Mogamulizumab and Concomitant Hypofractionated Low-Dose Total Skin Electron Beam Therapy (2 × 4 Gy) in Cutaneous T-Cell Lymphoma: Proof of Principle, Report of Two Cases
Abstract
1. Introduction
2. Patients and Methods
2.1. Case 1
2.1.1. Clinical Findings and Diagnostic Assessment
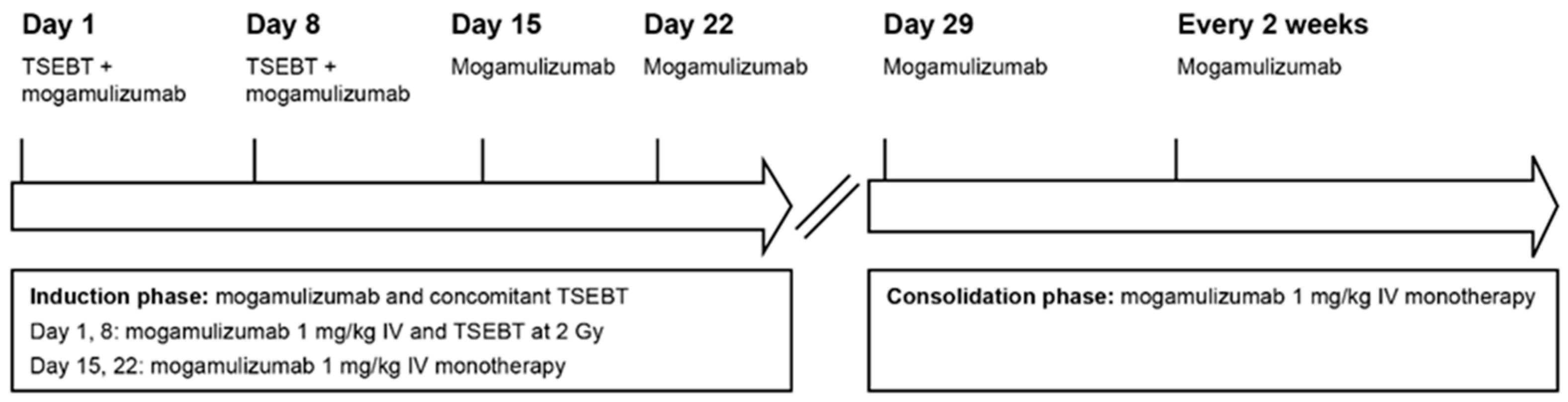
2.1.2. Therapeutic Intervention
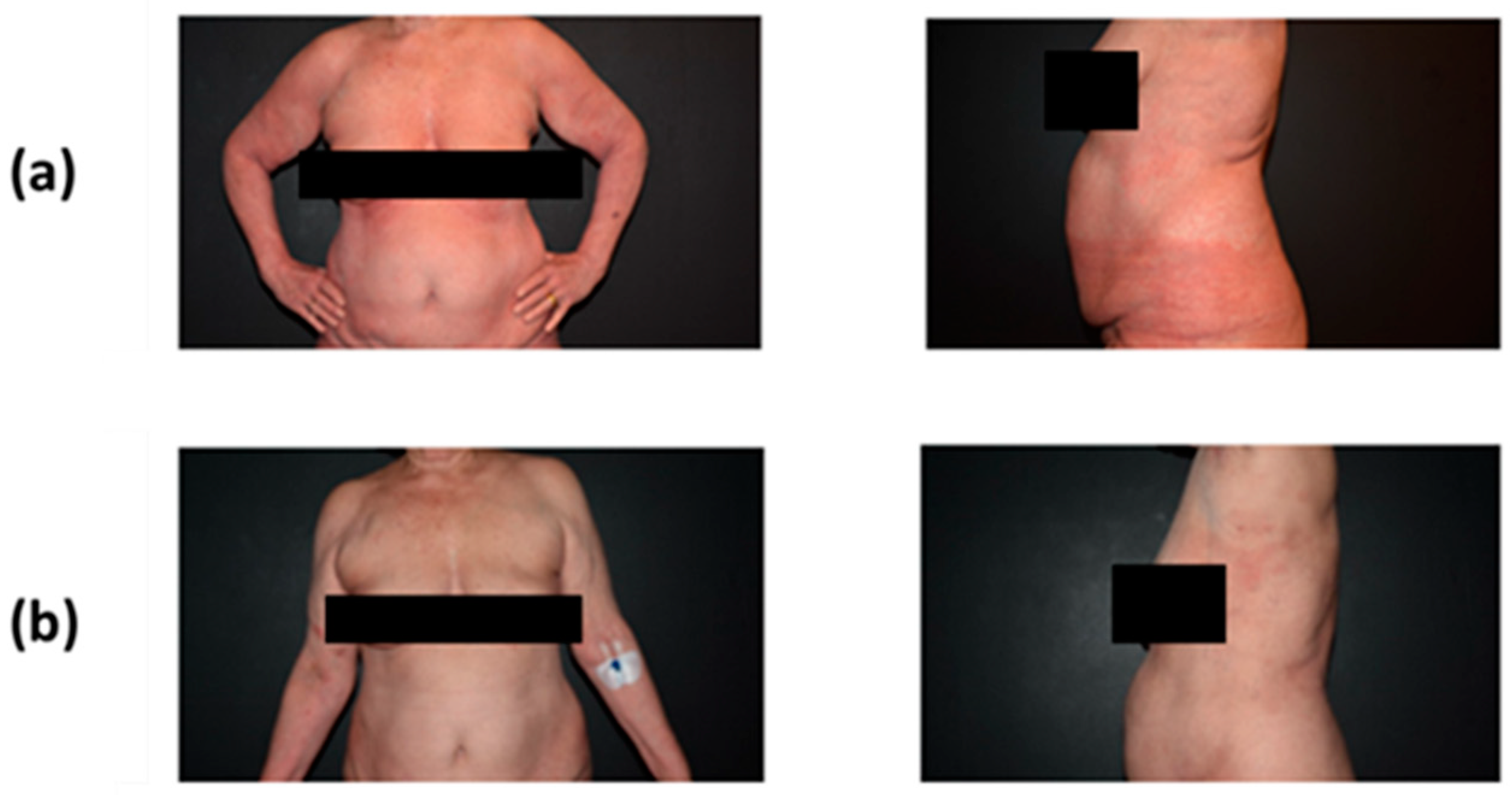
2.1.3. Follow-Up and Outcomes
2.2. Case 2
2.2.1. Clinical Findings and Diagnostic Assessment
2.2.2. Therapeutic Intervention
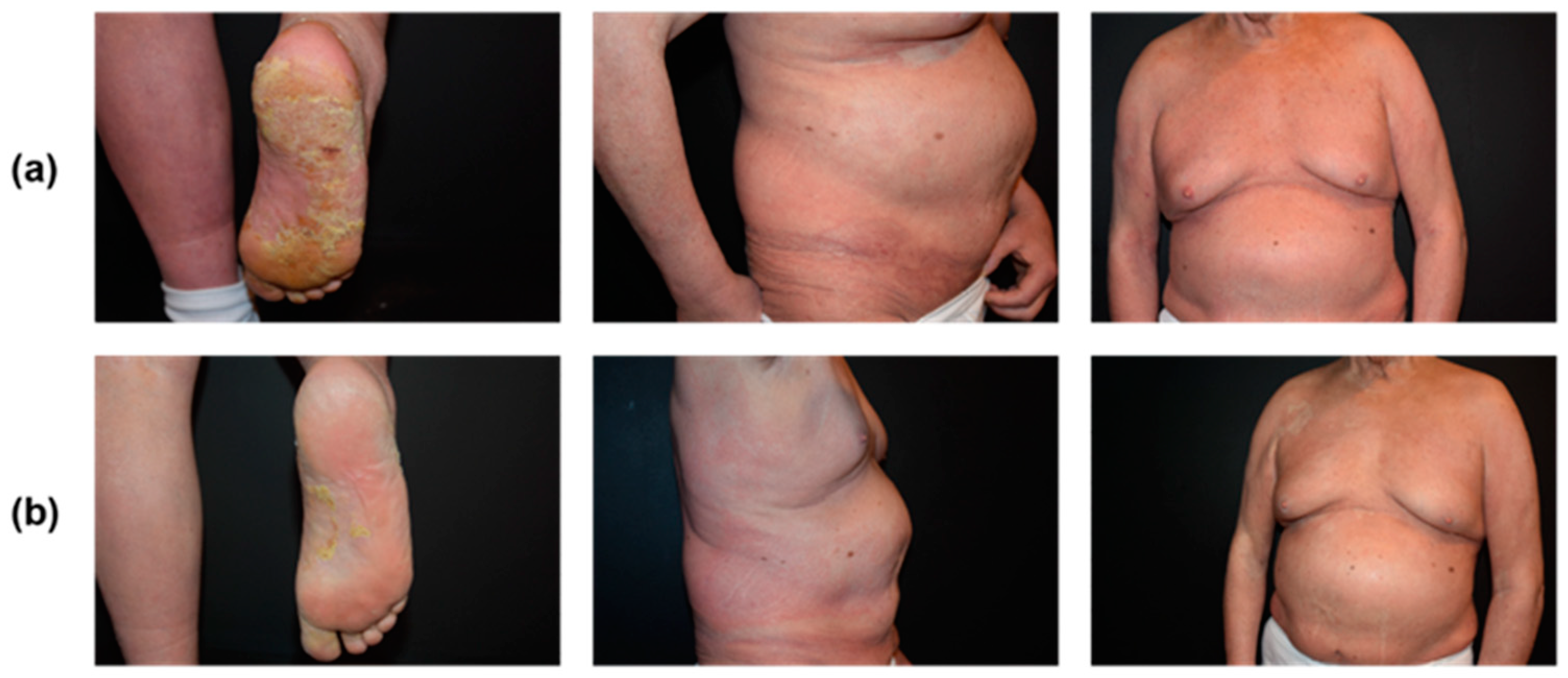
2.2.3. Follow-Up and Outcomes
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J.; Kim, Y.H.; Stonesifer, C.; Tensen, C.P.; Geskin, L.J.; Quaglino, P.; Ramelyte, E. Cutaneous T cell lymphoma. Nat. Rev. Dis. Prim. 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Assaf, C.; Gellrich, S.; Steinhoff, M.; Nashan, D.; Weisse, F.; Dippel, E.; Coors, E.; Stein, A.; Gollin, P.; Henke, U.; et al. Cutaneous lymphomas in Germany: An analysis of the Central Cutaneous Lymphoma Registry of the German Society of Dermatology (DDG). J. Dtsch. Dermatol. Ges. 2007, 5, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Dippel, E.; Assaf, C.; Becker, J.C.; von Bergwelt-Baildon, M.; Bernreiter, S.; Cozzio, A.; Eich, H.T.; Elsayad, K.; Follmann, M.; Grabbe, S.; et al. S2k-Guidelines—Cutaneous lymphomas (ICD10 C82-C86): Update 2021. J. Dtsch. Dermatol. Ges. 2022, 20, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.F.; Newland, K.; McCormack, C.; Lade, S.; Prince, H.M. Mycosis fungoides and Sezary syndrome: Current challenges in assessment, management and prognostic markers. Australas. J. Dermatol. 2016, 57, 182–191. [Google Scholar] [CrossRef]
- Agar, N.S.; Wedgeworth, E.; Crichton, S.; Mitchell, T.J.; Cox, M.; Ferreira, S.; Robson, A.; Calonje, E.; Stefanato, C.M.; Wain, E.M.; et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J. Clin. Oncol. 2010, 28, 4730–4739. [Google Scholar] [CrossRef]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Cutaneous T-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2021, 96, 1313–1328. [Google Scholar] [CrossRef]
- European Medicines Agency. POTELIGEO (Mogamulizumab) Summary of Product Characteristics. 2018. Available online: https://www.ema.europa.eu/en/documents/product-information/poteligeo-epar-product-information_en.pdf (accessed on 2 January 2024).
- US Food and Drug Administration. POTELIGEO (Mogamulizumab) Prescribing Information. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761051s000lbl.pdf (accessed on 2 January 2024).
- Bagot, M.; Dalle, S.; Sokol, L.; Tsianakas, A.; Musiek, A.; Ortiz-Romero, P.L.; Poligone, B.; Duvic, M.; Elmets, C.; Leoni, M.; et al. Long-term disease control and safety with the anti-CCR4 antibody mogamulizumab: Post-hoc analyses from the MAVORIC trial of patients with previously treated cutaneous T-cell lymphoma. Dermatol. Ther. 2022, 35, e15634. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Beylot-Barry, M.; Booken, N.; Weishaupt, C.; Scarisbrick, J.; Wu, W.; Rosen, J.; Medley, M.C. Impact of blood involvement on efficacy and time to response with mogamulizumab in mycosis fungoides and Sezary syndrome. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Beylot-Barry, M.; Quereux, G.; Nardin, C.; Duval-Modeste, A.B.; Dereure, O.; Dalac-Rat, S.; Dobos, G.; Pham-Ledard, A.; Ram-Wolff, C.; D’Incan, M.; et al. Effectiveness of mogamulizumab in patients with Mycosis Fungoides or Sezary syndrome: A multicentre, retrospective, real-world French study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Trum, N.A.; Zain, J.; Martinez, X.U.; Parekh, V.; Afkhami, M.; Abdulla, F.; Carson, K.R.; Rosen, S.T.; Bennett, C.L.; Querfeld, C. Mogamulizumab efficacy is underscored by its associated rash that mimics cutaneous T-cell lymphoma: A retrospective single-centre case series. Br. J. Dermatol. 2022, 186, 153–166. [Google Scholar] [CrossRef] [PubMed]
- De Masson, A.; Darbord, D.; Dobos, G.; Boisson, M.; Roelens, M.; Ram-Wolff, C.; Cassius, C.; Le Buanec, H.; de la Grange, P.; Jouenne, F.; et al. Macrophage-derived CXCL9 and CXCL11, T-cell skin homing, and disease control in mogamulizumab-treated CTCL patients. Blood 2022, 139, 1820–1832. [Google Scholar] [CrossRef]
- Avallone, G.; Roccuzzo, G.; Pileri, A.; Agostinelli, C.; Maronese, C.A.; Aquino, C.; Tavoletti, G.; Onida, F.; Fava, P.; Ribero, S.; et al. Clinicopathological definition, management and prognostic value of mogamulizumab-associated rash and other cutaneous events: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1738–1748. [Google Scholar] [CrossRef]
- Musiek, A.C.M.; Rieger, K.E.; Bagot, M.; Choi, J.N.; Fisher, D.C.; Guitart, J.; Haun, P.L.; Horwitz, S.M.; Huen, A.O.-L.; Kwong, B.Y.; et al. Dermatologic events associated with the anti-CCR4 antibody mogamulizumab: Characterization and management. Dermatol. Ther. 2022, 12, 29–40. [Google Scholar] [CrossRef]
- Chen, L.; Carson, K.R.; Staser, K.W.; Mehta-Shah, N.; Schaffer, A.; Rosman, I.S.; Musiek, A. Mogamulizumab-Associated Cutaneous Granulomatous Drug Eruption Mimicking Mycosis Fungoides but Possibly Indicating Durable Clinical Response. JAMA Dermatol. 2019, 155, 968–971. [Google Scholar] [CrossRef]
- Hansen, I.; Abeck, F.; Menz, A.; Schneider, S.W.; Booken, N. Mogamulizumab-associated rash–Case series and review of the literature. J. Der Dtsch. Dermatol. Ges. 2024, 22, 1079–1087. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Fuks, Z.; Bagshaw, M.A. The rationale for curative radiotherapy in mycosis fungoides. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 843–851. [Google Scholar] [CrossRef]
- Latzka, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Guenova, E.; Gniadecki, R.; Hodak, E.; Jonak, C.; Klemke, C.-D.; et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2023. Eur. J. Cancer 2023, 195, 113343. [Google Scholar] [CrossRef]
- Specht, L.; Dabaja, B.; Illidge, T.; Wilson, L.D.; Hoppe, R.T. Modern radiation therapy for primary cutaneous lymphomas: Field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Jones, G.W.; Kim, D.; Rosenthal, D.; Christensen, I.R.; Edelson, R.L.; Heald, P.W.; Kacinski, B.M. Experience with total skin electron beam therapy in combination with extracorporeal photopheresis in the management of patients with erythrodermic (T4) mycosis fungoides. J. Am. Acad. Dermatol. 2000, 43, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Durgin, J.S.; Jariwala, N.N.; Wysocka, M.; Zhang, K.K.; Maity, A.; Benoit, B.; Plastaras, J.P.; Lewis, D.J.; Rosenthal, J.M.; Teague, J.E.; et al. Low-Dose Total Skin Electron Beam Therapy as Part of a Multimodality Regimen for Treatment of Sézary Syndrome: Clinical, Immunologic, and Molecular Analysis. JAMA Dermatol. 2021, 157, 90–95. [Google Scholar] [CrossRef]
- Elsayad, K.; Rolf, D.; Sunderkötter, C.; Weishaupt, C.; Müller, E.C.; Nawar, T.; Stranzenbach, R.; Livingstone, E.; Stadler, R.; Steinbrink, K.; et al. Low-dose total skin electron beam therapy plus oral bexarotene maintenance therapy for cutaneous T-cell lymphoma. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. 2022, 20, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hosing, C.; Bassett, R.; Dabaja, B.; Talpur, R.; Alousi, A.; Ciurea, S.; Popat, U.; Qazilbash, M.; Shpall, E.J.; Oki, Y.; et al. Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: Updated results from a single institution. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Dunlop, J.D.; Samimi, S.S.; Morrissey, K.A.; Evans, K.G.; Gardner, J.M.; Introcaso, C.E.; Vittorio, C.C.; Rook, A.H.; Micaily, B.; et al. Improvement in peripheral blood disease burden in patients with Sezary syndrome and leukemic mycosis fungoides after total skin electron beam therapy. J. Am. Acad. Dermatol. 2013, 68, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Gorica, J.; De Feo, M.S.; Corica, F.; Sidrak, M.M.A.; Conte, M.; Filippi, L.; Schillaci, O.; De Vincentis, G.; Frantellizzi, V. Novel Theranostic Approaches Targeting CCR4-Receptor, Current Status and Translational Prospectives: A Systematic Review. Pharmaceuticals 2023, 16, 313. [Google Scholar] [CrossRef]
- Chiang, Y.; Lu, L.-F.; Tsai, C.-L.; Tsai, Y.-C.; Wang, C.-C.; Hsueh, F.-J.; Huang, C.-Y.; Chen, C.-H.; Pu, Y.-S.; Cheng, J.C.-H. CC chemokine receptor 4 (CCR4)-positive regulatory T cells interact with tumor-associated macrophages to facilitate metastatic potential after radiation. Eur. J. Cancer 2024, 198, 113521. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Harrison, C.; Tavallaee, M.; Bashey, S.; Sundram, U.; Li, S.; Million, L.; Dabaja, B.; Gangar, P.; Duvic, M.; et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: Results of a pooled analysis from 3 phase-II clinical trials. J. Am. Acad. Dermatol. 2015, 72, 286–292. [Google Scholar] [CrossRef]
- Kamstrup, M.R.; Gniadecki, R.; Iversen, L.; Skov, L.; Petersen, P.M.; Loft, A.; Specht, L. Low-dose (10-Gy) total skin electron beam therapy for cutaneous T-cell lymphoma: An open clinical study and pooled data analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 138–143. [Google Scholar] [CrossRef]
- Morris, S.; Scarisbrick, J.; Frew, J.; Irwin, C.; Grieve, R.; Humber, C.; Kuciejewska, A.; Bayne, S.; Weatherhead, S.; Child, F.; et al. The results of Low dose Total Skin Electron Beam Radiotherapy (TSEB), in patients with mycosis fungoides from the UK cutaneous lymphoma group. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Elsayad, K.; Kriz, J.; Moustakis, C.; Scobioala, S.; Reinartz, G.; Haverkamp, U.; Willich, N.; Weishaupt, C.; Stadler, R.; Sunderkötter, C.; et al. Total skin electron beam for primary cutaneous T-cell lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Rolf, D.; Elsayad, K.; Eich, H.T. Ultra-hypofractionated low-dose total skin electron beam followed by maintenance therapy: Preliminary findings from a prospective open-label study. J. Am. Acad. Dermatol. 2021, 85, 1601–1603. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.M.; West, L.; Atluri, P.S.; Sofia, F.Y.; Rizvi, S.; Geethakumari, P.R.; Awan, F.T.; Chen, W.; Shah, J.L.; Desai, N.B.; et al. Optimizing Palliative Focal Radiation Therapy Dose in Cutaneous T-Cell Lymphoma: How Low Can You Go? Pract. Radiat. Oncol. 2023, 13, e192–e199. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gilbert, M.; Lim, H.W.; McHargue, C.; Friedman, B.J.; Veenstra, J.J.; Siddiqui, F. Single-Fraction Radiation Therapy for Localized Cutaneous T-Cell Lymphoma. Pract. Radiat. Oncol. 2023, 13, 346–350. [Google Scholar] [CrossRef]
- Ward, J.; Prince, H.M.; McCormack, C.; Lade, S.; Buelens, O.; van der Weyden, C.; Bhabha, F.; Campbell, B.A. Excellent treatment outcomes from low dose radiation therapy for primary cutaneous CD4+ small/medium T-Cell lymphoproliferative disorder. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2023, 178, 109430. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whittaker, S.; Kim, Y.H.; Duvic, M.; Prince, H.M.; Lessin, S.R.; Wood, G.S.; Willemze, R.; Demierre, M.-F.; Pimpinelli, N.; et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J. Clin. Oncol. 2011, 29, 2598. [Google Scholar]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013, 2, 38–43. [Google Scholar]
- Elsayad, K.; Weishaupt, C.; Moustakis, C.; Danzer, M.F.; Müller, E.C.; Rolf, D.; Stranzenbach, R.; Livingstone, E.; Booken, N.; Stadler, R.; et al. Ultra-hypofractionated low-dose total skin electron beam in advanced stage mycosis fungoides and Sézary syndrome. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 164–170. [Google Scholar] [CrossRef]
- Bojanini, L.; Herrera, M.; Li, S.; Xu, N.; Ahmed, A.A.; Khodadoust, M.; Kim, Y.H. Long-term outcomes with mogamulizumab alone or in combination with other therapies for the treatment of cutaneous T-cell lymphoma. In Proceedings of the 17th International Conference on Malignant Lymphoma, Lugano, Switzerland, 13–17 June 2023. [Google Scholar]
- Polansky, M.; Talpur, R.; Daulat, S.; Hosing, C.; Dabaja, B.; Duvic, M. Long-Term Complete Responses to Combination Therapies and Allogeneic Stem Cell Transplants in Patients with Sezary Syndrome. Clin. Lymphoma Myeloma Leuk. 2015, 15, e83–e93. [Google Scholar] [CrossRef]
- Fong, S.; Hong, E.K.; Khodadoust, M.S.; Li, S.; Hoppe, R.T.; Kim, Y.H.; Hiniker, S.M. Low-dose total skin electron beam therapy combined with mogamulizumab for refractory mycosis fungoides and Sézary syndrome. Adv. Radiat. Oncol. 2021, 6, 100629. [Google Scholar] [CrossRef] [PubMed]
- Buckel, L.; Savariar, E.N.; Crisp, J.L.; Jones, K.A.; Hicks, A.M.; Scanderbeg, D.J.; Nguyen, Q.T.; Sicklick, J.K.; Lowy, A.M.; Tsien, R.Y.; et al. Tumor radiosensitization by monomethyl auristatin E: Mechanism of action and targeted delivery. Cancer Res. 2015, 75, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Oymanns, M.; Daum-Marzian, M.; Bellm, A.; Elsayad, K.; Eich, H.T.; Assaf, C. Near complete responses to concurrent brentuximab vedotin and ultra-hypofractionated low-dose total skin electron beam radiation in advanced cutaneous T-cell lymphoma. Br. J. Dermatol. 2023, 188, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Oymanns, M.; Assaf, C.; Daum-Marzian, M.; Eich, H.T.; Elsayad, K. Concurrent brentuximab vedotin and ultra-hypofractionated low-dose total skin electron beam therapy in 14 patients with tumor stage mycosis fungoides or Sézary syndrome. Eur. J. Cancer 2022, 173, S46. [Google Scholar] [CrossRef]
- European Organisation for Research and Treatment of Cancer. Anti-CCR4 Monoclonal Antibody (Mogamulizumab) and Total Skin Electron Beam Therapy (TSEB) in Patients with Stage IB-IIB Cutaneous T-Cell Lymphoma. 2023. Available online: https://ClinicalTrials.gov/show/NCT04128072 (accessed on 2 September 2024).
- Mogamulizumab + Low-Dose Total Skin Electron Beam Tx in Mycosis Fungoides & Sézary Syndrome. 2020. Available online: https://clinicaltrials.gov/study/NCT04256018 (accessed on 2 September 2024).

| Author | Combination | Evidence | Outcome |
|---|---|---|---|
| Fong S. et al., 2021 [44] | Mogamulizumab + low-dose TSEBT (2 × 12 Gy) (sequential) | Two SS patients (T4N2bM0B2/T4NxM0B2) | Both patients achieved a complete global response (CR) after 3 and 4 cycles (12 and 16 weeks, respectively) following the failure of 4 or 5 prior systemic therapies. The CR is ongoing after 43 and 72 weeks of follow-up |
| Oymanns M. et al., 2024 [this work] | Mogamulizumab + ultra-hypofractionated low-dose TSEBT (2 × 4 Gy) (concomittant) | Two SS Patients | Both patients achieved a complete response (CR) in the blood and a near CR in the skin during the first cycle (after 2 weeks). The near CR is ongoing after 70 weeks in one patient and was 22 weeks in the other. |
| Clinical Trials Number | Combination | Disease Type | Phase | Enrollment (Estimated) | Status | Primary Endpoint |
|---|---|---|---|---|---|---|
| NCT04256018 | Mogamulizumab + LD-TSEBT | MF/SS (MF Ib-IV, SS) | Phase 2 | 30 | Recruiting | ORR |
| NCT04128072 (MOGAT) | Mogamulizumab + TSEBT | CTCL (MF IB-IIB) | Phase 2 | 43 | Recruiting | PFS rate at 48 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oymanns, M.; Daum-Marzian, M.; Assaf, C. Mogamulizumab and Concomitant Hypofractionated Low-Dose Total Skin Electron Beam Therapy (2 × 4 Gy) in Cutaneous T-Cell Lymphoma: Proof of Principle, Report of Two Cases. Curr. Oncol. 2024, 31, 5412-5421. https://doi.org/10.3390/curroncol31090400
Oymanns M, Daum-Marzian M, Assaf C. Mogamulizumab and Concomitant Hypofractionated Low-Dose Total Skin Electron Beam Therapy (2 × 4 Gy) in Cutaneous T-Cell Lymphoma: Proof of Principle, Report of Two Cases. Current Oncology. 2024; 31(9):5412-5421. https://doi.org/10.3390/curroncol31090400
Chicago/Turabian StyleOymanns, Mathias, Michael Daum-Marzian, and Chalid Assaf. 2024. "Mogamulizumab and Concomitant Hypofractionated Low-Dose Total Skin Electron Beam Therapy (2 × 4 Gy) in Cutaneous T-Cell Lymphoma: Proof of Principle, Report of Two Cases" Current Oncology 31, no. 9: 5412-5421. https://doi.org/10.3390/curroncol31090400
APA StyleOymanns, M., Daum-Marzian, M., & Assaf, C. (2024). Mogamulizumab and Concomitant Hypofractionated Low-Dose Total Skin Electron Beam Therapy (2 × 4 Gy) in Cutaneous T-Cell Lymphoma: Proof of Principle, Report of Two Cases. Current Oncology, 31(9), 5412-5421. https://doi.org/10.3390/curroncol31090400






