The Role of Artificial Intelligence on Tumor Boards: Perspectives from Surgeons, Medical Oncologists and Radiation Oncologists
Abstract
:1. Introduction
2. Surgeon Perspective
2.1. AI to Enhance Surgical Performance and Training
2.2. AI to Enhance Preoperative Setting
2.3. AI for Intraoperative Support
3. Medical Oncologist’s Perspective
3.1. AI Applications in Molecular Profiling and Treatment Selection
3.2. Predictive Modeling for Drug Response and Personalized Therapy
3.3. Integrating AI in Clinical Trial Design and Patient Recruitment
4. Radiation Oncologist Perspectives
4.1. AI-Enhanced Radiotherapy Workflow (Contouring, Treatment Planning, Adaptive and Advanced Imaging Analysis)
4.2. Artificial Intelligence in Prediction of Radiotherapy Outcomes and Toxicity
4.3. Addressing Uncertainties and Limitations in AI for Radiation Oncology
5. MTBs Perspective
6. Discussion
- AI-based tools are already influencing surgical planning and predicting complications, recurrences, and therapeutic responses in medical imaging. This is advancing towards personalized medicine;
- AI’s ability to analyze big data can help discover new biomarkers and improve cancer screening, diagnosis, treatment, and prognosis. This can lead to better clinical outcomes [105];
- The use of deep learning-based AI in cancer pathology can enhance diagnostic accuracy, reduce the workload of pathologists, and support high-level decisions. Despite the challenges of algorithm validation and interpretation, this technology has the potential to revolutionize cancer diagnosis [106].
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Engelhardt, M.; Ihorst, G.; Schumacher, M.; Rassner, M.; Gengenbach, L.; Möller, M.; Shoumariyeh, K.; Neubauer, J.; Farthmann, J.; Herget, G.; et al. Multidisciplinary Tumor Boards and Their Analyses: The Yin and Yang of Outcome Measures. BMC Cancer 2021, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- El Saghir, N.S.; Keating, N.L.; Carlson, R.W.; Khoury, K.E.; Fallowfield, L. Tumor Boards: Optimizing the Structure and Improving Efficiency of Multidisciplinary Management of Patients with Cancer Worldwide. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e461–e466. [Google Scholar] [CrossRef] [PubMed]
- Basta, Y.L.; Baur, O.L.; van Dieren, S.; Klinkenbijl, J.H.; Fockens, P.; Tytgat, K.M. Is There a Benefit of Multidisciplinary Cancer Team Meetings for Patients with Gastrointestinal Malignancies? Ann. Surg. Oncol. 2016, 23, 2430–2437. [Google Scholar] [CrossRef]
- Winters, D.A.; Soukup, T.; Sevdalis, N.; Green, J.S.A.; Lamb, B.W. The Cancer Multidisciplinary Team Meeting: In Need of Change? History, Challenges and Future Perspectives. BJU Int. 2021, 128, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.N.; Kambhampati, C.; Monson, J.R.; Drew, P.J. Artificial Intelligence in Medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334–338. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.H. Artificial Intelligence-Driven Oncology Clinical Decision Support System for Multidisciplinary Teams. Sensors 2020, 20, 4693. [Google Scholar] [CrossRef]
- Walsh, S.; de Jong, E.E.; van Timmeren, J.E.; Ibrahim, A.; Compter, I.; Peerlings, J.; Sanduleanu, S.; Refaee, T.; Keek, S.; Larue, R.T.; et al. Decision Support Systems in Oncology. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Nagendran, M.; Chen, Y.; A Lovejoy, C.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; A Ioannidis, J.P.; Collins, G.S.; Maruthappu, M. Artificial Intelligence versus Clinicians: Systematic Review of Design, Reporting Standards, and Claims of Deep Learning Studies. BMJ 2020, 368, m689. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial Intelligence in Surgery: Promises and Perils. Ann. Surg. 2018, 268, 70–76. [Google Scholar] [CrossRef]
- Navarrete-Welton, A.J.; Hashimoto, D.A. Current Applications of Artificial Intelligence for Intraoperative Decision Support in Surgery. Front. Med. 2020, 14, 369–381. [Google Scholar] [CrossRef]
- Scally, C.P.; Varban, O.A.; Carlin, A.M.; Birkmeyer, J.D.; Dimick, J.B. Video Ratings of Surgical Skill and Late Outcomes of Bariatric Surgery. JAMA Surg. 2016, 151, e160428. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Finks, J.F.; O’Reilly, A.; Oerline, M.; Carlin, A.M.; Nunn, A.R.; Dimick, J.; Banerjee, M.; Birkmeyer, N.J. Surgical Skill and Complication Rates after Bariatric Surgery. N. Engl. J. Med. 2013, 369, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, P. Future Medicine Shaped by an Interdisciplinary New Biology. Lancet 2012, 379, 1544–1550. [Google Scholar] [CrossRef]
- Ward, T.M.; Mascagni, P.; Madani, A.; Padoy, N.; Perretta, S.; Hashimoto, D.A. Surgical Data Science and Artificial Intelligence for Surgical Education. J. Surg. Oncol. 2021, 124, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.R.; Norton, P.G.; Flintoft, V.; Blais, R.; Brown, A.; Cox, J.; Etchells, E.; Ghali, W.A.; Hébert, P.; Majumdar, S.R.; et al. The Canadian Adverse Events Study: The Incidence of Adverse Events among Hospital Patients in Canada. CMAJ 2004, 170, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.A.; Leape, L.L.; Laird, N.M.; Hebert, L.; Localio, A.R.; Lawthers, A.G.; Newhouse, J.P.; Weiler, P.C.; Hiatt, H.H. Incidence of Adverse Events and Negligence in Hospitalized Patients. Results of the Harvard Medical Practice Study I. N. Engl. J. Med. 1991, 324, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.J.; Asmis, T.R.; Clark, H.D.; Al Saied, G.; Code, C.C.; Caughey, S.C.; Baker, K.; Watters, J.; Worthington, J.; van Walraven, C. Ottawa Hospital Patient Safety Study: Incidence and Timing of Adverse Events in Patients Admitted to a Canadian Teaching Hospital. CMAJ 2004, 170, 1235–1240. [Google Scholar] [CrossRef]
- Gawande, A.A.; Thomas, E.J.; Zinner, M.J.; Brennan, T.A. The Incidence and Nature of Surgical Adverse Events in Colorado and Utah in 1992. Surgery 1999, 126, 66–75. [Google Scholar] [CrossRef]
- Madani, A.; Vassiliou, M.C.; Watanabe, Y.; Al-Halabi, B.; Al-Rowais, M.S.; Deckelbaum, D.L.; Fried, G.M.; Feldman, L.S. What Are the Principles That Guide Behaviors in the Operating Room?: Creating a Framework to Define and Measure Performance. Ann. Surg. 2017, 265, 255–267. [Google Scholar] [CrossRef]
- Figura, N.; Marano, L.; Moretti, E.; Ponzetto, A. Helicobacter Pylori Infection and Gastric Carcinoma: Not All the Strains and Patients Are Alike. World J. Gastrointest. Oncol. 2016, 8, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, O.; Rypel, A. Communicative Competence in Healthcare and Linguistic Theories: Insights and Applications. Acta Elbingensia 2023, 50, 26–31. [Google Scholar] [CrossRef]
- Januszko-Giergielewicz, B.; Wójcik-Kula, A.; Perliński, J. Dynamic Changes in Teaching and Learning Methods in the Fild of Study of Medicine—Evolution, Not Revolution. Acta Elbingensia 2023, 50, 77–82. [Google Scholar] [CrossRef]
- Way, L.W.; Stewart, L.; Gantert, W.; Liu, K.; Lee, C.M.; Whang, K.; Hunter, J.G. Causes and Prevention of Laparoscopic Bile Duct Injuries: Analysis of 252 Cases from a Human Factors and Cognitive Psychology Perspective. Ann. Surg. 2003, 237, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Grover, K.; Watanabe, Y. Measuring and Teaching Intraoperative Decision-Making Using the Visual Concordance Test: Deliberate Practice of Advanced Cognitive Skills. JAMA Surg. 2020, 155, 78–79. [Google Scholar] [CrossRef]
- Madani, A.; Namazi, B.; Altieri, M.S.; Hashimoto, D.A.; Rivera, A.M.; Pucher, P.H.; Navarrete-Welton, A.; Sankaranarayanan, G.; Brunt, L.M.; Okrainec, A.; et al. Artificial Intelligence for Intraoperative Guidance: Using Semantic Segmentation to Identify Surgical Anatomy During Laparoscopic Cholecystectomy. Ann. Surg. 2022, 276, 363–369. [Google Scholar] [CrossRef]
- Brunt, L.M.; Deziel, D.J.; Telem, D.A.; Strasberg, S.M.; Aggarwal, R.; Asbun, H.; Bonjer, J.; McDonald, M.; Alseidi, A.; Ujiki, M.; et al. Safe Cholecystectomy Multi-Society Practice Guideline and State of the Art Consensus Conference on Prevention of Bile Duct Injury During Cholecystectomy. Ann. Surg. 2020, 272, 3–23. [Google Scholar] [CrossRef]
- Madani, A.; Watanabe, Y.; Feldman, L.S.; Vassiliou, M.C.; Barkun, J.S.; Fried, G.M.; Aggarwal, R. Expert Intraoperative Judgment and Decision-Making: Defining the Cognitive Competencies for Safe Laparoscopic Cholecystectomy. J. Am. Coll. Surg. 2015, 221, 931–940. [Google Scholar] [CrossRef]
- Nijssen, M.A.; Schreinemakers, J.M.; Meyer, Z.; van der Schelling, G.P.; Crolla, R.M.; Rijken, A.M. Complications After Laparoscopic Cholecystectomy: A Video Evaluation Study of Whether the Critical View of Safety Was Reached. World J. Surg. 2015, 39, 1798–1803. [Google Scholar] [CrossRef]
- Stefanidis, D.; Chintalapudi, N.; Anderson-Montoya, B.; Oommen, B.; Tobben, D.; Pimentel, M. How Often Do Surgeons Obtain the Critical View of Safety during Laparoscopic Cholecystectomy? Surg. Endosc. 2017, 31, 142–146. [Google Scholar] [CrossRef]
- Mascagni, P.; Vardazaryan, A.; Alapatt, D.; Urade, T.; Emre, T.; Fiorillo, C.; Pessaux, P.; Mutter, D.; Marescaux, J.; Costamagna, G.; et al. Artificial Intelligence for Surgical Safety: Automatic Assessment of the Critical View of Safety in Laparoscopic Cholecystectomy Using Deep Learning. Ann. Surg. 2022, 275, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Guo, Y.; Shen, M.; Yang, G.Z. Application of Artificial Intelligence in Surgery. Front. Med. 2020, 14, 417–430. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, P.; Kazemi, E.; Imielinski, M.; Elemento, O.; Hajirasouliha, I. Deep Convolutional Neural Networks Enable Discrimination of Heterogeneous Digital Pathology Images. EBioMedicine 2018, 27, 317–328. [Google Scholar] [CrossRef]
- Żydowicz, W.M.; Skokowski, J.; Marano, L.; Polom, K. Current Trends and Beyond Conventional Approaches: Advancements in Breast Cancer Surgery through Three-Dimensional Imaging, Virtual Reality, Augmented Reality, and the Emerging Metaverse. J. Clin. Med. 2024, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Zverinski, D.; Pfahringer, B.; Kempfert, J.; Kuehne, T.; Sündermann, S.H.; Stamm, C.; Hofmann, T.; Falk, V.; Eickhoff, C. Machine Learning for Real-Time Prediction of Complications in Critical Care: A Retrospective Study. The Lancet. Respir. Med. 2018, 6, 905–914. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, Q.; Wei, X.; Pan, Y.; Zhao, J.; Xin, X.; Qin, C.; Wang, X.; Li, J.; et al. Diagnosis of Thyroid Cancer Using Deep Convolutional Neural Network Models Applied to Sonographic Images: A Retrospective, Multicohort, Diagnostic Study. Lancet Oncol. 2019, 20, 193–201. [Google Scholar] [CrossRef]
- Xia, J.; Cai, Z.; Heidari, A.A.; Ye, Y.; Chen, H.; Pan, Z. Enhanced Moth-Flame Optimizer with Quasi-Reflection and Refraction Learning with Application to Image Segmentation and Medical Diagnosis. Curr. Bioinform. 2022, 18, 109–142. [Google Scholar] [CrossRef]
- Xu, Z.; Heidari, A.A.; Kuang, F.; Khalil, A.; Mafarja, M.; Zhang, S.; Chen, H.; Pan, Z. Enhanced Gaussian Bare-Bones Grasshopper Optimization: Mitigating the Performance Concerns for Feature Selection. Expert Syst. Appl. 2023, 212, 118642. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Li, R.; Chen, H.; Turabieh, H.; Mafarja, M.; Pan, Z. Generalized Oppositional Moth Flame Optimization with Crossover Strategy: An Approach for Medical Diagnosis. J. Bionic Eng. 2021, 18, 991–1010. [Google Scholar] [CrossRef]
- Marano, L.; Ricci, A.; Savelli, V.; Verre, L.; Di Renzo, L.; Biccari, E.; Costantini, G.; Marrelli, D.; Roviello, F. From Digital World to Real Life: A Robotic Approach to the Esophagogastric Junction with a 3D Printed Model. BMC Surg. 2019, 19, 153. [Google Scholar] [CrossRef]
- Cool, D.; Downey, D.; Izawa, J.; Chin, J.; Fenster, A. 3D Prostate Model Formation from Non-Parallel 2D Ultrasound Biopsy Images. Med. Image Anal. 2006, 10, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Yang, G.Z.; Lee, S.L. A Real-Time and Registration-Free Framework for Dynamic Shape Instantiation. Med. Image Anal. 2018, 44, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Durr, N.J. Deep Learning and Conditional Random Fields-Based Depth Estimation and Topographical Reconstruction from Conventional Endoscopy. Med. Image Anal. 2018, 48, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Turan, M.; Almalioglu, Y.; Araujo, H.; Konukoglu, E.; Sitti, M. A Non-Rigid Map Fusion-Based Direct SLAM Method for Endoscopic Capsule Robots. Int. J. Intell. Robot. Appl. 2017, 1, 399–409. [Google Scholar] [CrossRef]
- Mountney, P.; Yang, G.Z. Soft Tissue Tracking for Minimally Invasive Surgery: Learning Local Deformation Online. Med. Image Comput. Comput. Assist. Interv. 2008, 2, 364–372. [Google Scholar]
- Ye, M.; Giannarou, S.; Meining, A.; Yang, G.Z. Online Tracking and Retargeting with Applications to Optical Biopsy in Gastrointestinal Endoscopic Examinations. Med. Image Anal. 2016, 30, 144–157. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Meng, X.; Geng, Z.; Wang, F.Y. 3-D Tracking for Augmented Reality Using Combined Region and Dense Cues in Endoscopic Surgery. IEEE J. Biomed. Health Inform. 2018, 22, 1540–1551. [Google Scholar] [CrossRef]
- Bernhardt, S.; Nicolau, S.A.; Soler, L.; Doignon, C. The Status of Augmented Reality in Laparoscopic Surgery as of 2016. Med. Image Anal. 2017, 37, 66–90. [Google Scholar] [CrossRef]
- Wang, J.; Suenaga, H.; Hoshi, K.; Yang, L.; Kobayashi, E.; Sakuma, I.; Liao, H. Augmented Reality Navigation with Automatic Marker-Free Image Registration Using 3-D Image Overlay for Dental Surgery. IEEE Trans. Biomed. Eng. 2014, 61, 1295–1304. [Google Scholar] [CrossRef]
- Pratt, P.; Ives, M.; Lawton, G.; Simmons, J.; Radev, N.; Spyropoulou, L.; Amiras, D. Through the HoloLensTM Looking Glass: Augmented Reality for Extremity Reconstruction Surgery Using 3D Vascular Models with Perforating Vessels. Eur. Radiol. Exp. 2018, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Wang, T.; Ji, X.; Shen, Y.; Sun, Z.; Zhang, X. A Markerless Automatic Deformable Registration Framework for Augmented Reality Navigation of Laparoscopy Partial Nephrectomy. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1285–1294. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, D.; Fang, M.; Zhu, Y.; Zang, Y.; Liu, Z.; Zhang, H.; Ying, J.; Zhao, X.; Tian, J. Can CT-Based Radiomics Signature Predict KRAS/NRAS/BRAF Mutations in Colorectal Cancer? Eur. Radiol. 2018, 28, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Lu, L.; Schwartz, L.H.; Qian, M.; Tejpar, S.; Eggleton, P.; Zhao, B.; Piessevaux, H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J. Natl. Cancer Inst. 2020, 112, 902–912. [Google Scholar] [CrossRef]
- Drukker, K.; Li, H.; Antropova, N.; Edwards, A.; Papaioannou, J.; Giger, M.L. Most-Enhancing Tumor Volume by MRI Radiomics Predicts Recurrence-Free Survival ``early on’’ in Neoadjuvant Treatment of Breast Cancer. Cancer Imaging Off. Publ. Int. Cancer ImagingSoc. 2018, 18, 12. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of Clinical Trial Success Rates and Related Parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Haddad, T.; Helgeson, J.M.; E Pomerleau, K.; Preininger, A.M.; Roebuck, M.C.; Dankwa-Mullan, I.; Jackson, G.P.; Goetz, M.P. Accuracy of an Artificial Intelligence System for Cancer Clinical Trial Eligibility Screening: Retrospective Pilot Study. JMIR Med. Inform. 2021, 9, e27767. [Google Scholar] [CrossRef]
- Zhou, M.; Leung, A.; Echegaray, S.; Gentles, A.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Plevritis, S.K.; Rubin, D.L.; Napel, S.; et al. Non-Small Cell Lung Cancer Radiogenomics Map Identifies Relationships between Molecular and Imaging Phenotypes with Prognostic Implications. Radiology 2018, 286, 307–315. [Google Scholar] [CrossRef]
- Forghani, R.; Savadjiev, P.; Chatterjee, A.; Muthukrishnan, N.; Reinhold, C.; Forghani, B. Radiomics and Artificial Intelligence for Biomarker and Prediction Model Development in Oncology. Comput. Struct. Biotechnol. J. 2019, 17, 995–1008. [Google Scholar] [CrossRef]
- Fraser, M.; Sabelnykova, V.Y.; Yamaguchi, T.N.; Heisler, L.E.; Livingstone, J.; Huang, V.; Shiah, Y.-J.; Yousif, F.; Lin, X.; Masella, A.P.; et al. Genomic Hallmarks of Localized, Non-Indolent Prostate Cancer. Nature 2017, 541, 359–364. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.M.; Jiang, Y.; Fan, X.; Wang, J.; Antic, T.; Prior, F.; VanderWeele, D.; Oto, A. Quantitative Multiparametric MRI Features and PTEN Expression of Peripheral Zone Prostate Cancer: A Pilot Study. AJR Am. J. Roentgenol. 2016, 206, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Renard-Penna, R.; Cancel-Tassin, G.; Comperat, E.; Varinot, J.; Léon, P.; Roupret, M.; Mozer, P.; Vaessen, C.; Lucidarme, O.; Bitker, M.-O.; et al. Multiparametric Magnetic Resonance Imaging Predicts Postoperative Pathology but Misses Aggressive Prostate Cancers as Assessed by Cell Cycle Progression Score. J. Urol. 2015, 194, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Tahoun, M.; Klaan, B.; Thierfelder, K.M.; Weber, M.-A.; Krause, B.J.; Hakenberg, O.; Fuellen, G.; Hamed, M. A Radiogenomic Approach for Decoding Molecular Mechanisms Underlying Tumor Progression in Prostate Cancer. Cancers 2019, 11, 1293. [Google Scholar] [CrossRef]
- Jiang, L.; You, C.; Xiao, Y.; Wang, H.; Su, G.-H.; Xia, B.-Q.; Zheng, R.-C.; Zhang, D.-D.; Jiang, Y.-Z.; Gu, Y.-J.; et al. Radiogenomic Analysis Reveals Tumor Heterogeneity of Triple-Negative Breast Cancer. Cell Rep. Med. 2022, 3, 100694. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial Intelligence in Digital Pathology—New Tools for Diagnosis and Precision Oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Huang, W.; Randhawa, R.; Jain, P.; Hubbard, S.; Eickhoff, J.; Kummar, S.; Wilding, G.; Basu, H.; Roy, R. A Novel Artificial Intelligence-Powered Method for Prediction of Early Recurrence of Prostate Cancer After Prostatectomy and Cancer Drivers. JCO Clin. Cancer Inform. 2022, 6, e2100131. [Google Scholar] [CrossRef]
- Marmorino, F.; Faggioni, L.; Rossini, D.; Gabelloni, M.; Goddi, A.; Ferrer, L.; Conca, V.; Vargas, J.; Biagiarelli, F.; Daniel, F.; et al. The Prognostic Value of Radiomic Features in Liver-Limited Metastatic Colorectal Cancer Patients from the TRIBE2 Study. Future Oncol. 2023, 19, 1601–1611. [Google Scholar] [CrossRef]
- Shi, L.; He, Y.; Yuan, Z.; Benedict, S.; Valicenti, R.; Qiu, J.; Rong, Y. Radiomics for Response and Outcome Assessment for Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2018, 17, 1533033818782788. [Google Scholar] [CrossRef]
- Khorrami, M.; Prasanna, P.; Gupta, A. Changes in CT Radiomic Features Associated with Lymphocyte Distribution Predict Overall Survival and Response to Immunotherapy in Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 108–119. [Google Scholar] [CrossRef]
- Park, S.; Ock, C.-Y.; Kim, H.; Pereira, S.; Park, S.; Ma, M.; Choi, S.; Kim, S.; Shin, S.; Aum, B.J.; et al. Artificial Intelligence-Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Askin, S.; Burkhalter, D.; Calado, G.; El Dakrouni, S. Artificial Intelligence Applied to Clinical Trials: Opportunities and Challenges. Health Technol. 2023, 13, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Pea, A.; Scarpa, A. Artificial Intelligence in Oncology: Current Applications and Future Perspectives. Br. J. Cancer 2022, 126, 4–9. [Google Scholar] [CrossRef]
- Woo, M. An AI Boost for Clinical Trials. Nature 2019, 573, S100–S102. [Google Scholar] [CrossRef] [PubMed]
- Sangari, N.; Qu, Y. A Comparative Study on Machine Learning Algorithms for Predicting Breast Cancer Prognosis in Improving Clinical Trials. In Proceedings of the 2020 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 16–18 December 2020. [Google Scholar]
- Schperberg, A.V.; Boichard, A.; Tsigelny, I.F.; Richard, S.B.; Kurzrock, R. Machine Learning Model to Predict Oncologic Outcomes for Drugs in Randomized Clinical Trials. Int. J. Cancer 2020, 147, 2537–2549. [Google Scholar] [CrossRef]
- Kolla, L.; Gruber, F.K.; Khalid, O.; Hill, C.; Parikh, R.B. The Case for AI-Driven Cancer Clinical Trials—The Efficacy Arm in Silico. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188572. [Google Scholar] [CrossRef]
- Haddad, T.C.; Helgeson, J.; Pomerleau, K.; Makey, M.; Lombardo, P.; Coverdill, S.; Urman, A.; Rammage, M.; Goetz, M.P.; LaRusso, N. Impact of a Cognitive Computing Clinical Trial Matching System in an Ambulatory Oncology Practice. J. Clin. Oncol. 2018, 36, 6550. [Google Scholar] [CrossRef]
- Feijoo, F.; Palopoli, M.; Bernstein, J.; Siddiqui, S.; Albright, T.E. Key Indicators of Phase Transition for Clinical Trials through Machine Learning. Drug Discov. Today 2020, 25, 414–421. [Google Scholar] [CrossRef]
- Chen, Z.H.; Lin, L.; Wu, C.F.; Li, C.F.; Xu, R.H.; Sun, Y. Artificial Intelligence for Assisting Cancer Diagnosis and Treatment in the Era of Precision Medicine. Cancer Commun. 2021, 41, 1100–1115. [Google Scholar]
- Huynh, E.; Hosny, A.; Guthier, C.; Bitterman, D.S.; Petit, S.F.; Haas-Kogan, D.A.; Kann, B.; Aerts, H.J.W.L.; Mak, R.H. Artificial Intelligence in Radiation Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 771–781. [Google Scholar]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Arimura, H.; Soufi, M.; Kamezawa, H.; Ninomiya, K.; Yamada, M. Radiomics with Artificial Intelligence for Precision Medicine in Radiation Therapy. J. Radiat. Res. 2019, 60, 150–157. [Google Scholar] [CrossRef]
- Dercle, L.; Henry, T.; Carré, A.; Paragios, N.; Deutsch, E.; Robert, C. Reinventing Radiation Therapy with Machine Learning and Imaging Bio-Markers (Radiomics): State-of-the-Art, Challenges and Perspectives. Methods 2021, 188, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, L.J.; Pepa, M.; Zaffaroni, M.; Marvaso, G.; Alterio, D.; Volpe, S.; Corrao, G.; Augugliaro, M.; Starzyńska, A.; Leonardi, M.C.; et al. Machine Learning-Based Models for Prediction of Toxicity Outcomes in Radiotherapy. Front. Oncol. 2020, 10, 790. [Google Scholar] [CrossRef]
- Zomkowska, E.; Zakrzewska, M.; Pilewski, D.; Zajączkiewicz, H. Assessment of Nervomuscle Coordination in the Act of Swallowing Using Dynamic Imaging Tests Performed by Cone Beam Computer Tomography. Acta Elbingensia 2023, 50, 6–9. [Google Scholar] [CrossRef]
- Bourbonne, V.; Da-Ano, R.; Jaouen, V.; Lucia, F.; Dissaux, G.; Bert, J.; Pradier, O.; Visvikis, D.; Hatt, M.; Schick, U. Radiomics Analysis of 3D Dose Distributions to Predict Toxicity of Radiotherapy for Lung Cancer. Radiother. Oncol. 2021, 155, 144–150. [Google Scholar] [CrossRef]
- Kerns, S.L.; Kundu, S.; Oh, J.H.; Singhal, S.K.; Janelsins, M.; Travis, L.B.; Deasy, J.O.; Janssens, A.C.J.; Ostrer, H.; Parliament, M.; et al. The Prediction of Radiotherapy Toxicity Using Single Nucleotide Polymorphism-Based Models: A Step Toward Prevention. Semin. Radiat. Oncol. 2015, 25, 281–291. [Google Scholar] [CrossRef]
- de Biase, A.; Sourlos, N.; van Ooijen, P.M.A. Standardization of Artificial Intelligence Development in Radiotherapy. Semin. Radiat. Oncol. 2022, 32, 415–420. [Google Scholar] [CrossRef]
- Vandewinckele, L.; Claessens, M.; Dinkla, A.; Brouwer, C.; Crijns, W.; Verellen, D.; van Elmpt, W. Overview of Artificial Intelligence-Based Applications in Radiotherapy: Recommendations for Implementation and Quality Assurance. Radiother. Oncol. 2020, 153, 55–66. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Hong, J.C.; Zheng, D. Artificial Intelligence in Radiotherapy Treatment Planning: Present and Future. Technol. Cancer Res. Treat. 2019, 18, 1533033819873922. [Google Scholar] [CrossRef]
- Feng, M.; Valdes, G.; Dixit, N.; Solberg, T.D. Machine Learning in Radiation Oncology: Opportunities, Requirements, and Needs. Front. Oncol. 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Somashekhar, S.; Sepúlveda, M.-J.; Puglielli, S.; Norden, A.; Shortliffe, E.; Kumar, C.R.; Rauthan, A.; Kumar, N.A.; Patil, P.; Rhee, K.; et al. Watson for Oncology and Breast Cancer Treatment Recommendations: Agreement with an Expert Multidisciplinary Tumor Board. Ann. Oncol. 2018, 29, 418–423. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Ma, X.; Chen, Y.; Xi, J.; Yin, X.; Kang, H.; Guan, H.; Dai, Z.; Liu, D.; et al. Concordance between Treatment Recommendations Provided by IBM Watson for Oncology and a Multidisciplinary Tumor Board for Breast Cancer in China. Jpn. J. Clin. Oncol. 2020, 50, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Park, H.-Y.; Kho, B.-G.; Park, C.-K.; Oh, I.-J.; Kim, Y.-C.; Kim, S.; Yun, J.-S.; Song, S.-Y.; Na, K.-J.; et al. Artificial Intelligence and Lung Cancer Treatment Decision: Agreement with Recommendation of Multidisciplinary Tumor Board. Transl. Lung Cancer Res. 2020, 9, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Le Thien, M.A.; Redjdal, A.; Bouaud, J.; Séroussi, B. Using Machine Learning on Imbalanced Guideline Compliance Data to Optimize Multidisciplinary Tumour Board Decision Making for the Management of Breast Cancer Patients. Stud. Health Technol. Inform. 2022, 290, 787–788. [Google Scholar]
- Botsis, T.; Murray, J.; Leal, A.; Palsgrove, D.; Wang, W.; White, J.R.; Velculescu, V.E.; Anagnostou, V.; Johns Hopkins Molecular Tumor Board Investigators. Natural Language Processing Approaches for Retrieval of Clinically Relevant Genomic Information in Cancer. Stud. Health Technol. Inform. 2022, 295, 350–353. [Google Scholar]
- Ng, S.S.T.; Oehring, R.; Ramasetti, N.; Roller, R.; Thomas, P.; Chen, Y.; Moosburner, S.; Winter, A.; Maurer, M.-M.; Auer, T.A.; et al. Concordance of a Decision Algorithm and Multidisciplinary Team Meetings for Patients with Liver Cancer-a Study Protocol for a Randomized Controlled Trial. Trials 2023, 24, 577. [Google Scholar] [CrossRef]
- Park, Y.E.; Chae, H. The Fidelity of Artificial Intelligence to Multidisciplinary Tumor Board Recommendations for Patients with Gastric Cancer: A Retrospective Study. J. Gastrointest. Cancer 2024, 55, 365–372. [Google Scholar] [CrossRef]
- Lukac, S.; Dayan, D.; Fink, V.; Leinert, E.; Hartkopf, A.; Veselinovic, K.; Janni, W.; Rack, B.; Pfister, K.; Heitmeir, B.; et al. Evaluating ChatGPT as an Adjunct for the Multidisciplinary Tumor Board Decision-Making in Primary Breast Cancer Cases. Arch. Gynecol. Obstet. 2023, 308, 1831–1844. [Google Scholar] [CrossRef]
- Griewing, S.; Gremke, N.; Wagner, U.; Lingenfelder, M.; Kuhn, S.; Boekhoff, J. Challenging ChatGPT 3.5 in Senology—An Assessment of Concordance with Breast Cancer Tumor Board Decision Making. J. Pers. Med. 2023, 13, 1502. [Google Scholar] [CrossRef]
- Griewing, S.; Knitza, J.; Boekhoff, J.; Hillen, C.; Lechner, F.; Wagner, U.; Wallwiener, M.; Kuhn, S. Evolution of Publicly Available Large Language Models for Complex Decision-Making in Breast Cancer Care. Arch. Gynecol. Obstet. 2024, 310, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, J.; Westphalen, C.B.; Frey, S.; Schmitt, Y.; Heinemann, V.; Fey, T.; Nasseh, D. Supporting the Decision to Perform Molecular Profiling for Cancer Patients Based on Routinely Collected Data through the Use of Machine Learning. Clin. Exp. Med. 2024, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Horsfall, H.L.; Funnell, J.P.; Hanrahan, J.G.; Khan, D.Z.; Muirhead, W.; Stoyanov, D.; Marcus, H.J. Artificial Intelligence in Brain Tumour Surgery-An Emerging Paradigm. Cancers 2021, 13, 5010. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, X.; Gan, Y.; Han, S.; Rong, P.; Wang, W.; Li, W.; Zhou, L. Artificial Intelligence Assists Precision Medicine in Cancer Treatment. Front. Oncol. 2022, 12, 998222. [Google Scholar] [CrossRef]
- McDougall, R.J. Computer Knows Best? The Need for Value-Flexibility in Medical AI. J. Med. Ethics 2019, 45, 156–160. [Google Scholar] [CrossRef]
- Marano, L.; Fusario, D.; Savelli, V.; Marrelli, D.; Roviello, F. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses. Updates Surg. 2021, 73, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Grunhut, J. Artificial Intelligence: The Elephant in the Tumor Board Room. Acad. Med. 2023, 98, 542. [Google Scholar] [CrossRef]
- Burr, C.; Leslie, D. Ethical Assurance: A Practical Approach to the Responsible Design, Development, and Deployment of Data-Driven Technologies. AI Ethics 2023, 3, 73–98. [Google Scholar] [CrossRef]
- Burrell, J. How the Machine ‘Thinks’: Understanding Opacity in Machine Learning Algorithms. Big Data Soc. 2016, 3, 2053951715622512. [Google Scholar] [CrossRef]
- Żydowicz, W.M.; Skokowski, J.; Marano, L.; Polom, K. Navigating the Metaverse: A New Virtual Tool with Promising Real Benefits for Breast Cancer Patients. J. Clin. Med. 2024, 13, 4337. [Google Scholar] [CrossRef]
- Sap, M.; Card, D.; Gabriel, S.; Choi, Y.; Smith, N.A. The Risk of Racial Bias in Hate Speech Detection. In Proceedings of the ACL 2019—57th Annual Meeting of the Association for Computational Linguistics, Florence, Italy, 28 July–2 August 2019; pp. 1668–1678. [Google Scholar] [CrossRef]
- Ahmet, E. The Impact of Artificial Intelligence on Social Problems and Solutions: An Analysis on the Context of Digital Divide and Exploitation. Yeni Medya 2022, 2022, 247–264. [Google Scholar]
- Lee, M.K. Understanding Perception of Algorithmic Decisions: Fairness, Trust, and Emotion in Response to Algorithmic Management. Big Data Soc. 2018, 5, 2053951718756684. [Google Scholar] [CrossRef]
- Selbst, A.D.; Boyd, D.; Friedler, S.A.; Venkatasubramanian, S.; Vertesi, J. Fairness and Abstraction in Sociotechnical Systems. In Proceedings of the FAT* 2019—Proceedings of the 2019 Conference on Fairness, Accountability, and Transparency, Atlanta, GA, USA, 29–31 January 2019; pp. 59–68. [Google Scholar] [CrossRef]
- Ferrer, X.; Van Nuenen, T.; Such, J.M.; Cote, M.; Criado, N. Bias and Discrimination in AI: A Cross-Disciplinary Perspective. IEEE Technol. Soc. Mag. 2021, 40, 72–80. [Google Scholar] [CrossRef]
- Felzmann, H.; Fosch-Villaronga, E.; Lutz, C.; Tamò-Larrieux, A. Towards Transparency by Design for Artificial Intelligence. Sci. Eng. Ethics 2020, 26, 3333–3361. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; Rakova, B.; Ayesh, A.; Fanti, A.; Lennon, M. Principles to Practices for Responsible AI: Closing the Gap. arXiv 2020, arXiv:2006.04707. [Google Scholar]
- Schmitt, L. Mapping Global AI Governance: A Nascent Regime in a Fragmented Landscape. AI Ethics 2022, 2, 303–314. [Google Scholar] [CrossRef]
- Benjamins, R.; Rubio Viñuela, Y.; Alonso, C. Social and Ethical Challenges of the Metaverse: Opening the Debate. AI Ethics 2023, 3, 689–697. [Google Scholar] [CrossRef]
- Habbal, A.; Ali, M.K.; Abuzaraida, M.A. Artificial Intelligence Trust, Risk and Security Management (AI TRiSM): Frameworks, Applications, Challenges and Future Research Directions. Expert Syst. Appl. 2024, 240, 122442. [Google Scholar] [CrossRef]
- Kaissis, G.A.; Makowski, M.R.; Rückert, D.; Braren, R.F. Secure, Privacy-Preserving and Federated Machine Learning in Medical Imaging. Nat. Mach. Intell. 2020, 2, 305–311. [Google Scholar] [CrossRef]
- ISO/IEC 27001:2022; Information Security, Cybersecurity and Privacy Protection—Information Security Management Systems—Requirements. ISO: Geneva, Switzerland, 2022.
- Dayan, I.; Roth, H.R.; Zhong, A.; Harouni, A.; Gentili, A.; Abidin, A.Z.; Liu, A.; Costa, A.B.; Wood, B.J.; Tsai, C.S.; et al. Federated Learning for Predicting Clinical Outcomes in Patients with COVID-19. Nat. Med. 2021, 27, 1735–1743. [Google Scholar] [CrossRef]
- Ghafur, S.; Grass, E.; Jennings, N.R.; Darzi, A. The Challenges of Cybersecurity in Health Care: The UK National Health Service as a Case Study. Lancet Digit. Health 2019, 1, e10–e12. [Google Scholar] [CrossRef]


| Category | Details |
|---|---|
| AI to Enhance Surgical Performance and Education | High rates of preventable adverse events in surgery highlight the need for improved judgment and decision-making. AI and machine learning, particularly computer vision, are used to develop algorithms for error reduction and real-time guidance. Challenges include variability in anatomical structures and expert cognitive behaviors. Tools like GoNoGoNet and CholeNet offer real-time guidance by identifying safe and hazardous areas during surgeries like laparoscopic cholecystectomy. DeepCVS model predicts critical views of safety (CVS) in surgical procedures. |
| AI to Enhance Preoperative Setting | AI aids in surgical planning using medical records and imaging (X-ray, CT, MRI). Techniques include anatomical classification, detection, segmentation, and registration. Deep learning enhances these tasks but faces challenges like generalizability and explainability. Collaborative efforts and personalized data integration are essential for early detection and treatment. |
| AI for Intraoperative Support | AI in MIS provides improved visualization and localization through shape instantiation, endoscopic navigation, tissue tracking, and augmented reality. Advances in 3D reconstruction from 2D images and navigation techniques like SLAM help guide endoscopes. Tissue tracking is improved with learning-based methods. Augmented reality enhances intraoperative vision by overlaying preoperative images. Challenges include textureless surfaces, variable illumination, and organ deformation during surgery. Future AI must integrate multimodal data and adapt to micro- and nanorobotics. |
| Category | Details |
|---|---|
| AI in Molecular Profiling and Treatment Selection | AI algorithms analyze multi-OMICs data for molecular characterization, tumor grading, and clinical decision-making. Radiogenomics integrates imaging-derived parameters with genomic data. Radiomics extracts quantitative features from medical images to predict treatment response and patient outcomes. AI platforms optimize clinical trial protocols and patient recruitment. Examples include radiomic features predicting RAS mutation status in colorectal cancer, and radiogenomics identifying EGFR expression in lung cancer. |
| Predictive Modeling for Drug Response and Personalized Therapy | AI predicts tumor response to treatments, aiding personalized therapy. Radiomic signatures can predict response to treatments like FOLFIRI and detect EGFR-resistant tumors. In NSCLC, AI evaluates treatment efficacy and predicts outcomes, aiding in immunotherapy selection. Radiomic features from MRI can predict recurrence-free survival in breast cancer patients undergoing chemotherapy. AI can identify immune phenotypes in NSCLC, predicting response to immune checkpoint inhibitors. |
| Integrating AI in Clinical Trial Design and Patient Recruitment | AI improves clinical trial design and patient recruitment. Natural language processing (NLP) software analyzes large datasets to optimize trial protocols. AI predicts progression-free survival and overall survival in clinical trials, potentially replacing control arms with virtual arms. AI enhances patient recruitment by matching patients with suitable trials based on molecular profiles. AI platforms, like Watson for Clinical Trial Matching, have increased patient accrual in trials. Challenges include data standardization, reproducibility, and regulatory frameworks for health data. |
| Category | Details |
|---|---|
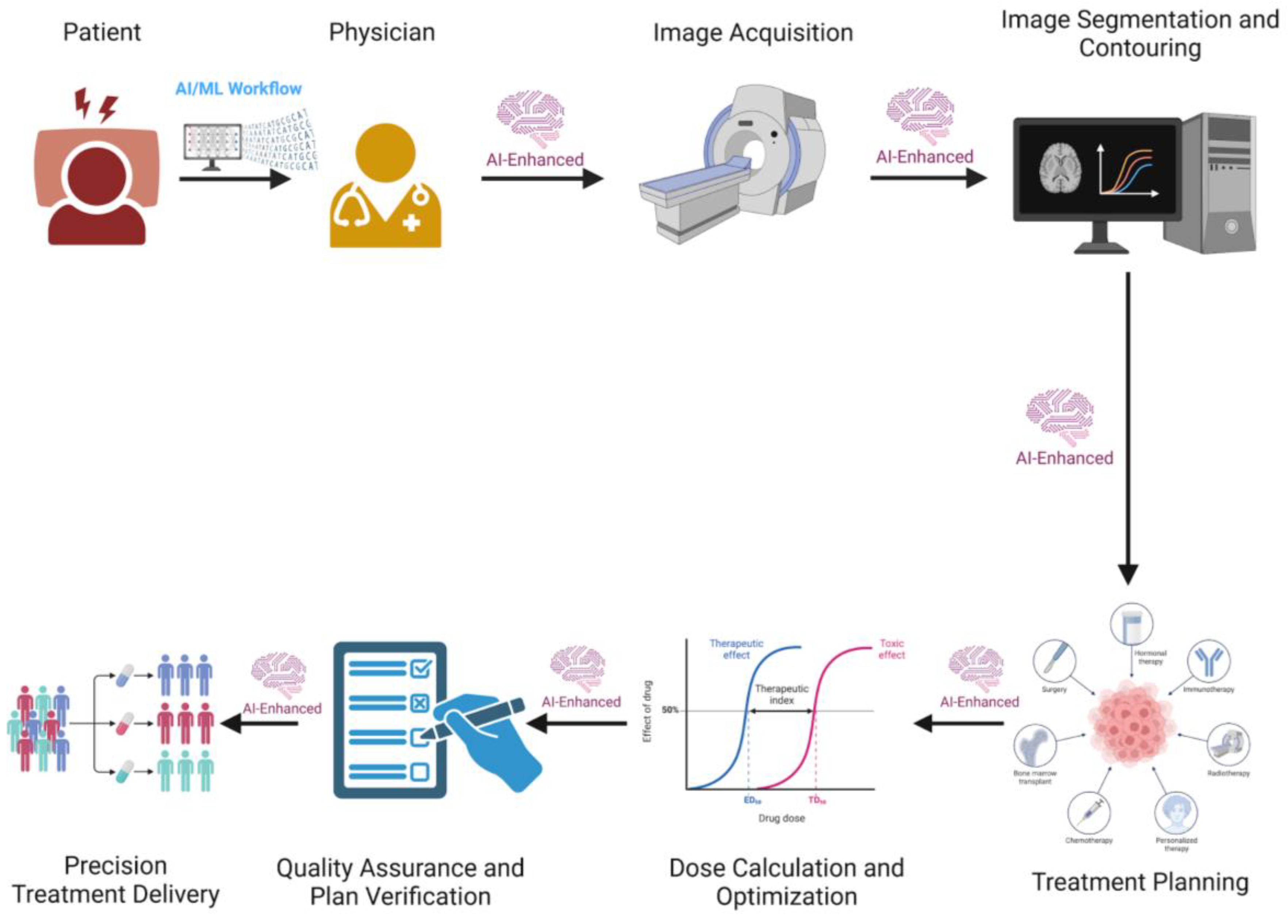
| AI-enhanced Radiotherapy Workflow | AI, particularly deep learning, enhances contouring, treatment planning, optimization, and adaptive workflows. AI-based tools like the Radiation Planning Assistant (RPA) automate key components of radiotherapy planning, improving efficiency and accessibility, especially in low- and middle-income countries (LMICs). AI can automate repetitive tasks, optimize time, and improve clinical outcomes through predictive models and decision support systems (DSS). AI aids in all steps of radiotherapy, from patient consultation to treatment delivery, reducing clinical workload and improving quality assurance. |
| Prediction of Radiotherapy Outcomes and Toxicity | AI and machine learning (ML) predict radiotherapy outcomes and toxicity by analyzing complex medical data. Radiomics extract quantitative features from medical images, improving decision-making in precision medicine. AI models enhance prediction of side effects, integrating radiomic features, genetic factors, and imaging analyses. AI can predict treatment outcomes and toxicity, contributing to more personalized and accurate radiotherapy. Challenges include interpretability, validation, and standardization of AI models. |
| Addressing Uncertainties and Limitations in AI for Radiation Oncology | AI in radiation oncology faces challenges like lack of standardized protocols, small datasets, and need for regular model updates. Extensive clinical trials and standardized protocols are essential for effective integration of AI in clinical settings. Human intervention remains crucial to ensure quality and safety. Despite potential, AI’s full realization requires addressing uncertainties and constraints, ensuring patient safety, and efficacy in oncological radiotherapy. |
| Category | Details |
|---|---|
| Enhancing Efficiency and Effectiveness | AI can analyze large datasets, including genomic, imaging, and clinical records, to identify patterns and correlations, providing valuable insights into tumor characteristics, treatment responses, and patient prognoses. This capability allows for more informed decision-making in cancer care. |
| Multidisciplinary Teams (MDTs) | MDTs, comprising medical professionals from various specialties, collaborate to define treatment plans for patients. AI-driven Clinical Decision Support Systems (CDSS) can reduce the time spent evaluating evidence-based practices, integrating clinical, imaging, biological, genetic, and cost-related data to produce predictive models. |
| Streamlining Administrative Tasks | AI can automate the collection and organization of patient data, reducing manual data entry and allowing clinicians to focus on critical case discussions. This improves the efficiency of the Multidisciplinary Tumor Board (MTB). |
| AI Tools in MTB | IBM’s Watson for Oncology, now equipped with advanced generative AI capabilities, has shown high concordance with MTB decisions and facilitates a multidisciplinary approach, saving time for simpler cases. Other AI tools and chatbots like ChatGPT are being explored to standardize language and interpretation of MTB discussions. |
| Optimizing Decision-Making | AI models, such as those developed by Kasprzak et al., can optimize decisions like whether molecular profiling should be performed, impacting patient outcomes. |
| Overall Impact of AI | Integrating AI in MTB can enhance diagnostic precision, personalize treatment plans, predict patient outcomes, and streamline administrative tasks, leading to more comprehensive, timely, and effective cancer care, and ultimately improving patient outcomes and advancing oncology. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, V.; Marmorino, F.; Germani, M.M.; Cichowska-Cwalińska, N.; Menditti, V.S.; Gallo, P.; Studiale, V.; Taravella, A.; Landi, M.; Reginelli, A.; et al. The Role of Artificial Intelligence on Tumor Boards: Perspectives from Surgeons, Medical Oncologists and Radiation Oncologists. Curr. Oncol. 2024, 31, 4984-5007. https://doi.org/10.3390/curroncol31090369
Nardone V, Marmorino F, Germani MM, Cichowska-Cwalińska N, Menditti VS, Gallo P, Studiale V, Taravella A, Landi M, Reginelli A, et al. The Role of Artificial Intelligence on Tumor Boards: Perspectives from Surgeons, Medical Oncologists and Radiation Oncologists. Current Oncology. 2024; 31(9):4984-5007. https://doi.org/10.3390/curroncol31090369
Chicago/Turabian StyleNardone, Valerio, Federica Marmorino, Marco Maria Germani, Natalia Cichowska-Cwalińska, Vittorio Salvatore Menditti, Paolo Gallo, Vittorio Studiale, Ada Taravella, Matteo Landi, Alfonso Reginelli, and et al. 2024. "The Role of Artificial Intelligence on Tumor Boards: Perspectives from Surgeons, Medical Oncologists and Radiation Oncologists" Current Oncology 31, no. 9: 4984-5007. https://doi.org/10.3390/curroncol31090369








