Effects of Hydrocodone Rescheduling on Pain Management Practices Among Older Breast Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Cohort
2.3. Utilization of Pharmacotherapy for Pain Management
2.4. Patient Characteristics
2.5. Statistical Analysis
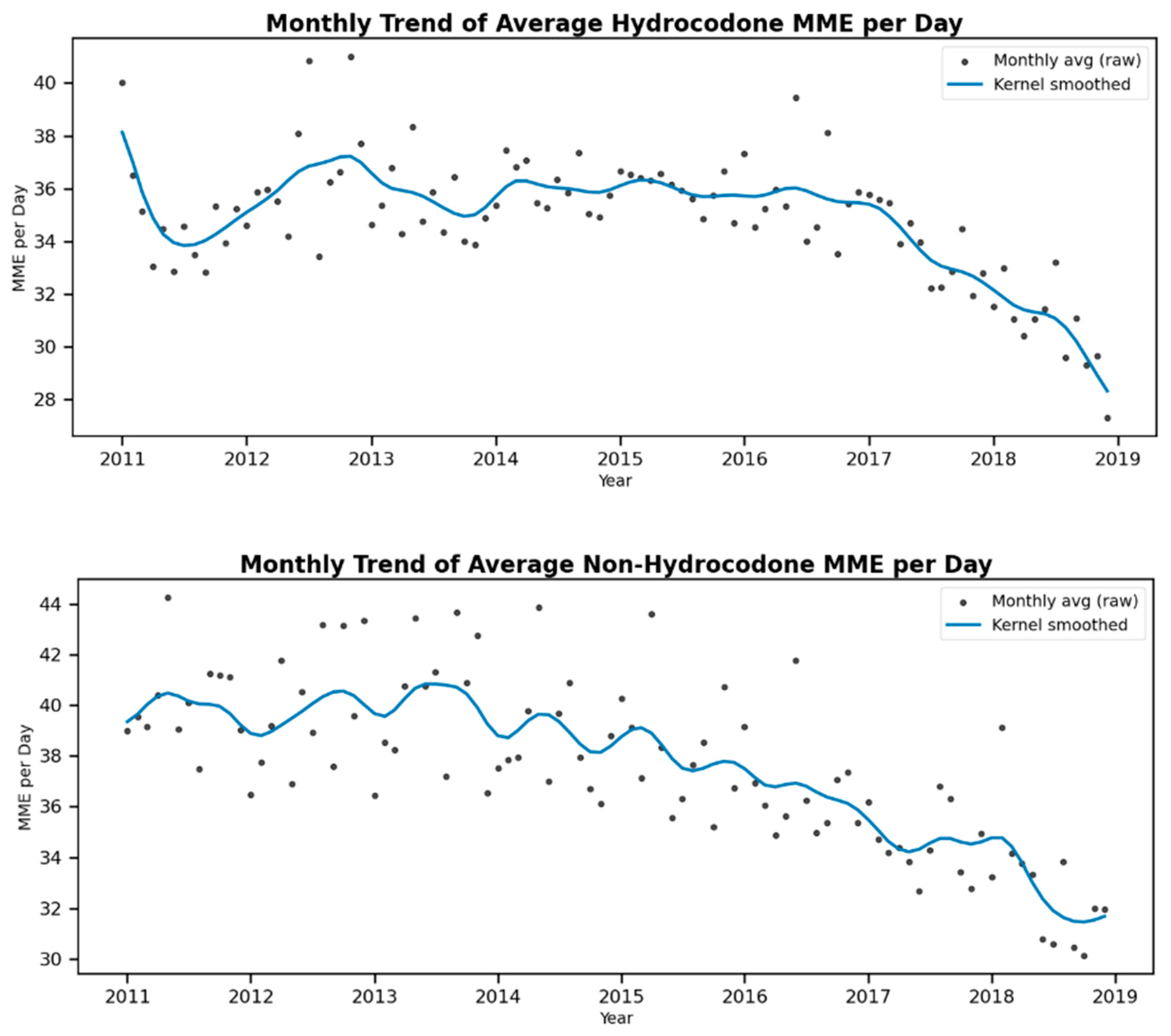
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mojtabai, R.; Amin-Esmaeili, M.; Nejat, E.; Olfson, M. Misuse of prescribed opioids in the United States. Pharmacoepidemiol. Drug Saf. 2019, 28, 345–353. [Google Scholar] [CrossRef]
- Biancuzzi, H.; Dal Mas, F.; Brescia, V.; Campostrini, S.; Cascella, M.; Cuomo, A.; Cobianchi, L.; Dorken-Gallastegi, A.; Gebran, A.; Kaafarani, H.M. Opioid misuse: A review of the main issues, challenges, and strategies. Int. J. Environ. Res. Public Health 2022, 19, 11754. [Google Scholar] [CrossRef]
- Han, B.; Jones, C.M.; Einstein, E.B.; Dowell, D.; Compton, W.M. Prescription opioid use disorder among adults reporting prescription opioid use with or without misuse in the United States. J. Clin. Psychiatry 2024, 85, 56054. [Google Scholar] [CrossRef]
- Owusu, D.N.; Brooks, B.; Ahuja, M.; Goodin, K.; Mensah, E.A. Association between pain medication misuse, psychological distress, and opioid use disorder among adults with lifetime cancer diagnosis in the United States. Support. Care Cancer 2025, 33, 638. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 64, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Bolshakova, M.; Bluthenthal, R.; Sussman, S. Opioid use and misuse: Health impact, prevalence, correlates and interventions. Psychol. Health 2019, 34, 1105–1139. [Google Scholar] [CrossRef]
- International Narcotics Control Board. Comments on the Reported Statistics on Narcotic Drugs. International Narcotics Control Board: Vienna, Austria, 2018. Available online: https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2021/2_NAR_2021-Part_II_Comments-E.pdf (accessed on 25 August 2024).
- Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed. Regist. 2014, 79, 49661–49682. [Google Scholar]
- Gabay, M. Federal controlled substances act: Controlled substances prescriptions. Hosp. Pharm. 2013, 48, 644. [Google Scholar] [CrossRef] [PubMed]
- Kenny, B.J.; Preuss, C.V. Pharmacy prescription requirements. In StatPearls [Internet]; StatPearls Publishing: Saint Petersburg, FL, USA, 2024. [Google Scholar]
- Raji, M.A.; Kuo, Y.F.; Adhikari, D.; Baillargeon, J.; Goodwin, J.S. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol. Drug Saf. 2018, 27, 513–519. [Google Scholar] [CrossRef]
- Tran, S.; Lavitas, P.; Stevens, K.; Greenwood, B.C.; Clements, K.; Alper, C.J.; Lenz, K.; Price, M.; Hydery, T.; Arnold, J.L. The effect of a federal controlled substance act schedule change on hydrocodone combination products claims in a Medicaid population. J. Manag. Care Spec. Pharm. 2017, 23, 532–539. [Google Scholar] [CrossRef]
- Tan, W.H.; Feaman, S.; Milam, L.; Garber, V.; McAllister, J.; Blatnik, J.A.; Brunt, L.M. Postoperative opioid prescribing practices and the impact of the hydrocodone schedule change. Surgery 2018, 164, 879–886. [Google Scholar] [CrossRef]
- Karami, S.; Ajao, A.; Wong, J.; Zhang, D.; Meyer, T.; Ding, Y.; Secora, A.; Major, J.M.; Gill, R.; Chai, G.P. The impact of hydrocodone rescheduling on utilization, abuse, misuse, and overdose deaths. Pharmacoepidemiol. Drug Saf. 2023, 32, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Statistics. Available online: https://www.cdc.gov/breast-cancer/statistics/?CDC_AAref_Val=https://www.cdc.gov/cancer/breast/statistics/index.htm (accessed on 28 January 2024).
- Changes Over Time: All Types of Cancer. Available online: https://gis.cdc.gov/Cancer/USCS/#/Trends/ (accessed on 26 August 2024).
- U.S. Cancer Statistics Breast Cancer Stat Bite. Available online: https://www.cdc.gov/united-states-cancer-statistics/publications/breast-cancer-stat-bite.html (accessed on 26 August 2024).
- Wang, K.; Yee, C.; Tam, S.; Drost, L.; Chan, S.; Zaki, P.; Rico, V.; Ariello, K.; Dasios, M.; Lam, H. Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast 2018, 42, 113–127. [Google Scholar] [CrossRef]
- Leysen, L.; Beckwee, D.; Nijs, J.; Pas, R.; Bilterys, T.; Vermeir, S.; Adriaenssens, N. Risk factors of pain in breast cancer survivors: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 3607–3643. [Google Scholar] [CrossRef]
- Gibson, D.C.; Chou, L.N.; Raji, M.A.; Baillargeon, J.G.; Kuo, Y.F. Opioid Prescribing Trends in Women Following Mastectomy or Breast-Conserving Surgery Before and After the 2014 Federal Reclassification of Hydrocodone. Oncologist 2020, 25, 281–289. [Google Scholar] [CrossRef]
- Busby, J.; Mills, K.; Zhang, S.-D.; Liberante, F.G.; Cardwell, C.R. Selective serotonin reuptake inhibitor use and breast cancer survival: A population-based cohort study. Breast Cancer Res. 2018, 20, 4. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results. Available online: https://seer.cancer.gov/ (accessed on 25 August 2024).
- Friedman, S.; Negoita, S. History of the surveillance, epidemiology, and end results (SEER) program. JNCI Monogr. 2024, 2024, 105–109. [Google Scholar] [CrossRef]
- McDonagh, M.; Selph, S.; Buckley, D.; Holmes, R.; Mauer, K.; Ramirez, S.; Hsu, F.; Dana, T.; Fu, R.; Chou, R. Nonopioid Pharmacologic Treatments for Chronic Pain; Comparative Effectiveness Review No.228; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020.
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef] [PubMed]
- Comorbidity SAS Macro (2021 Version). Available online: https://healthcaredelivery.cancer.gov/seermedicare/considerations/macro-2021.html (accessed on 10 September 2025).
- Usmani, S.A.; Hollmann, J.; Goodin, A.; Hincapie-Castillo, J.M.; Adkins, L.E.; Ourhaan, N.; Oueini, R.; Bhagwandass, H.; Easey, T.; Vouri, S.M. Effects of hydrocodone rescheduling on opioid use outcomes: A systematic review. J. Am. Pharm. Assoc. 2021, 61, e20–e44. [Google Scholar] [CrossRef] [PubMed]
- Jairam, V.; Yang, D.X.; Pasha, S.; Soulos, P.R.; Gross, C.P.; Yu, J.B.; Park, H.S. Temporal trends in opioid prescribing patterns among oncologists in the Medicare population. JNCI J. Natl. Cancer Inst. 2021, 113, 274–281. [Google Scholar] [CrossRef]
- Sabik, L.M.; Eom, K.Y.; Sun, Z.; Merlin, J.S.; Bulls, H.W.; Moyo, P.; Pruskowski, J.A.; van Londen, G.; Rosenzweig, M.; Schenker, Y. Patterns and trends in receipt of opioids among patients receiving treatment for cancer in a large health system. J. Natl. Compr. Cancer Netw. 2022, 20, 460–467.e1. [Google Scholar] [CrossRef]
- Baum, L.V.M.; KC, M.; Soulos, P.R.; Jeffery, M.; Ruddy, K.J.; Leapman, M.; Jairam, V.; Dinan, M.A.; Lerro, C.; Woods, C. Trends in new and persistent opioid use in older adults with cancer. J. Clin. Oncol. 2023, 41, 16. [Google Scholar] [CrossRef]
- Grasso, M.A.; Dezman, Z.D.; Grasso, C.T.; Jerrard, D.A. Opioid pain medication prescriptions obtained through emergency medical visits in the Veterans Health Administration. J. Opioid Manag. 2017, 13, 77–84. [Google Scholar] [CrossRef]
- Murimi, I.B.; Chang, H.Y.; Bicket, M.; Jones, C.M.; Alexander, G.C. Using trajectory models to assess the effect of hydrocodone upscheduling among chronic hydrocodone users. Pharmacoepidemiol. Drug Saf. 2019, 28, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.S.; Khan, J.S.; Dhaliwal, J.; Busse, J.W.; Choi, S.; Devereaux, P.; Clarke, H. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-F.; Raji, M.A.; Liaw, V.; Baillargeon, J.; Goodwin, J.S. Opioid prescriptions in older Medicare beneficiaries following the 2014 hydrocodone rescheduling. In Proceedings of the Pharmacoepidemiology and Drug Safety, Prague, Czech Republic, 22–26 August 2018; p. 427. [Google Scholar]
- Piper, B.J.; Shah, D.T.; Simoyan, O.M.; McCall, K.L.; Nichols, S.D. Trends in medical use of opioids in the US, 2006–2016. Am. J. Prev. Med. 2018, 54, 652–660. [Google Scholar] [CrossRef]
- Hatfield, M.; Sansgiry, S.; Johnson, M.; Essien, E.; Todd, K.; Fleming, M. Rescheduling hydrocodone combination products: Assessing the impact on opioid utilization. Value Health 2016, 19, A255. [Google Scholar] [CrossRef]
- Simpson, K.J.; Moran, M.T.; Foster, M.L.; Shah, D.T.; Chung, D.Y.; Nichols, S.D.; McCall, K.L.; Piper, B.J. Descriptive, observational study of pharmaceutical and non-pharmaceutical arrests, use, and overdoses in Maine. BMJ Open 2019, 9, e027117. [Google Scholar] [CrossRef]
- Habbouche, J.; Lee, J.; Steiger, R.; Dupree, J.M.; Khalsa, C.; Englesbe, M.; Brummett, C.; Waljee, J. Association of hydrocodone schedule change with opioid prescriptions following surgery. JAMA Surg. 2018, 153, 1111–1119. [Google Scholar] [CrossRef]
- Habbouche, J.A.; Lee, J.S.; Khalsa, C.; Howard, R.; Hu, H.M.; Englesbe, M.J.; Brummett, C.; Waljee, J.F. Effect of opioid schedule change on prescribing habits of surgeons. J. Am. Coll. Surg. 2017, 225, S82. [Google Scholar] [CrossRef]
- Anderson, J.E.; Cocanour, C.S.; Galante, J.M. Trauma and acute care surgeons report prescribing less opioids over time. Trauma Surg. Acute Care Open 2019, 4, e000255. [Google Scholar] [CrossRef]
- Luby, A.O.; Alessio-Bilowus, D.; Hu, H.M.; Brummett, C.M.; Waljee, J.F.; Bicket, M.C. Trends in opioid prescribing and new persistent opioid use after surgery in the United States. Ann. Surg. 2025, 281, 347–352. [Google Scholar] [CrossRef]
- Veerabagu, S.A.; Nugent, S.T.; Geng, Z.; Yanes, A.F.; Fischer, J.P.; Kovell, R.; Kuntz, A.F.; Rajasekaran, K.; VanderBeek, B.L.; Miller, C.J. National opioid prescription decline across outpatient specialty surgeries: A claims database study. J. Am. Acad. Dermatol. 2025, 93, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P. Vital signs: Changes in opioid prescribing in the United States, 2006–2015. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.W.; Brett, A.S. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern. Med. 2019, 179, 695–701. [Google Scholar] [CrossRef]
- Williams, C.; Al-Jammali, Z.; Herink, M. Gabapentinoids for pain: A review of published comparative effectiveness trials and data submitted to the FDA for approval. Drugs 2023, 83, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, B.J.; Gleeson, B.; Morford, K.L.; Adams, Z.; Funaro, M.C.; Becker, W.C.; Merlin, J.S. Long-term use of muscle relaxant medications for chronic pain: A systematic review. JAMA Netw. Open 2024, 7, e2434835. [Google Scholar] [CrossRef]
- Cashin, A.G.; Folly, T.; Bagg, M.K.; Wewege, M.A.; Jones, M.D.; Ferraro, M.C.; Leake, H.B.; Rizzo, R.R.; Schabrun, S.M.; Gustin, S.M. Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: Systematic review and meta-analysis. BMJ 2021, 374, n1446. [Google Scholar] [CrossRef]
- Soprano, S.E.; Hennessy, S.; Bilker, W.B.; Leonard, C.E. Assessment of physician prescribing of muscle relaxants in the United States, 2005–2016. JAMA Netw. Open 2020, 3, e207664. [Google Scholar] [CrossRef]

| Total (N = 52,272) | |
|---|---|
| Age | |
| 66–69 | 12,716 (24.3%) |
| 70–74 | 15,269 (29.2%) |
| 75–79 | 11,225 (21.5%) |
| >=80 | 13,062 (25.0%) |
| Race/Ethnicity | |
| White | 43,430 (83.1%) |
| AA | 3187 (6.1%) |
| Hispanic | 2978 (5.7%) |
| Other race | 2677 (5.1%) |
| Hydrocodone | |
| Yes | 23,967 (45.9%) |
| No | 28,305 (54.1%) |
| Non-Hydrocodone opioids | |
| Yes | 24,880 (47.6%) |
| No | 27,392 (52.4%) |
| NSAIDs | |
| Yes | 13,095 (25.1%) |
| No | 39,177 (74.9%) |
| Antidepressants | |
| Yes | 10,754 (20.6%) |
| No | 41,518 (79.4%) |
| Radiation therapy | |
| Yes | 30,439 (58.2%) |
| No | 21,833 (41.8%) |
| Chemotherapy | |
| Yes | 14,895 (28.5%) |
| No | 37,377 (71.5%) |
| Immunotherapy | |
| Yes | 7350 (14.1%) |
| No | 44,922 (85.9%) |
| Hormonal therapy | |
| Yes | 46,853 (89.6%) |
| No | 5419 (10.4%) |
| Dual Eligibility | |
| Yes | 9391 (18.0%) |
| No | 42,881 (82.0%) |
| Depression | |
| Yes | 11,196 (21.4%) |
| No | 41,076 (78.6%) |
| Charlson comorbidity | |
| No | 20,245 (42.3%) |
| 1 | 13,422 (28.1%) |
| 2 | 8104 (16.9%) |
| 3 or more | 6051 (12.7%) |
| Variables | AOR | 95% CI | p-Value |
|---|---|---|---|
| Hydrocodone Use | |||
| Policy Change | |||
| Post policy change | 0.81 | [0.75, 0.86] | <0.001 |
| Before policy change (reference) | |||
| Time Trend | |||
| in 12 months | 0.91 | [0.90, 0.92] | <0.001 |
| Non-hydrocodone Opioids Use | |||
| Policy Change | |||
| Post policy change | 1.25 | [1.17, 1.34] | <0.001 |
| Before policy change (reference) | |||
| Time Trend | |||
| in 12 months | 1 | [0.99, 1.02] | 0.614 |
| NSAIDs Use | |||
| Policy Change | |||
| Post policy change | 0.94 | [0.87, 1.02] | 0.139 |
| Before policy change (reference) | |||
| Time Trend | |||
| in 12 months | 1.01 | [0.99, 1.03] | 0.359 |
| Antidepressants Use | |||
| Policy Change | |||
| Post policy change | 1.02 | [0.93, 1.12] | 0.659 |
| Before policy change (reference) | |||
| Time Trend | |||
| in 12 months | 0.99 | [0.97, 1.01] | 0.604 |
| Variables | Estimate | Standard Error | p-Value | |
|---|---|---|---|---|
| Hydrocodone Use | ||||
| Policy Change | ||||
| Post policy change | −1.637 | 0.535 | 0.002 | |
| Pre policy change (reference) | ||||
| Time trend | ||||
| in 12 months | −0.95 | 0.117 | <0.001 | |
| Non-hydrocodone Opioids Use | ||||
| Policy Change | ||||
| Post policy change | −0.856 | 0.909 | 0.346 | |
| Before policy change (reference) | ||||
| Time trend | ||||
| in 12 months | −1.624 | 0.199 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Ikram, M.; Zhou, S.; Klein, R.; Leslie, D.; Thornton, J.D. Effects of Hydrocodone Rescheduling on Pain Management Practices Among Older Breast Cancer Patients. Curr. Oncol. 2025, 32, 593. https://doi.org/10.3390/curroncol32110593
Shen C, Ikram M, Zhou S, Klein R, Leslie D, Thornton JD. Effects of Hydrocodone Rescheduling on Pain Management Practices Among Older Breast Cancer Patients. Current Oncology. 2025; 32(11):593. https://doi.org/10.3390/curroncol32110593
Chicago/Turabian StyleShen, Chan, Mohammad Ikram, Shouhao Zhou, Roger Klein, Douglas Leslie, and James Douglas Thornton. 2025. "Effects of Hydrocodone Rescheduling on Pain Management Practices Among Older Breast Cancer Patients" Current Oncology 32, no. 11: 593. https://doi.org/10.3390/curroncol32110593
APA StyleShen, C., Ikram, M., Zhou, S., Klein, R., Leslie, D., & Thornton, J. D. (2025). Effects of Hydrocodone Rescheduling on Pain Management Practices Among Older Breast Cancer Patients. Current Oncology, 32(11), 593. https://doi.org/10.3390/curroncol32110593






