Abstract
COVID-19, a novel infectious disease caused by the emergence of the SARS-CoV-2 virus in 2020, has had a profound impact on healthcare, both at the individual and population level. The impact at the population level was felt most acutely during the emergency phase of the pandemic, with hospital capacity issues leading to widespread disruptions and delays in the delivery of healthcare services such as screening programs and elective surgeries. While hospitals are no longer being acutely overwhelmed by COVID-19 patients, the impact of the virus on vulnerable patient populations such as cancer patients continues to be of ongoing consequence. Cancer patients remain at high risk of hospitalization, ICU admission, and death due to COVID-19, even in the era of vaccination. Infection prevention and risk mitigation strategies such air quality control, masking, testing, vaccination, and treatment should therefore be integrated into the usual care and counseling of cancer patients moving forward to avoid preventable morbidity and mortality from this infection and ensure the safety of this vulnerable cohort as they navigate their cancer diagnosis and treatment in the era of COVID-19.
1. Introduction
COVID-19, caused by the SARS-CoV-2 virus, has had a profound impact on global health since its emergence in 2019. The pandemic has not only overwhelmed healthcare systems but has also posed severe risks to vulnerable populations, including patients with cancer. GLOBOCAN estimates that close to 20 million people were diagnosed with cancer in 2022 and that approximately one in five people will develop cancer in their lifetime. These individuals are particularly susceptible to COVID-19 due to immune system compromise from the malignancy itself, side effects of anti-cancer treatments, and increased contact with the healthcare system, which may result in greater exposure to the SARS-CoV-2 virus. The interplay between COVID-19 and cancer care is multi-faceted and complex. This review aims to provide a broad overview of the existing literature on this topic, highlighting the impact of the COVID-19 pandemic on cancer care, the clinical outcomes of COVID-19 in cancer patients, and the prevention and therapeutic strategies that can be used to mitigate COVID-19-related risks for these patients, with a focus on North American guidelines. This practical guide will help inform clinical practice and emphasize the need for ongoing vigilance and patient counseling with regards to the SARS-CoV-2 virus.
2. The Impact of the COVID-19 Pandemic on Cancer Care
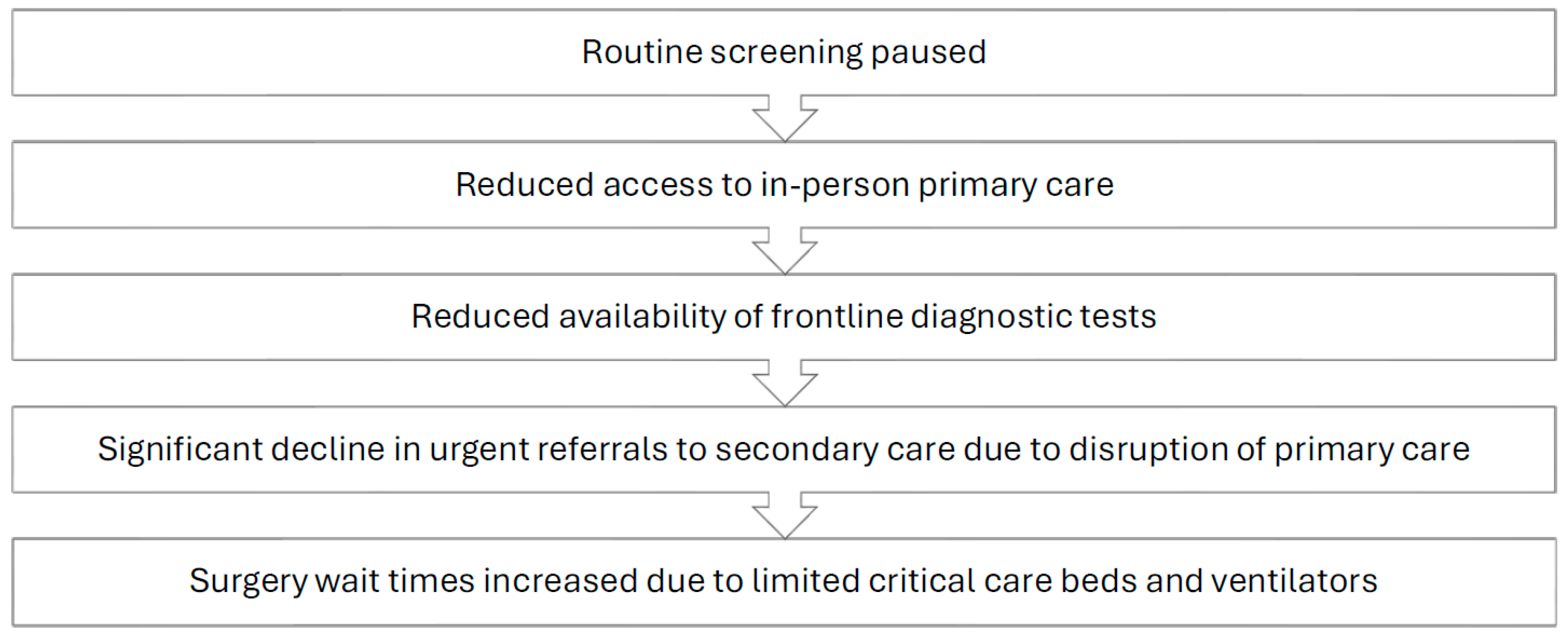
The screening and workup of malignancies is a time-sensitive matter that requires prompt implementation of guideline-oriented protocols by competent staff who work in a well-functioning healthcare system. Unfortunately, the COVID-19 pandemic resulted in significant disruptions to the standard pathway commonly followed by patients seeking cancer screening, as well as patients with existing malignancies requiring treatment (Figure 1). Proficient cancer care is a result of a dynamic interplay between three key entities: screening protocols, healthcare workers, and the healthcare system. The aforementioned key players were all impacted by the global pandemic to varying degrees.
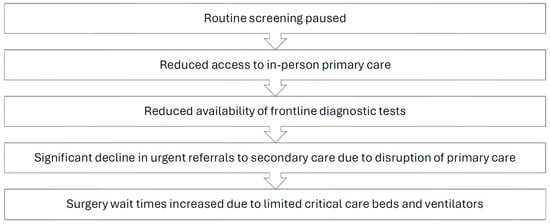
Figure 1.
Impact of the COVID-19 pandemic on the oncology care pathway.
The COVID-19 pandemic greatly impacted the lives of patients suffering from various medical and surgical conditions, with oncology patients being a particularly affected group [1,2]. The large-scale lockdowns that occurred in many countries, in addition to the heightened level of fear due to a rapidly spreading novel pathogen at the time, led to an increased reluctance among individuals to visit their primary care practitioners for routine testing and screening [3,4]. A survey conducted through the Council of Academic Family Medicine’s Educational Research Alliance, an American group, found that up to 34.5% of respondents reported postponing cancer screening, with many physicians reporting that patients were afraid to come into the office [5,6]. Additionally, several medical governing bodies, including the European Society for Medical Oncology and the American Cancer Society, recommended that at-risk patients cease routine screening protocols during the peak of the pandemic. This, combined with the aforementioned factors, led to a seismic decrease in the number of screened patients for commonly prevalent cancers [7,8,9]. In the United States (US), between the months of January and April 2020, breast, colon, and cervical cancer screening rates dropped by a massive 94%, 86%, and 94%, respectively [10]. The province of Ontario, Canada showed similar findings, with a 41% reduction in screening rates in 2020 compared to the prior year [11]. This observable decrease in screening across several jurisdictions and various countries posed a massive problem to at-risk groups, as their malignancies would go undetected during the peak of the COVID-19 pandemic. The interruption of breast cancer screening, even for a period of only 3 months, may lead to a 7% decrease in total diagnosed cases per year, with a 6-month interruption potentially leading to a 14% decrease [12]. A national survey conducted in the United States estimated that over 134,000 cancer cases may have gone undiagnosed between March 2020 and December 2020 [13]. According to the National Cancer Institute in the US, the significant screening delays and cancellations during the spring of 2020 are expected to result in nearly 10,000 excess deaths from breast and colorectal cancer alone within the next decade [14].
In addition to the disruption of screening programs during the pandemic, access to general practitioners and primary care physicians was restricted in an effort to control the outbreak of SARS-CoV-2. As a result, referrals dropped significantly, with institutions such as the National Health Services (NHS) in the UK observing a 60% reduction in referrals to secondary care centers [15]. Furthermore, the difficult working conditions associated with the crisis increased the prevalence of physician burnout, depression, and overall stress [16]. In a survey of 206 large healthcare organizations in the US, 50% of healthcare workers met the criteria for burnout, with nurses being the most affected group at 56%. Nurses were also the group most likely to report their intent to leave at 41% [17]. These alarming figures illustrate the tremendous burden carried by key actors of the healthcare system since 2020. The psychological distress experienced by healthcare workers on the front lines was multifactorial in etiology, with prominent causes including significant personal protective equipment (PPE) shortages, increased documentation requirements, fear of a newly spreading disease, and high patient volumes, which, combined with previously described disruptions and delays, contributed to reduced healthcare service quality and moral injury [14,18].
The SARS-CoV-2 virus itself also contributed to the negative toll of the pandemic on the healthcare workforce. A study by the Institut national de la santé du Quebec (INSPQ) that was presented at the 1st Canadian Symposium on Long Covid found that 10% of all healthcare workers suffered from the effects of long COVID lasting more than 12 weeks, with a third experiencing severe symptoms. More than half of the healthcare workers in the study had been experiencing long COVID symptoms, such as shortness of breath, fatigue, memory loss, and confusion for longer than a year. The majority developed long COVID after January 2022, subsequent to an omicron infection or reinfection. Seventy percent reported that their symptoms had an impact on their work [19].
The staffing issues that have plagued hospitals since the start of the pandemic, with a survey of American healthcare workers revealing that 18% had left their jobs during the pandemic [20], in addition to the high volume of COVID-19 patients that required critical care beds and ventilators, resulted in significantly increased wait times for diagnostic tests and elective surgeries, including cancer surgeries [15]. It is estimated, based on global surgical data, that over a 12-week period of peak disruption during the pandemic, 37.7% of cancer surgeries were postponed or canceled [21]. These disruptions in cancer care, which have occurred at multiple points in the patient care pathway and on more than one occasion since the start of the pandemic, can be expected to result in an aftershock period where physicians and healthcare systems must contend with an increased burden of more advanced stage cancers due to disease progression from delays in care and from undetected malignancies eventually presenting at more advanced stages. It is also hypothesized that some patients may experience rapid disease progression after COVID-19 infection due to immune dysregulation [22,23], which could potentially further add to the burden of advanced staged disease.
3. COVID-19 Outcomes in Cancer Patients
Patients living with cancer are very often immunocompromised because of their disease and/or their anti-cancer treatments. They are also often older than 60 years old and have other co-morbidities in addition to their cancer diagnosis and treatment. These compounding risk factors make this patient population highly vulnerable to COVID-19 infections. It is thus of vital importance for both cancer patients and their physicians to be aware of this new risk as these patients will require enhanced preventive measures, surveillance, and counseling going forward.
A systematic review and meta-analysis published in the first year of the pandemic found that the rate of severe or critical disease in cancer patients with COVID-19 was 45.5% (odds ratio (OR) = 3.91), the rate of admission to the intensive care unit (ICU) was 14.5% (OR = 3.10), the rate of mechanical ventilation was 11.7% (OR = 4.86), and the mortality rate was 21.1% (OR = 3.23) when compared to non-cancer patients [24]. Similar findings have been reported in other large-scale systematic reviews and meta-analyses, as well [25,26]. This increased risk of severe COVID-19 outcomes was echoed by a prospective study highlighting a higher risk of severe events (ICU admission, mechanical ventilation, and death) among patients with cancer compared to those without cancer, at 39% versus 8%, respectively [27]; the increased risk persisted after adjusting for age and smoking status [28], although one systematic review found that adjusting for age consistently resulted in lower estimated ORs of COVID-19-related death in cancer patients (adjusted OR = 1.37) [29]. Interestingly, COVID-19-related mortality may decrease with time since initial diagnosis and treatment of cancer (adjusted OR 1.55 versus 0.98 at 1 year versus 5 years since cancer diagnosis/treatment, respectively) [30]. Finally, hematological malignancies have been shown to have a higher mortality risk when compared to solid tumors (OR = 1.86) [31].
The clinical outcomes of cancer patients with COVID-19 may also be influenced by the type of treatment they are receiving. Large studies have found that patients with hematologic malignancies undergoing chemotherapy treatment and patients receiving surgical treatments for their cancer had a higher risk of clinically severe events compared to patients who were not receiving these treatments. No significant differences were found in COVID-19 outcomes in patients receiving cancer therapies for solid tumors [27,32]. Similarly, evidence suggests that there is no increase in mortality or ICU/hospitalization rates in cancer patients receiving immune checkpoint inhibitors compared to cancer patients who are not [33,34,35]. COVID-19 infection in the context of hematopoietic cell transplantation (HCT), on the other hand, has been shown to be associated with increased mortality, mechanical ventilation, and ICU admission. Higher death rates were found in patients who developed COVID-19 within 12 months of HCT (risk ratio (RR) = 2.11), within 6 months of receiving immunosuppressant drugs (RR = 2.11), and in the context of graft vs. host disease (RR = 2.38) [36].
COVID-19 infections may also negatively impact cancer treatment in other ways. For example, SARS-CoV-2-positive cancer patients have been shown to be significantly more likely to experience hematotoxicity to their anti-cancer treatments when compared to patients that did not test positive for SARS-CoV-2 (73% vs. 35%), leading to more treatment delays in the patients that contracted COVID-19 [37]. As with the general population, COVID-19 infections in cancer patients also carry the risk of developing long-COVID. The rate of long-COVID in this patient population, defined as the persistence/worsening of symptoms or one or more sequalae following acute COVID-19 infection, varies considerably across studies. Long-COVID has been reported to occur in 51.3% (n = 80), 16.6% (n = 186), 12.4% (n = 97), 60% (n = 312), and 8% (n = 186) of cancer patients at 4 weeks, 2.3 months, 12 weeks, 7 months, and 12 months after the acute COVID-19 diagnosis, respectively [38,39,40,41]. It should be noted, however, that these studies lacked control groups, and many symptoms of long-COVID overlap with known side effects of anti-cancer therapies and cancer itself. More research is needed in this area to quantify the risk of long-COVID in this patient population.
Many of the data available on the risks associated with COVID infections in cancer patients stems from research conducted before the vaccine rollout; however, it is important to note that vaccination benefit may be reduced in this patient population as many cancer patients, such as those with hematologic malignancies and HCT recipients, experience reduced immunogenicity to COVID-19 vaccines [42,43,44,45]. Nevertheless, vaccination has been shown to significantly reduce the risk of poor COVID-19 outcomes in this vulnerable population. An observational cohort study comparing unvaccinated cancer patients to those that had been vaccinated found that the incidence of hospitalization was 42% vs. 29%, respectively; the incidence of mechanical ventilation was 8.4% vs. 4.6%; and all-cause mortality within 30 days of COVID-19 diagnosis was 17% vs. 4.65% [46]. In a case–control study of vaccinated patients with hematologic malignancies, those who had received a booster vaccine dose had reduced odds of severe disease (aOR = 0.73); however, the proportion of patients that experienced severe COVID-19 (21.3%) or died within 28 days of a positive SARS-CoV-2 test result (3.5%) remained high in this highly vaccinated cohort, suggesting that these patients remain extremely vulnerable to COVID-19 even in the post-vaccination era [47].
4. Treatment of COVID-19 Infections in Cancer Patients
Given the risks associated with COVID-19 in cancer patients, if infection should occur, treatment options to reduce the risk of a negative outcome should be carefully considered. As we previously described, it is well-documented that cancer, either from the pathology itself or its treatment, can increase the risk of progression to severe COVID-19 disease and all-cause mortality [25,48]. Since the start of the pandemic, multiple treatments have been studied, some with beneficial results and others with disappointing outcomes. In this section, the available evidence on COVID-19 treatments will be reviewed to provide insight on how to treat cancer patients with confirmed COVID-19 infections.
Upon initial evaluation, it is crucial to determine where the patient lies on the clinical spectrum of disease (i.e., asymptomatic, pre-symptomatic, mild, moderate, severe, or critical). In addition, whether the patient is hospitalized or requires supplemental oxygen will also influence the therapeutic algorithm [48]. As a guiding principle, since cancer patients are considered a high-risk group, they are eligible to receive anti-COVID-19 medication in the outpatient setting for mild-to-moderate disease. COVID-19 treatments can be divided into several categories, including antiviral agents, anti-SARS-CoV-2 monoclonal antibodies, immunomodulatory agents, antithrombotic therapies, and miscellaneous drugs. While a comprehensive review of all COVID-19 therapies reported in the literature is beyond the scope of this review, this section will focus on the therapeutics that have been most studied or used to date. Table 1, adapted from the National Institutes of Health (NIH) COVID-19 guidelines, summarizes the recommendations and level of evidence for each of these treatment options [48]. These guidelines were last updated in February 2024 and come from the consensus of numerous American-based federal agencies and professional societies. The level of evidence “A” designates a strong recommendation, “B” for moderate, and “C” for weak.

Table 1.
Summary of recommendations for anti-COVID therapies in cancer patients.
Nirmatrelvir/Ritonavir (PaxlovidMC) is an anti-viral agent approved by Health Canada and strongly recommended by the World Health Organization (WHO) for the treatment of patients with mild-to-moderate COVID-19 infection at high risk of progression to severe disease [49]. The approval of this agent was based on the EPIC-HR phase 2/3 trial, which enrolled nonhospitalized adults with mild-to-moderate disease who were not vaccinated and at high risk of progressing to severe disease. It demonstrated an 89% relative risk reduction in COVID-associated hospitalization or death with twice-daily treatment for five days, when initiated within five days of symptoms onset [50]. However, it is worth mentioning that ritonavir is a potent P450 3A4 inhibitor, which may cause drug–drug interactions with patients’ other medications, including their anti-cancer medications [51]. If in doubt, it is recommended to consult a pharmacist for assistance.
The second anti-viral agent commonly used to treat COVID-19 is Remdesivir. It is FDA- and Health Canada-approved for the treatment of COVID infection in nonhospitalized patients with mild-to-moderate disease who are at high risk of progression to severe disease [49]. It should be started within seven days of symptom onset and administered for three days [48]. If the patient is hospitalized, the treatment should be continued for five days or until the end of their hospital stay. Multiple clinical trials were conducted to provide these recommendations, including the PINETREE trial in nonhospitalized patients, which showed that three consecutive days of Remdesivir resulted in an 87% relative reduction in the risk of hospitalization or death when compared to that in a placebo group [52]. In hospitalized patients, the ACTT-1 trial demonstrated that the time to recovery was reduced in patients with severe COVID [53]. The benefits were greater when treatment was initiated within 10 days of symptom onset and in patients receiving supplemental oxygen. Its intravenous formulation makes Remdesivir less convenient for the outpatient setting, unfortunately; however, multiple provinces in Canada have organized community infusion centers that enable delivery of this medication in the outpatient setting without the need for hospitalization [53,54].
The next category includes monoclonal antibodies against SARS-CoV-2 spike proteins. Many of these products received emergency FDA approval during the early pandemic period for the treatment of mild-to-moderate disease, including bamlanivimab plus etesevimab, casirivimab plus imdevimab, sotrovimab, and bebtelovimab [55,56,57,58]. However, none of these are currently FDA-approved because they were judged to be non-efficacious for the later COVID variants and subvariants such as Omicron [48].
Another broad class of COVID treatments includes immunomodulators such as systemic corticosteroids. There are strong data for the use of corticosteroids from several trials that demonstrated improved clinical outcomes and reduced mortality in hospitalized COVID patients requiring supplemental oxygen [59,60]. The underlying mechanism is thought to be decreased systemic inflammation which, when left unabated, can lead to lung injury and multiorgan dysfunction in severe disease. The landmark trial, RECOVERY, showed reduced mortality in the cohort receiving 6 mg dexamethasone for 10 days plus the standard of care compared to those receiving the standard of care alone. However, there are no available data to support its use in nonhospitalized patients not requiring supplemental oxygen [61,62]. Other systemic corticosteroids such as methylprednisone and hydrocortisone have been studied, but evidence for their use is limited due to small sample sizes. Therefore, these should only be used in situations where dexamethasone is not readily available [48]. Additional immunomodulators that can be used to treat COVID-19 are tocilizumab (interleukin-6 inhibitor), baricitinib (janus kinase inhibitor), abatacept, and infliximab. Combining their use with systemic steroids is recommended for patients with severe-to-critical disease exhibiting increased oxygen requirements or systemic inflammation despite already being on dexamethasone [48]. In this scenario, baricitinib or tocilizumab are preferred [63,64].
The final category of COVID-19 treatments for discussion includes various miscellaneous drugs studied in randomized trials. This includes fluvoxamine, intravenous immunoglobulins (IVIG), ivermectin, and metformin. Fluvoxamine, a selective serotonin reuptake inhibitor, was studied in at least six clinical trials [48]; however, no significant benefit was found for preventing hospitalization or death either in vaccinated or unvaccinated patients [65,66]. A similar scenario also applies for IVIG. Studies showed uneven levels of neutralizing activity against SARS-CoV-2 variants which was likely related to which variant was dominant at the time of plasma collection [67]. The inconsistent results render the data too weak to support its use [48]. As for ivermectin, which is FDA-approved as an antiparasitic medication, at least four randomized trials compared it against a placebo in the outpatient setting. However, all trials failed to demonstrate clinical benefits in terms of progression to severe disease, hospitalization, or death. It is consequently not recommended in the treatment of COVID [48]. The last agent is metformin, identified because of its antiviral, anti-inflammatory, and antithrombotic properties [68]. The TOGETHER and COVID-OUT trials assessed its efficacy for nonhospitalized patients [65,69] and found that it did not reduce the risk of hospitalization or death. Consequently, the data are insufficient to support its use [48].
5. COVID-19 Infection Prevention Strategies
As discussed in previous sections, the evidence overwhelmingly demonstrates that cancer patients remain vulnerable to severe outcomes of COVID-19 infections, even in the era of vaccination. While there are treatment options that may mitigate the risk of negative outcomes as discussed above, the benefit and accessibility of these treatments is limited. Infection prevention strategies are thus essential for oncology patients. It is incumbent for physicians and healthcare workers to ensure the safety of these patients in the healthcare setting, while offering counseling and education on strategies to protect themselves from COVID-19 in the community. The following section will review current recommendations and evidence regarding indoor air quality, masking, rapid antigen testing, and vaccination guidelines.
Although the World Health Organization (WHO) recommended that COVID-19 no longer fits the definition of a Public Health Emergency of International Concern in May 2023, the organization has continued to emphasize that the pandemic is not over. As recently as February 2024, Maria Van Kerkhove, interim director of the WHO’s Department of Epidemic and Pandemic Preparedness and Prevention, has stated publicly that we are still in a pandemic and that COVID-19 is still a global health risk, and she expressed concern at the complacency seen at the government level in many countries [70]. This complacency is of notable concern for the safety of high-risk groups such as oncology patients, particularly in healthcare settings, given that these patients are in frequent contact with the healthcare system.
In April 2024, the WHO released a technical report recognizing that COVID-19 is predominantly spread through the air by airborne inhalation/transmission, with direct deposition (formerly known as droplet transmission) representing a much smaller risk [71]. Recognizing that the mode of transmission of COVID-19 is primarily through airborne inhalation is central to providing high-impact practical guidance to patients and healthcare providers to limit the spread of COVID-19 in the community and in healthcare settings.
The Public Health Agency of Canada (PHAC) recommends applying a hierarchy of controls to prevent the spread of respiratory infectious diseases in healthcare settings [72]. The hierarchy of controls, which is similar to the “Swiss cheese” model of infection prevention, utilizes multiple, layered interventions to prevent the spread of infection. In the case of respiratory pathogens like SARS-CoV-2, the hierarchy of controls involves administrative controls, engineering controls, and personal protective equipment. Administrative controls are policies and procedures that are intended to decrease the chances that the virus is present in an establishment. Examples of administrative controls include policies that encourage workers to stay home when ill and facilitating access to testing to detect and remove cases of infection from the environment, as well as infrastructure that allows patients greater access to high-quality telemedicine where possible [72].
Since asymptomatic transmission accounts for more than 50% of COVID-19 infections, symptom-based administrative controls are insufficient in isolation [73,74]. The risk of asymptomatic transmission in healthcare facilities can be mitigated with testing and universal masking policies which will be further discussed below. Rapid antigen tests (RAT) provide another tool that can allow oncology patients to minimize the risk of being inadvertently exposed to the virus by those they interact with in the community. While the sensitivity of a single RAT is 83% in symptomatic individuals and only 39% in those without symptoms, the sensitivity of these tests was significantly improved with repeat testing in a recent study. In this study, testing twice, 48 h apart, increased the sensitivity of RAT to 93% in symptomatic participants, while testing three times increased the sensitivity to 79% in asymptomatic participants [75]. It should be noted that a positive RAT result is correlated with infectivity, and therefore, patients should avoid contact with positive individuals where possible until their RAT is negative on two separate occasions, at least 24 h apart [76,77].
Occasionally, oncology patients may find themselves unable to avoid contact with positive contacts, such as when someone in their home has COVID-19. In this instance, patients can be referred to patient-friendly online resources such as the Clean Air Crew website that details how to isolate from an infectious occupant inside a home or apartment [78].
The next broad category in the hierarchy of controls, or Swiss cheese model, is engineering controls [72]. In the healthcare setting, engineering controls include optimizing indoor air ventilation and filtration to reduce the viral load in the air should an infectious person be present in the establishment. While an in-depth overview of indoor air engineering controls is beyond the scope of this review, Public Health Ontario has published a detailed guide on this topic, Heating, Ventilation and Air Conditioning (HVAC) Systems in Buildings and COVID-19, that notes that improper or insufficient ventilation has been frequently reported as a risk factor in outbreak investigations [79]. Patients should also be encouraged to improve the indoor ventilation of their homes if possible, especially if an infectious person is in the house or if there are high levels of community spread, which can be assessed using public wastewater monitoring data and resources such as COVID-19 Resources Canada’s biweekly COVID forecast [80]. The Public Health Agency of Canada (PHAC) has created patient-friendly resources on this topic that can be found on the COVID-19: Improving indoor ventilation page [81]. This page includes a video on ways to improve ventilation and air filtration in the home, as well as a printable guide on choosing the best air purifier for your home, among other resources.
The final category in the hierarchy of controls is masking [72]. It is important to note that masks, both in the healthcare setting and the community, serve dual purposes: source control and personal protective equipment (PPE). Source control means lowering the risk that an infected person infects others. PPE means lowering the risk that an uninfected person gets infected. When using masks as PPE to control the hazard at the individual level, they are intended to limit the inhalation of infectious particles. While masking is no longer mandated by law, organizations such as the National Cancer Institute and PHAC continue to stress the importance of wearing a mask in the community setting for those at increased risk of more severe disease or outcomes, when around others who are at high risk of more-severe disease or outcomes, when visiting a group living setting, and/or when in a crowded or poorly ventilated setting [81]. In the community setting, the PHAC recommends choosing the best quality and best-fitting mask, which essentially means a respirator mask such as an N95, KN95, or elastomeric mask. PHAC notes that respirators do not require formal fit testing for use in the community [82]. Masks are considered well-fitted when there are minimal gaps on the edges, including around the nose and sides, to ensure air is properly filtered through the mask. Cloth masks and non-medical masks provide limited and inconsistent protection, and patients and their caregivers should therefore be encouraged to use well-fitting medical-grade masks or respirators [82].
The other usefulness of masks in the hierarchy of controls is as source control to limit the spread of the wearer’s exhaled respiratory particles. Much like masks in the community setting, masks used for the purpose of source control are not required to be fit-tested, but they should be as well-constructed and well-fitting as possible, as noted by groups such as the Canadian Center for Occupational Health and Safety (CCOHS) [83]. While universal masking has been discontinued outside of healthcare contexts for some time, there is ongoing debate about the ethics of discontinuing masking in healthcare facilities, with several patient advocates and advocacy groups calling for continued masking in light of the fact that COVID-19 is here to stay and is now in circulation year-round [84]. These calls for vigilance are notable in the oncology milieu given the high risk of severe outcomes previously described in this article. It is also important to note that for many, attending healthcare spaces is not a choice but is required for active illness management. One advocate, Christine Mitchell, a public health researcher and caregiver to her father who is battling colorectal cancer, remarked that “It’s very jarring to have so much fear about going to a place that I am going to protect my health or my father’s going to get treatment, and being fearful that it’s actually endangering our health” [85].
The reasons cited for reverting to masking only in certain circumstances in healthcare settings are often similar to those given for community settings. It is felt that since immunity acquired through vaccination and infection has greatly reduced the morbidity and mortality of COVID-19, the risk is now similar to that of other respiratory infections, and these risks have long been tolerated. A perspective piece in the New England Journal of Medicine noted that this framing has limited application in healthcare settings for two main reasons. The first is that hospitalized patients, or in the case of this review, oncology patients, are different from nonhospitalized patients, or non-cancer patients. Hospitals and healthcare facilities, by definition, aggregate subgroups of the population that continue to be at elevated risk for severe disease and death. The second reason is that nosocomial infections caused by viruses other than SARS-CoV-2 are common, underappreciated, and are also associated with adverse health effects in vulnerable populations [86]. These include influenza, RSV, or other infections that may delay or negatively affect treatment outcomes. Healthcare workers should therefore seek to reduce the risk of nosocomial transmission of all respiratory pathogens to vulnerable patients. The authors note that viewed through this lens, continued masking in healthcare settings makes sense, especially given that viral illnesses can be spread by staff and visitors with mild or asymptomatic infections. The authors go on to acknowledge that healthcare workers may be experiencing masking fatigue and that therefore masking requirements can be tied to levels of viral transmission and the individual patients’ risk of severe disease. When applying these recommendations to the care of cancer patients, a patient population known to be at increased risk of severe disease and death, universal masking for the protection of these patients seems to be a prudent and justified course of action. Indeed, the Centers for Disease Control and Prevention (CDC) and PHAC both continue to recommend that healthcare facilities consider broad masking for source control in certain clinical contexts, such as when working with immunocompromised persons or those at greater risk of acquiring an infection [87,88].
Unfortunately, oncology patients may acquire COVID-19 despite the prevention measures discussed as part of the hierarchy of controls. Up-to-date vaccination therefore remains an essential harm-reduction tool in the care of oncology patients. Beginning in the fall of 2024, the National Advisory Committee on Immunization (NACI) has recommended the most recently updated COVID-19 vaccination for previously vaccinated and unvaccinated individuals at increased risk of SARS-CoV-2 infection or severe COVID-19 disease such as those with cancer (among other groups) [89]. For previously unvaccinated individuals who are moderately-to-severely immunocompromised, the NACI recommends that patients receive three doses. New recipients of hematopoietic stem cell transplantation (HSCT) or chimeric antigen receptor (CAR) T-cell therapy are considered immunologically naïve and the NACI recommends that they be vaccinated with three doses beginning at 3 to 6 months post-HSCT/CAR T-cell therapy, regardless of vaccination or infection history prior to transplant/therapy [88]. Finally, previously vaccinated individuals that are moderately-to-severely immunocompromised are most often eligible for additional booster COVID-19 vaccine doses after 3–6 months. In the spring of 2024, NACI guidelines allowed for this patient population to receive a first dose of the updated XBB.1.5 vaccine in the fall of 2023 and a second dose in the spring of 2024, with a recommended interval of 6 months [90]. Similarly, the CDC’s current guidelines state that immunocompromised individuals under the age of 65 may receive an additional (second) dose of any updated vaccine, and immunocompromised individuals over the age of 65 should receive an additional (second) dose of any updated vaccine at least 2 months after the last updated vaccine dose [91]. It is important for clinicians to review these guidelines periodically to ensure that oncology patients are properly counseled on the most recent COVID-19 vaccination eligibility guidelines and recommendations.
6. Conclusions
Since the introduction of the SARS-CoV-2 virus into circulation in 2020, cancer patients have faced new risks and unique challenges related to their health status and the ongoing circulation of this novel pathogen. The COVID-19 pandemic compromised access to healthcare services, disrupting screening programs, negatively affecting surgical wait times, and introducing a new nosocomial and community infection risk that oncology patients and healthcare teams must contend with. Numerous studies have shown that cancer patients are at increased risk of severe outcomes and death in the event of a COVID-19 infection, even after vaccination. The risk is therefore ongoing and ever-present for these patients. Fortunately, there is quality evidence to support the use of multi-pronged disease prevention measures, both at the individual level and through broader public health strategies. These include air quality engineering controls, testing and other administrative controls, masking, and vaccination. Additionally, with the assistance of their care teams, patients can also access antiviral therapies in the outpatient setting that may limit progression to severe COVID-19 disease and decrease mortality should they contract the virus. As the situation is still evolving, and new vaccines and treatments are expected to emerge in the future, continuous monitoring of new tools and evidence and adherence to updated recommendations and guidelines are essential components of modern-day cancer care that can mitigate risk and ensure optimal outcomes for this vulnerable patient population in the era of COVID-19.
Author Contributions
Writing—original draft preparation, S.C., F.M., B.A. and L.I.; writing—review and editing, J.J.W.K., C.W., J.V. and L.I.; supervision, L.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Cheryl White and Joe Vipond are founders and volunteer board members of the Canadian Covid Society (CCS), and Cheryl White is a founder and member of Community Access to Ventilation Information (CAVI). Both are not-for-profit organizations and neither author receives any form of financial compensation for their volunteer work.
References
- Peacock, H.M.; Tambuyzer, T.; Verdoodt, F.; Calay, F.; Poirel, H.A.; De Schutter, H.; Francart, J.; Van Damme, N.; Van Eycken, L. Decline and Incomplete Recovery in Cancer Diagnoses during the COVID-19 Pandemic in Belgium: A Year-Long, Population-Level Analysis. ESMO Open 2021, 6, 100197. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; Visser, O.; Verhoeven, R.H.A.; Louwman, M.W.J.; van Nederveen, F.H.; Willems, S.M.; Merkx, M.A.W.; Lemmens, V.E.P.P.; Nagtegaal, I.D.; Siesling, S. Fewer Cancer Diagnoses during the Covid-19 Epidemic in the Netherlands. Lancet Oncol. 2020, 21, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Maeta, A.; Takaoka, Y.; Saeki, K.; Hamada, M.; Hiraguchi, Y.; Kawakami, T.; Okafuji, I.; Takemura, Y.; Takahashi, K.; et al. Parents’ Fears About Hospital Visits and Trait Anxiety in the COVID-19 Pandemic. Healthcare 2023, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Bostan, S.; Kaya, A.; Karaman, Ö.; Karataş, A.; Dereli, S. Effect of COVID-19 Pandemic on Anxiety Depression and Intention to Go to Hospital in Chronic Patients. Int. J. Clin. Pract. 2021, 75, e14219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Loftus, R. The Forgotten “C”? The Impact of COVID-19 on Cancer Care. Available online: https://www.macmillan.org.uk/_images/forgotten-c-impact-of-covid-19-on-cancer-care_tcm9-359174.pdf (accessed on 7 June 2024).
- Price, S.T.; Mainous, A.G.; Rooks, B.J. Survey of Cancer Screening Practices and Telehealth Services Among Primary Care Physicians during the COVID-19 Pandemic. Prev. Med. Rep. 2022, 27, 101769. [Google Scholar] [CrossRef]
- Van den Puttelaar, R.; Lansdorp-Vogelaar, I.; Hahn, A.I.; Rutter, C.M.; Levin, T.R.; Zauber, A.G.; Meester, R.G.S. Impact and Recovery from COVID-19–Related Disruptions in Colorectal Cancer Screening and Care in the US: A Scenario Analysis. Cancer Epidemiol. Biomark. Prev. 2023, 32, 22–29. [Google Scholar] [CrossRef]
- Peters, S.; Karin, K.; Brandt, J.; Lordick, F.; Pentheroudakis, G.; Curigliano, G.; Soo, R.; Bramley, C.; Vyas, M.; Jezdic, S.; et al. Cancer Care during the COVID-19 Pandemic: An ESMO Guide for Patients. Available online: https://www.esmo.org/for-patients/cancer-care-during-the-covid-19-pandemic (accessed on 7 June 2024).
- Bartlett, N.; Dickson-Watts, K.; Fiorese, B.; Budd, A.; Meere, D. Cancer Screening and COVID-19 in Australia. Available online: https://www.aihw.gov.au/reports/cancer-screening/cancer-screening-and-covid-19-in-australia-inbrief/contents/summary (accessed on 7 June 2024).
- Cancino, R.S.; Su, Z.; Mesa, R.; Tomlinson, G.E.; Wang, J. The Impact of COVID-19 on Cancer Screening: Challenges and Opportunities. JMIR Cancer 2020, 6, e21697. [Google Scholar] [CrossRef]
- Walker, M.J.; Meggetto, O.; Gao, J.; Espino-Hernández, G.; Jembere, N.; Bravo, C.A.; Rey, M.; Aslam, U.; Sheppard, A.J.; Lofters, A.K.; et al. Measuring the Impact of the COVID-19 Pandemic on Organized Cancer Screening and Diagnostic Follow-Up Care in Ontario, Canada: A Provincial, Population-Based Study. Prev. Med. 2021, 151, 106586. [Google Scholar] [CrossRef]
- Yong, J.H.; Mainprize, J.G.; Yaffe, M.J.; Ruan, Y.; Poirier, A.E.; Coldman, A.; Nadeau, C.; Iragorri, N.; Hilsden, R.J.; Brenner, D.R. The Impact of Episodic Screening Interruption: COVID-19 and Population-Based Cancer Screening in Canada. J. Med. Screen. 2021, 28, 100–107. [Google Scholar] [CrossRef]
- Burus, T.; Lei, F.; Huang, B.; Christian, W.J.; Hull, P.C.; Ellis, A.R.; Slavova, S.; Tucker, T.C.; Lang Kuhs, K.A. Undiagnosed Cancer Cases in the US during the First 10 Months of the COVID-19 Pandemic. JAMA Oncol. 2024, 10, 500–507. [Google Scholar] [CrossRef]
- Sharpless, N.E. COVID-19 and Cancer. Science 2020, 368, 1290. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.K.; Riches, J.C. The Impact of the COVID-19 Pandemic on Oncology Care and Clinical Trials. Cancers 2021, 13, 5924. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.G.; Soylu, T.; Hoffman, C.F.; Kishton, R.E.; Cronholm, P.F. ‘Anxiety, COVID, Burnout and Now Depression’: A Qualitative Study of Primary Care Clinicians’ Perceptions of Burnout. J. Gen. Intern. Med. 2024, 39, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Rotenstein, L.S.; Brown, R.; Sinsky, C.; Linzer, M. The Association of Work Overload with Burnout and Intent to Leave the Job Across the Healthcare Workforce during COVID-19. J. Gen. Intern. Med. 2023, 38, 1920–1927. [Google Scholar] [CrossRef]
- Patrick, A.; Pryor, R. COVID-19, Burnout, and Healthcare-Associated Infections: A Focus on Wellness as a Top Safety Priority. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e155. [Google Scholar] [CrossRef]
- Fonds de Recherche du Québec. 1st Canadian Symposium on Long COVID: Research Excellence as a Vehicle for Solutions. Available online: https://frq.gouv.qc.ca/en/event/1st-canadian-symposium-on-long-covid-research-excellence-as-a-vehicle-for-solutions/ (accessed on 7 June 2024).
- Galvin, G. Nearly 1 in 5 Health Care Workers Have Quit Their Jobs during the Pandemic; Volume 1. Available online: https://thepcc.org/2022/01/07/nearly-1-5-health-care-workers-have-quit-their-jobs-during-pandemic (accessed on 7 June 2024).
- COVID Surg Collaborative. Elective Surgery Cancellations Due to the COVID-19 Pandemic: Global Predictive Modelling to Inform Surgical Recovery Plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar] [CrossRef]
- Gregory, T.A.; Knight, S.R.; Aaroe, A.E.; Highsmith, K.N.; Janatpour, Z.C.; O’Brien, B.J.; Majd, N.K.; Loghin, M.E.; Patel, C.B.; Weathers, S.-P.; et al. Accelerated Tumor Progression After COVID-19 Infection in Patients with Glioblastoma: A Retrospective Case–Control Study. Neurooncol. Pract. 2024, 11, npae029. [Google Scholar] [CrossRef]
- Becker, S.; Jonigk, D.; Luft, A.; Dübbel, L.; Werlein, C.; Malik, E.; Schild-Suhren, M. COVID-19 Can Lead to Rapid Progression of Cervical Intraepithelial Neoplasia by Dysregulating the Immune System: A Hypothesis. J. Reprod. Immunol. 2022, 154, 103763. [Google Scholar] [CrossRef]
- ElGohary, G.M.; Hashmi, S.; Styczynski, J.; Kharfan-Dabaja, M.A.; Alblooshi, R.M.; de la Cámara, R.; Mohmed, S.; Alshaibani, A.; Cesaro, S.; Abd El-Aziz, N.; et al. The Risk and Prognosis of COVID-19 Infection in Cancer Patients: A Systematic Review and Meta-analysis. Hematol. Oncol. Stem Cell Ther. 2022, 15, 45–53. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients with COVID-19: A Meta-analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, Q.; Chiang, J.; Tan, S.H.; Chua, G.W.Y.; Xie, C.; Chua, M.L.K.; Soon, Y.Y.; Yang, V.S. Impact of Cancer Diagnoses on the Outcomes of Patients with COVID-19: A Systematic Review and Meta-analysis. BMJ Open 2022, 12, e044661. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer Patients in SARS-CoV-2 Infection: A Nationwide Analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and Its Impact on 1590 Patients with COVID-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Freeman, V.; Hughes, S.; Carle, C.; Campbell, D.; Egger, S.; Hui, H.; Yap, S.; Deandrea, S.; Caruana, M.; Onyeka, T.C.; et al. Are Patients with Cancer at Higher Risk of COVID-19-Related Death? A Systematic Review and Critical Appraisal of the Early Evidence. J. Cancer Policy 2022, 33, 100340. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.; Hughes, S.; Hui, H.; Allsop, M.J.; Egger, S.; David, M.; Caruana, M.; Coxeter, P.; Carle, C.; Onyeka, T.; et al. Risk of COVID-19 Death for People with a Pre-existing Cancer Diagnosis Prior to COVID-19-Vaccination: A Systematic Review and Meta-analysis. Int. J. Cancer 2024, 154, 1394–1412. [Google Scholar] [CrossRef]
- Hardy, N.; Vegivinti, C.T.R.; Mehta, M.; Thurnham, J.; Mebane, A.; Pederson, J.M.; Tarchand, R.; Shivakumar, J.; Olaniran, P.; Gadodia, R.; et al. Mortality of COVID-19 in Patients with Hematological Malignancies Versus Solid Tumors: A Systematic Literature Review and Meta-analysis. Clin. Exp. Med. 2023, 23, 1945–1959. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Chen, X.; Sun, Z.; Zou, Y.; Chen, C.; Sun, S. The Effect of Anticancer Treatment on Cancer Patients with COVID-19: A Systematic Review and Meta-analysis. Cancer Med. 2021, 10, 1043–1056. [Google Scholar] [CrossRef]
- Cao, C.; Gan, X.; Hu, X.; Su, Y.; Zhang, Y.; Peng, X. Association of Active Immunotherapy with Outcomes in Cancer Patients with COVID-19: A Systematic Review and Meta-analysis. Aging 2022, 14, 2062–2080. [Google Scholar] [CrossRef]
- Lazarus, G.; Budiman, R.A.; Rinaldi, I. Does Immune Checkpoint Inhibitor Increase the Risks of Poor Outcomes in COVID-19-Infected Cancer Patients? A Systematic Review and Meta-analysis. Cancer Immunol. Immunother. 2022, 71, 373–386. [Google Scholar] [CrossRef]
- Ruiz, J.I.; Lopez-Olivo, M.A.; Geng, Y.; Suarez-Almazor, M.E. COVID-19 Outcomes in Patients with Cancer Receiving Immune Checkpoint Inhibitors: A Systematic Review. J. Immunother. Precis Oncol. 2023, 6, 103–110. [Google Scholar] [CrossRef]
- Lim, Y.J.; Khan, U.; Karpha, I.; Ross, A.; Saif, M.; Remberger, M.; Kalakonda, N.; Pettitt, A.R.; Floisand, Y. COVID-19 Outcomes in Haematopoietic Cell Transplant Recipients: A Systematic Review and Meta-analysis. EJHaem 2022, 3, 862–872. [Google Scholar] [CrossRef] [PubMed]
- van Marcke, C.; Honoré, N.; van der Elst, A.; Beyaert, S.; Derouane, F.; Dumont, C.; Aboubakar Nana, F.; Baurain, J.F.; Borbath, I.; Collard, P.; et al. Safety of Systemic Anti-cancer Treatment in Oncology Patients with Non-severe COVID-19: A Cohort Study. BMC Cancer 2021, 21, 578. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Salazar, R.; Gennari, A.; Aguilar-Company, J.; Bower, M.; Bertuzzi, A.; Brunet, J.; Lambertini, M.; Maluquer, C.; Pedrazzoli, P.; et al. Persistence of Long-Term COVID-19 Sequelae in Patients with Cancer: An Analysis from the OnCovid Registry. Eur. J. Cancer 2022, 170, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Iglesias, M.J.; Tremble, K.; Russell, B.; Moss, C.; Dolly, S.; Sita-Lumsden, A.; Cortellini, A.; Pinato, D.J.; Rigg, A.; Karagiannis, S.N.; et al. Long-Term Effects of COVID-19 on Cancer Patients: The Experience from Guy’s Cancer Centre. Future Oncol. 2022, 18, 3585–3594. [Google Scholar] [CrossRef]
- Lasagna, A.; Albi, G.; Figini, S.; Basile, S.; Sacchi, P.; Bruno, R.; Pedrazzoli, P. Long-COVID in Patients with Cancer Previously Treated with Early Anti-SARS-CoV-2 Therapies in an Out-of-Hospital Setting: A Single-Center Experience. Cancers 2023, 15, 1269. [Google Scholar] [CrossRef]
- Dagher, H.; Chaftari, A.-M.; Subbiah, I.M.; Malek, A.E.; Jiang, Y.; Lamie, P.; Granwehr, B.; John, T.; Yepez, E.; Borjan, J.; et al. Long COVID in Cancer Patients: Preponderance of Symptoms in Majority of Patients over Long Time Period. eLife 2023, 12, e81182. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T Cell Immune Responses Following mRNA COVID-19 Vaccination in Patients with Cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef]
- Shem-Tov, N.; Yerushalmi, R.; Danylesko, I.; Litachevsky, V.; Levy, I.; Olmer, L.; Lusitg, Y.; Avigdor, A.; Nagler, A.; Shimoni, A.; et al. Immunogenicity and Safety of the BNT162b2 mRNA COVID-19 Vaccine in Haematopoietic Stem Cell Transplantation Recipients. Br. J. Haematol. 2022, 196, 884–891. [Google Scholar] [CrossRef]
- Dhakal, B.; Abedin, S.; Fenske, T.; Chhabra, S.; Ledeboer, N.; Hari, P.; Hamadani, M. Response to SARS-CoV-2 Vaccination in Patients After Hematopoietic Cell Transplantation and CAR T-Cell Therapy. Blood 2021, 138, 1278–1281. [Google Scholar] [CrossRef]
- Passamonti, F.; Nicastri, E.; Di Rocco, A.; Guarini, A.; Ibatici, A.; Luminari, S.; Mikulska, M.; Visco, C. Management of Patients with Lymphoma and COVID-19: Narrative Review and Evidence-Based Practical Recommendations. Hematol. Oncol. 2023, 41, 3–15. [Google Scholar] [CrossRef]
- Ospina, A.V.; Brugés, R.; Triana, I.; Sánchez-Vanegas, G.; Barrero, A.; Mantilla, W.; Ramos, P.; Bernal, L.; Aruachán, S.; González, M.; et al. Impact of Vaccination Against COVID-19 on Patients with Cancer in ACHOC-C19 Study: Real World Evidence from One Latin American Country. J. Cancer 2023, 14, 2410–2416. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.T.; Vo, A.D.; La, J.; Do, N.V.; Fillmore, N.R.; Brophy, M.; Branch-Elliman, W.; Monach, P.A. Severe COVID-19 in Vaccinated Adults with Hematologic Cancers in the Veterans Health Administration. JAMA Netw. Open 2024, 7, e240288. [Google Scholar] [CrossRef] [PubMed]
- COVID 19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Stapleton, NY, USA. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 3 June 2024).
- Gouvernement du Canada. Canada.ca, 18 October 2022. Available online: https://www.canada.ca/fr/sante-canada/services/medicaments-produits-sante/covid19-industrie/medicaments-vaccins-traitements/traitements.html (accessed on 3 June 2024).
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Quercia, R.; Di Perri, G.; Pein, C.; Bodie, J.; Singh, R.S.P.; Hendrick, V.; Boffito, M. Ritonavir: 25 Years’ Experience of Concomitant Medication Management. A Narrative Review. Infect. Dis. Ther. 2024, 13, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Hedskog, C.; Rodriguez, L.; Roychoudhury, P.; Huang, M.-L.; Jerome, K.R.; Hao, L.; Ireton, R.C.; Li, J.; Perry, J.K.; Han, D.; et al. Viral Resistance Analyses from the Remdesivir Phase 3 Adaptive COVID-19 Treatment Trial-1 (ACTT-1). J. Infect. Dis. 2023, 228, 1263–1273. [Google Scholar] [CrossRef]
- Access to Antiviral Treatments for COVID-19 in the Community, n.d. Available online: https://www.ontariohealth.ca/sites/ontariohealth/files/Access_to_antiviral_treatments_for_COVID-19_in_the_community.pdf (accessed on 3 June 2024).
- Food and Drug Administration. Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. 2022. Available online: https://www.fda.gov/media/145802/download (accessed on 3 June 2024).
- Food and Drug Administration. Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of REGEN-COV (Casirivimab and Imdevimab). 2021. Available online: https://www.fda.gov/media/145611/download (accessed on 3 June 2024).
- Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization (EUA) of Sotrovimab. 2023. Available online: https://www.fda.gov/media/149534/download (accessed on 3 June 2024).
- Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Bebtelovimab. 2022. Available online: https://www.fda.gov/media/156152/download (accessed on 3 June 2024).
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-Analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Li, H.; Yan, B.; Gao, R.; Ren, J.; Yang, J. Effectiveness of Corticosteroids to Treat Severe COVID-19: A Systematic Review and Meta-analysis of Prospective Studies. Int. Immunopharmacol. 2021, 100, 108121. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Crothers, K.; DeFaccio, R.; Tate, J.; Alba, P.R.; Goetz, M.B.; Jones, B.; King, J.T.; Marconi, V.; Ohl, M.E.; Rentsch, C.T.; et al. Dexamethasone in Hospitalised COVID-19 Patients Not on Intensive Respiratory Support. Eur. Respir. J. 2022, 60, 2102532. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Tocilizumab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Reis, G.; Dos Santos Moreira-Silva, E.A.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; Dos Santos, C.V.Q.; de Souza Campos, V.H.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of Early Treatment with Fluvoxamine on Risk of Emergency Care and Hospitalisation Among Patients with COVID-19: The TOGETHER Randomised, Platform Clinical Trial. Lancet Glob. Health 2022, 10, e42–e51. [Google Scholar] [CrossRef] [PubMed]
- Reiersen, A.M.; Mattar, C.; Bender Ignacio, R.A.; Boulware, D.R.; Lee, T.C.; Hess, R.; Lankowski, A.J.; McDonald, E.G.; Miller, J.P.; Powderly, W.G.; et al. The STOP COVID 2 Study: Fluvoxamine vs Placebo for Outpatients with Symptomatic COVID-19, a Fully Remote Randomized Controlled Trial. Open Forum Infect. Dis. 2023, 10, ofad419. [Google Scholar] [CrossRef]
- Cousins, K.; Sano, K.; Lam, B.; Röltgen, K.; Bhavsar, D.; Singh, G.; McRae, O.; Jeong, S.; Aboelregal, N.; Ho, H.-E.; et al. Detection of SARS-CoV-2 Antibodies in Immunoglobulin Products. J. Allergy Clin. Immunol. Pract. 2023, 11, 2534–2541.e2. [Google Scholar] [CrossRef]
- Karam, B.S.; Morris, R.S.; Bramante, C.T.; Puskarich, M.; Zolfaghari, E.J.; Lotfi-Emran, S.; Ingraham, N.E.; Charles, A.; Odde, D.J.; Tignanelli, C.J. mTOR Inhibition in COVID-19: A Commentary and Review of Efficacy in RNA Viruses. J. Med. Virol. 2021, 93, 1843–1846. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33314219 (accessed on 2 June 2024). [CrossRef]
- Bramante, C.T.; Huling, J.D.; Tignanelli, C.J.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Cohen, K.; Puskarich, M.A.; Belani, H.K.; Proper, J.L.; et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for COVID-19. N. Engl. J. Med. 2022, 387, 599–610. [Google Scholar] [CrossRef]
- Rampant COVID Poses New Challenges in the Fifth Year of the Pandemic. Available online: https://www.scientificamerican.com/article/rampant-covid-poses-new-challenges-in-the-fifth-year-of-the-pandemic/ (accessed on 22 June 2024).
- Global Technical Consultation Report on Proposed Terminology for Pathogens That Transmit Through the Air. Available online: https://www.who.int/publications/m/item/global-technical-consultation-report-on-proposed-terminology-for-pathogens-that-transmit-through-the-air (accessed on 12 June 2024).
- Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/routine-practices-precautions-healthcare-associated-infections/routine-practices-precautions-healthcare-associated-infections-2016-FINAL-eng.pdf (accessed on 2 June 2024).
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission from People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef]
- Subramanian, R.; He, Q.; Pascual, M. Quantifying Asymptomatic Infection and Transmission of COVID-19 in New York City Using Observed Cases, Serology, and Testing Capacity. Proc. Natl. Acad. Sci. USA 2021, 118, e2019716118. [Google Scholar] [CrossRef]
- Soni, A.; Herbert, C.; Lin, H.; Yan, Y.; Pretz, C.; Stamegna, P.; Wang, B.; Orwig, T.; Wright, C.; Tarrant, S.; et al. Performance of Rapid Antigen Tests to Detect Symptomatic and Asymptomatic SARS-CoV-2 Infection: A Prospective Cohort Study. Ann. Intern. Med. 2023, 176, 975–982. [Google Scholar] [CrossRef]
- Tu, Y.-P.; Green, C.; Hao, L.; Greninger, A.L.; Morton, J.F.; Sights, H.A.; Gale, M.; Drain, P.K. COVID-19 Antigen Results Correlate with the Quantity of Replication-Competent SARS-CoV-2 in a Cross-Sectional Study of Ambulatory Adults during the Delta Wave. Microbiol. Spectr. 2023, 11, e0006423. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.E.; Riedel, S.; Dutta, S.; Arnaout, R.; Cheng, A.; Ditelberg, S.; Hamel, D.J.; Chang, C.A.; Kanki, P.J. Sars-Cov-2 Antigen Tests Predict Infectivity Based on Viral Culture: Comparison of Antigen, PCR Viral Load, and Viral Culture Testing on a Large Sample Cohort. Clin. Microbiol. Infect. 2023, 29, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Someone in My Home Has COVID. How do We Isolate Safely? Available online: https://cleanaircrew.org/someone-in-my-home-has-covid-how-do-we-isolate-safely/ (accessed on 8 June 2024).
- Heating, Ventilation and Air Conditioning (HVAC) Systems in Buildings and COVID-19. Available online: https://www.publichealthontario.ca/-/media/documents/ncov/ipac/2020/09/covid-19-hvac-systems-in-buildings.pdf (accessed on 8 June 2024).
- Canadian COVID-19 Hazard Index. Available online: https://covid19resources.ca/covid-hazard-index/ (accessed on 23 June 2024).
- Improve Indoor Air Quality in Your Home. Available online: https://www.canada.ca/en/health-canada/services/air-quality/improve-indoor-air-quality-in-your-home.html (accessed on 8 June 2024).
- COVID-19 Mask Use: Advice for Community Settings. Available online: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/prevention-risks/about-non-medical-masks-face-coverings.html (accessed on 8 June 2024).
- Controlling Respiratory Infectious Diseases in the Workplace. Available online: https://www.ccohs.ca/images/products/infographics/download/respiratory-infectious-diseases.png (accessed on 9 June 2024).
- ACTION/CARE/EQUITY. Keep Masks in Healthcare. Available online: https://www.actioncareequity.org/ (accessed on 9 June 2024).
- Mask Mandates Have Kept One Cancer Center Free from COVID-19. Others Have Lifted Masking Requirements Despite Patient Concerns. Available online: https://www.cancertherapyadvisor.com/features/mask-mandates-have-kept-one-cancer-center-free-from-covid-19-others-have-lifted-masking-requirements-despite-patient-concerns/ (accessed on 9 June 2024).
- Klompas, M.; Baker, M.A.; Rhee, C.; Baden, L.R. Strategic Masking to Protect Patients from All Respiratory Viral Infections. N. Engl. J. Med. 2023, 389, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 20 August 2024).
- Updated Guidance for Infection Prevention and Control in Health Care Settings When COVID-19 Is Suspected or Confirmed, April 2024. Available online: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/omicron-infection-prevention-control-health-care-settings-covid-19-suspected-confirmed.html (accessed on 20 June 2024).
- An Advisory Committee Statement (ACS); National Advisory Committee on Immunization (NACI). Guidance on the Use of COVID-19 Vaccines during the Fall of 2024. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/vaccines-immunization/national-advisory-committee-immunization-guidance-covid-19-vaccines-fall-2024/naci-statement-2024-05-03.pdf (accessed on 20 June 2024).
- COVID-19 Vaccines: Canadian Immunization Guide. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html (accessed on 22 June 2024).
- Use of COVID-19 Vaccines in the U.S. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#table-02 (accessed on 20 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).