, KRAS, BRAF, ALK, and cMET genetic alterations in 1440 Sardinian patients with lung adenocarcinoma. BMC Pulm. Med. 2019, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 8 May 2024).
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef]
- Putzu, C.; Canova, S.; Paliogiannis, P.; Lobrano, R.; Sala, L.; Cortinovis, D.L.; Colonese, F. Duration of immunotherapy in non-small cell lung cancer survivors: A lifelong commitment? Cancers 2023, 15, 689. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Rashid, S.; Al-Bozom, I.A. PD-L1 immunostaining: What pathologists need to know. Diagn. Pathol. 2021, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, B.; Pau, M.C.; Zinellu, E.; Mangoni, A.A.; Paliogiannis, P.; Pirina, P.; Fois, A.G.; Carru, C.; Zinellu, A. Association between red blood cell distribution width and obstructive sleep apnea syndrome: A systematic review and meta-analysis. J. Clin. Med. 2023, 12, 3302. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, J.; Ma, X.; Pan, L. The predictive value of neutrophil-to-lymphocyte ratio for chronic obstructive pulmonary disease: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2020, 14, 929–936. [Google Scholar] [CrossRef]
- Shojaan, H.; Kalami, N.; Ghasempour Alamdari, M.; Emami Alorizy, S.M.; Ghaedi, A.; Bazrgar, A.; Khanzadeh, M.; Lucke-Wold, B.; Khanzadeh, S. Diagnostic value of the neutrophil lymphocyte ratio in discrimination between tuberculosis and bacterial community acquired pneumonia: A meta-analysis. J. Clin. Tuberc. Other Mycobact. Dis. 2023, 33, 100395. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Cheng, Y. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in limited-stage small-cell lung cancer: A meta-analysis. Future Oncol. 2023, 19, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, J.; Zhang, Y.; Chen, Z.; Chen, Y.; Ye, S. Platelet-lymphocyte ratio is a prognostic marker in small cell lung cancer-A systemic review and meta-analysis. Front. Oncol. 2023, 12, 1086742. [Google Scholar] [CrossRef]
- Putzu, C.; Cortinovis, D.L.; Colonese, F.; Canova, S.; Carru, C.; Zinellu, A.; Paliogiannis, P. Blood cell count indexes as predictors of outcomes in advanced non-small-cell lung cancer patients treated with Nivolumab. Cancer Immunol. Immunother. 2018, 67, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Ginesu, G.C.; Paliogiannis, P.; Feo, C.F.; Cossu, M.L.; Scanu, A.M.; Fancellu, A.; Fois, A.G.; Zinellu, A.; Perra, T.; Veneroni, S.; et al. Inflammatory indexes as predictive biomarkers of postoperative complications in oncological thoracic surgery. Curr. Oncol. 2022, 29, 3425–3432. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Ginesu, G.C.; Tanda, C.; Feo, C.F.; Fancellu, A.; Fois, A.G.; Mangoni, A.A.; Sotgia, S.; Carru, C.; Porcu, A.; et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J. Surg. 2018, 88, 616–620. [Google Scholar] [CrossRef]
- Tanaka, S.; Uchino, J.; Yokoi, T.; Kijima, T.; Goto, Y.; Suga, Y.; Katayama, Y.; Nakamura, R.; Morimoto, K.; Nakao, A.; et al. Prognostic Nutritional Index and Lung Immune Prognostic Index as prognostic predictors for combination therapies of immune checkpoint inhibitors and cytotoxic anticancer chemotherapy for patients with advanced non-small cell lung cancer. Diagnostics 2022, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Ferrara, R.; Rebuzzi, S.E.; Proto, C.; Signorelli, D.; Galli, G.; De Toma, A.; Randon, G.; Pagani, F.; Viscardi, G.; et al. EPSILoN: A prognostic score for immunotherapy in advanced non-small-cell lung cancer: A validation cohort. Cancers 2019, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Punekar, S.R.; Shum, E.; Grello, C.M.; Lau, S.C.; Velcheti, V. Immunotherapy in non-small cell lung cancer: Past, present, and future directions. Front. Oncol. 2022, 12, 877594. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus Pemetrexed and Platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 2021, 6, 100273. [Google Scholar] [CrossRef]
- Nishio, M.; Ohe, Y.; Ikeda, S.; Yokoyama, T.; Hayashi, H.; Fukuhara, T.; Sato, Y.; Tanaka, H.; Hotta, K.; Sugawara, S.; et al. First-line nivolumab plus ipilimumab in metastatic non-small cell lung cancer: 5-year outcomes in Japanese patients from CheckMate 227 Part 1. Int. J. Clin. Oncol. 2023, 28, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Kiriu, T.; Yamamoto, M.; Nagano, T.; Hazama, D.; Sekiya, R.; Katsurada, M.; Tamura, D.; Tachihara, M.; Kobayashi, K.; Nishimura, Y. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS ONE 2018, 13, e0193018. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- Hawinkels, L.J.; Zuidwijk, K.; Verspaget, H.W.; de Jonge-Muller, E.S.; van Duijn, W.; Ferreira, V.; Fontijn, R.D.; David, G.; Hommes, D.W.; Lamers, C.B.; et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur. J. Cancer 2008, 44, 1904–1913. [Google Scholar] [CrossRef]
- Malhab, L.J.B.; Saber-Ayad, M.M.; Al-Hakm, R.; Nair, V.A.; Paliogiannis, P.; Pintus, G.; Abdel-Rahman, W.M. Chronic inflammation and cancer: The role of endothelial dysfunction and vascular inflammation. Curr. Pharm. Des. 2021, 27, 2156–2169. [Google Scholar] [CrossRef] [PubMed]
- Schmied, L.; Höglund, P.; Meinke, S. Platelet-mediated protection of cancer cells from immune surveillance—Possible implications for cancer immunotherapy. Front. Immunol. 2021, 12, 640578. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Liu, J.; Zhang, D.; Liu, X.; Yue, Y.; Zhou, Q.; Gao, X.; Chen, M.; Xu, Y.; et al. Correlations between peripheral blood biomarkers and clinical outcomes in advanced non-small cell lung cancer patients who received immunotherapy-based treatments. Transl. Lung Cancer Res. 2021, 10, 4477–4493. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, J.; Jiang, L.; Zhang, L.; Huang, J.; Tian, Y.; Zhao, Y.; Liu, X.; Xia, L.; Gao, P.; et al. The predictive value of inflammatory biomarkers for major pathological response in non-small cell lung cancer patients receiving neoadjuvant chemoimmunotherapy and its association with the immune-related tumor microenvironment: A multi-center study. Cancer Immunol. Immunother. 2023, 72, 783–794. [Google Scholar] [CrossRef]
- Bi, H.; Ren, D.; Xiao, Y.; Zhou, Y.; Yi, B.; Han, W.; Shao, Y.; Wang, J.; Zhang, C.; Wang, H. Prognostic implications of neutrophil-to-lymphocyte ratio in patients with extensive-stage small cell lung cancer receiving chemoimmunotherapy: A multicenter, real-world study. Thorac. Cancer 2024, 15, 559–569. [Google Scholar] [CrossRef]
- Mahiat, C.; Bihin, B.; Duplaquet, F.; Stanciu Pop, C.; Dupont, M.; Vander Borght, T.; Rondelet, B.; Vanderick, J.; André, B.; Pirard, L.; et al. Systemic inflammation/nutritional status scores are prognostic but not predictive in metastatic non-small-cell lung cancer treated with first-line immune checkpoint inhibitors. Int. J. Mol. Sci. 2023, 24, 3618. [Google Scholar] [CrossRef]
- Mountzios, G.; Samantas, E.; Sengha, K.; Zervas, E.; Krisam, J.; Samitas, K.; Bozorgmehr, F.; Kuon, J.; Agelaki, S.; Baka, S.; et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open 2021, 6, 100254. [Google Scholar] [CrossRef] [PubMed]
| Global Cohort (n = 62) | Responders (n = 47) | Non-Responders (n = 15) | p-Value | |
|---|---|---|---|---|
| Age, years | 68.5 (62.0–74.0) | 66.0 (61.3–72.8) | 71.0 (64.8–75.0) | 0.17 |
| Gender (M/F) | 45/17 | 34/13 | 11/4 | 0.94 |
| Smoking status, n (no/former/yes) | 3/45/11 | 3/33/9 | 0/12/2 | 0.51 |
| Histological type, n (ADK/SQ) | 53/9 | 41/6 | 12/3 | 0.49 |
| PD-L1, n (1–49%/<1%) | 30/30 | 23/22 | 7/8 | 0.77 |
| Stage T, n (T1/T2/T3/T4) | 4/2/3/53 | 4/2/2/39 | 0/0/1/14 | 0.53 |
| Stage N, n (N0/N1/N2/N3) | 3/9/11/38 | 3/9/8/26 | 0/0/3/12 | 0.18 |
| Deceased, n (yes/no) | 23/37 | 10/35 | 13/2 | <0.0001 |
| Overall survival, (months) | 12.1 (7.4–24.3) | 14.5 (9.1–31.9) | 7.5 (4.2–11.2) | 0.0015 |
| Hb (g/dL) | 12.3 ± 1.7 | 12.6 ± 1.7 | 11.9 ± 1.8 | 0.47 |
| RDW, (%) | 14.7 (13.8–15.8) | 14.7 (13.4–15.8) | 14.8 (14.1–15.6) | 0.53 |
| WBC, n (×109 L) | 8.86 (7.40–11.15) | 8.74 (6.91–10.72) | 8.96 (7.96–13.73) | 0.29 |
| Neutrophils, n (×109 L) | 6.00 (4.10–7.60) | 5.62 (3.80–7.37) | 6.40 (5.59–11.95) | 0.074 |
| Lymphocytes, n (×109 L) | 1.70 (1.30–2.20) | 1.80 (1.40–2.44) | 1.40 (1.10–1.98) | 0.10 |
| Monocytes, n (×109 L) | 0.60 (0.50–0.80) | 0.60 (0.50–0.80) | 0.60 (0.50–0.80) | 0.55 |
| Platelets, n (×109 L) | 287 (253–355) | 287 (254–362) | 293 (247–349) | 0.91 |
| NLR | 3.45 (2.18–5.47) | 3.31 (2.15–4.12) | 5.36 (2.78–10.82) | 0.019 |
| NMR | 9.75 (7.60–11.80) | 9.20 (7.45–11.20) | 14.00 (8.82–21.20) | 0.013 |
| MLR | 0.33 (0.23–0.53) | 0.33 (0.23–0.51) | 0.40 (0.21–0.55) | 0.67 |
| PLR | 169 (118–246) | 163 (114–244)) | 209 (131–248) | 0.17 |
| SII | 985 (624–1838) | 945 (552–1373) | 1395 (929–3334) | 0.025 |
| AISI | 543 (277–1072) | 487 (273–955) | 837 (357–1524) | 0.20 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age, years | 1.0424 | 0.9735 to 1.1162 | 0.23 |
| Gender (M/F) | 0.9510 | 0.2564 to 3.5275 | 0.94 |
| Smoking status, n (no/former/yes) | 1.0444 | 0.2906 to 3.7533 | 0.95 |
| Histological type, n (ADK/SQ) | 1.7083 | 0.3707 to 7.8732 | 0.49 |
| PD-L1, n (1–49%/<1%) | 1.1948 | 0.3706 to 3.8525 | 0.77 |
| Stage T, n (T1/T2/T3/T4) | 2.3437 | 0.5289 to 10.3860 | 0.26 |
| Stage N, n (N0/N1/N2/N3) | 2.7685 | 0.9610 to 7.9752 | 0.06 |
| Hb (g/dL) | 0.7851 | 0.5462 to 1.1286 | 0.19 |
| RDW, (%) | 1.1248 | 0.8202 to 1.5426 | 0.47 |
| WBC, n | 1.0731 | 0.9289 to 1.2395 | 0.34 |
| Neutrophils, n | 1.1335 | 0.9724 to 1.3213 | 0.11 |
| Lymphocytes, n | 0.4280 | 0.1620 to 1.1309 | 0.09 |
| Monocytes, n | 0.2614 | 0.0253 to 2.7002 | 0.26 |
| Platelets, n | 0.9987 | 0.9939 to 1.0036 | 0.60 |
| NLR | 1.2561 | 1.0519 to 1.4998 | 0.012 |
| NMR | 1.1410 | 1.0121 to 1.2864 | 0.03 |
| MLR | 1.8104 | 0.1299 to 25.2236 | 0.66 |
| PLR | 1.0018 | 0.9983 to 1.0053 | 0.32 |
| SII | 1.0002 | 0.9999 to 1.0005 | 0.27 |
| AISI | 1.0000 | 0.9997 to 1.0003 | 0.84 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| NLR | 1.3210 | 1.0648 to 1.6387 | 0.01 | 1.8300 | 1.1236 to 2.9806 | 0.02 |
| NMR | 1.1585 | 1.0070 to 1.3328 | 0.04 | 1.1698 | 1.0019 to 1.3657 | 0.047 |
| Global Cohort (n = 60) | Survivors (n = 37) | Non-Survivors (n = 23) | p-Value | |
|---|---|---|---|---|
| Age, years | 68.0 (62.0–73.5) | 66.0 (62.0–73.3) | 70.0 (61.5–74.5) | 0.37 |
| Gender (M/F) | 44/16 | 27/10 | 17/6 | 0.94 |
| Smoking status, n (no/former/yes) | 3/43/11 | 3/25/7 | 0/18/4 | 0.35 |
| Histological type, n (ADK/SQ) | 51/9 | 33/4 | 18/5 | 0.25 |
| PD-L1, n (1–49%/<1%) | 29/29 | 18/17 | 11/12 | 0.79 |
| Stage T, n (T1/T2/T3/T4) | 4/2/3/51 | 4/2/2/29 | 0/0/1/22 | 0.23 |
| Stage N, n (N0/N1/N2/N3) | 3/8/10/38 | 3/7/5/21 | 0/1/5/17 | 0.15 |
| Hb (g/dL) | 12.3 ± 1.7 | 12.6 ± 1.8 | 12.2 ± 1.7 | 0.43 |
| RDW, (%) | 14.6 (13.6–15.7) | 14.4 (13.3–15.8) | 14.8 (14.1–15.6) | 0.51 |
| WBC, n (×109 L) | 8.94 (7.41–11.53) | 8.26 (6.36–9.92) | 9.69 (7.97–14.03) | 0.026 |
| Neutrophils, n (×109 L) | 6.00 (4.10–7.81) | 5.30 (3.48–7.03) | 7.00 (6.00–11.95) | 0.001 |
| Lymphocytes, n (×109 L) | 1.70 (1.30–2.23) | 1.80 (1.40–2.50) | 1.50 (1.13–2.00) | 0.14 |
| Monocytes, n (×109 L) | 0.60 (0.50–0.80) | 0.60 (0.50–0.80) | 0.80 (0.50–0.80) | 0.32 |
| Platelets, n (×109 L) | 287 (252–353) | 270 (238–336) | 314 (281–407) | 0.052 |
| NLR | 3.45 (2.20–5.42) | 2.94 (1.92–3.88) | 4.56 (3.07–9.49) | 0.012 |
| NMR | 9.60 (7.60–11.75) | 9.00 (7.08–11.05) | 10.40 (8.80–18.18) | 0.007 |
| MLR | 0.34 (0.24–0.54) | 0.33 (0.24–0.41) | 0.43 (0.24–0.58) | 0.16 |
| PLR | 169 (119–246) | 136 (107–201) | 220 (145–273) | 0.016 |
| SII | 985 (626–1709) | 849 (488–1081) | 1493 (1000–2578) | 0.0004 |
| AISI | 594 (279–1168) | 351 (256–794) | 1016 (470–1836) | 0.006 |
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age, years | 1.0268 | 0.9706 to 1.0863 | 0.36 |
| Gender (M/F) | 0.9529 | 0.2928 to 3.1016 | 0.94 |
| Smoking status, n (no/former/yes) | 1.3482 | 0.4379 to 4.1505 | 0.60 |
| Histological type, n (ADK/SQ) | 2.2917 | 0.5458 to 9.6219 | 0.26 |
| PD-L1, n (1–49%/<1%) | 1.1551 | 0.4030 to 3.3107 | 0.79 |
| Stage T, n (T1/T2/T3/T4) | 3.4877 | 0.6931 to 17.5496 | 0.13 |
| Stage N, n (N0/N1/N2/N3) | 2.0009 | 0.9653 to 4.1472 | 0.06 |
| Hb (g/dL) | 0.8804 | 0.6468 to 1.1982 | 0.42 |
| RDW, (%) | 1.1224 | 0.8410 to 1.4978 | 0.43 |
| WBC, n | 1.2202 | 1.0339 to 1.4400 | 0.019 |
| Neutrophils, n | 1.2916 | 1.0692 to 1.5604 | 0.008 |
| Lymphocytes, n | 0.5819 | 0.2719 to 1.2454 | 0.16 |
| Monocytes, n | 1.7055 | 0.2449 to 11.8784 | 0.59 |
| Platelets, n | 1.0014 | 0.9977 to 1.0052 | 0.45 |
| NLR | 1.3601 | 1.0949 to 1.6896 | 0.005 |
| NMR | 1.2159 | 1.0396 to 1.4221 | 0.015 |
| MLR | 5.6613 | 0.4789 to 66.9198 | 0.17 |
| PLR | 1.0023 | 0.9988 to 1.0058 | 0.19 |
| SII | 1.0004 | 1.0000 to 1.0007 | 0.054 |
| AISI | 1.0002 | 0.9999 to 1.0005 | 0.20 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p-Value | aOR | 95% CI | p-Value | |
| WBC | 1.2596 | 1.0458 to 1.5171 | 0.015 | 1.2475 | 1.0317 to 1.5084 | 0.023 |
| Neutrophils | 1.3112 | 1.0698 to 1.6071 | 0.009 | 1.2990 | 1.0527 to 1.6028 | 0.015 |
| NLR | 1.3498 | 1.0758 to 1.6936 | 0.01 | 1.3489 | 1.0632 to 1.7114 | 0.014 |
| NMR | 1.2502 | 1.0311 to 1.5158 | 0.02 | 1.5685 | 1.0901 to 2.2568 | 0.015 |
| AUC | 95% CI | p-Value | Cut-Off | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| WBC | 0.672 | 0.539 to 0.788 | 0.017 | >11.98 | 39 | 89 |
| Neutrophils | 0.746 | 0.617 to 0.849 | 0.0001 | >5.7 | 83 | 65 |
| NLR | 0.749 | 0.620 to 0.852 | 0.0001 | >4.0 | 61 | 81 |
| NMR | 0.707 | 0.576 to 0.818 | 0.0038 | >11.8 | 48 | 92 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| aHR | 95% CI | p-Value | aHR | 95% CI | p-Value | |
| WBC | 1.1966 | 1.0443 to 1.3711 | 0.01 | 1.1191 | 0.9944 to 1.2594 | 0.062 |
| Neutrophils | 1.2297 | 1.0745 to 1.4074 | 0.003 | 1.1480 | 1.0162 to 1.2970 | 0.027 |
| NLR | 1.3016 | 1.1267 to 1.5037 | 0.003 | 1.2141 | 1.0666 to 1.3819 | 0.003 |
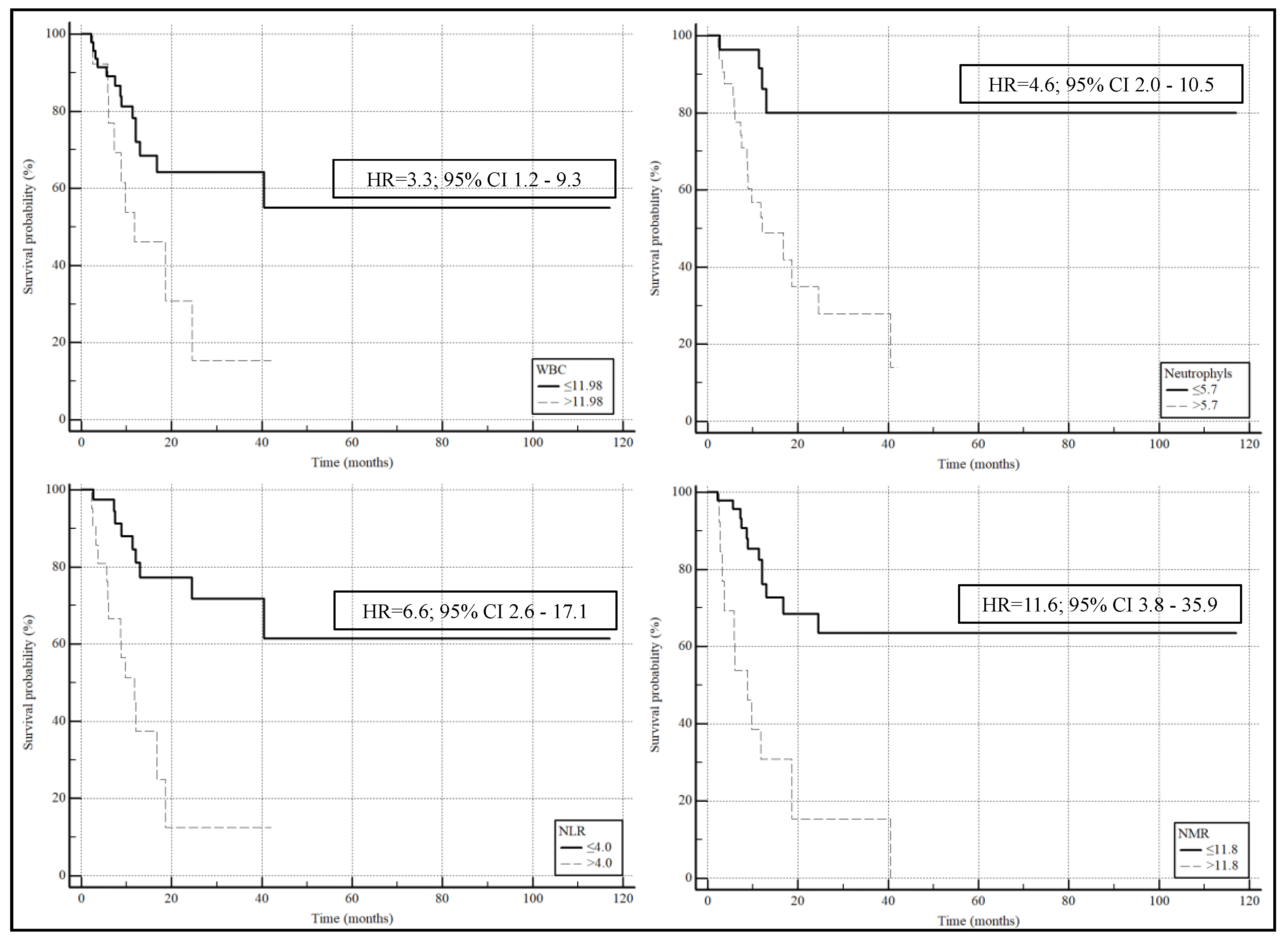
| NMR | 1.0217 | 1.0056 to 1.0380 | 0.008 | 1.0174 | 1.0027 to 1.0324 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putzu, C.; Serra, R.; Campus, R.; Fadda, G.M.; Sini, C.; Marongiu, A.; Ginesu, G.C.; Fois, A.G.; Palmieri, G.; Zinellu, A.; et al. Complete Blood Count-Based Biomarkers as Predictors of Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients with PD-L1 < 50% Treated with First-Line Chemoimmunotherapy. Curr. Oncol. 2024, 31, 4955-4967. https://doi.org/10.3390/curroncol31090367
Putzu C, Serra R, Campus R, Fadda GM, Sini C, Marongiu A, Ginesu GC, Fois AG, Palmieri G, Zinellu A, et al. Complete Blood Count-Based Biomarkers as Predictors of Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients with PD-L1 < 50% Treated with First-Line Chemoimmunotherapy. Current Oncology. 2024; 31(9):4955-4967. https://doi.org/10.3390/curroncol31090367
Chicago/Turabian StylePutzu, Carlo, Riccardo Serra, Rachele Campus, Giovanni Maria Fadda, Claudio Sini, Andrea Marongiu, Giorgio Carlo Ginesu, Alessandro Giuseppe Fois, Giuseppe Palmieri, Angelo Zinellu, and et al. 2024. "Complete Blood Count-Based Biomarkers as Predictors of Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients with PD-L1 < 50% Treated with First-Line Chemoimmunotherapy" Current Oncology 31, no. 9: 4955-4967. https://doi.org/10.3390/curroncol31090367
APA StylePutzu, C., Serra, R., Campus, R., Fadda, G. M., Sini, C., Marongiu, A., Ginesu, G. C., Fois, A. G., Palmieri, G., Zinellu, A., Cossu, A., & Paliogiannis, P. (2024). Complete Blood Count-Based Biomarkers as Predictors of Clinical Outcomes in Advanced Non-Small Cell Lung Cancer Patients with PD-L1 < 50% Treated with First-Line Chemoimmunotherapy. Current Oncology, 31(9), 4955-4967. https://doi.org/10.3390/curroncol31090367








