Outcomes of Allogeneic Stem Cell Transplant in Patients with Relapsed/Refractory Hodgkin Lymphoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Definition of Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient and Transplant Characteristics
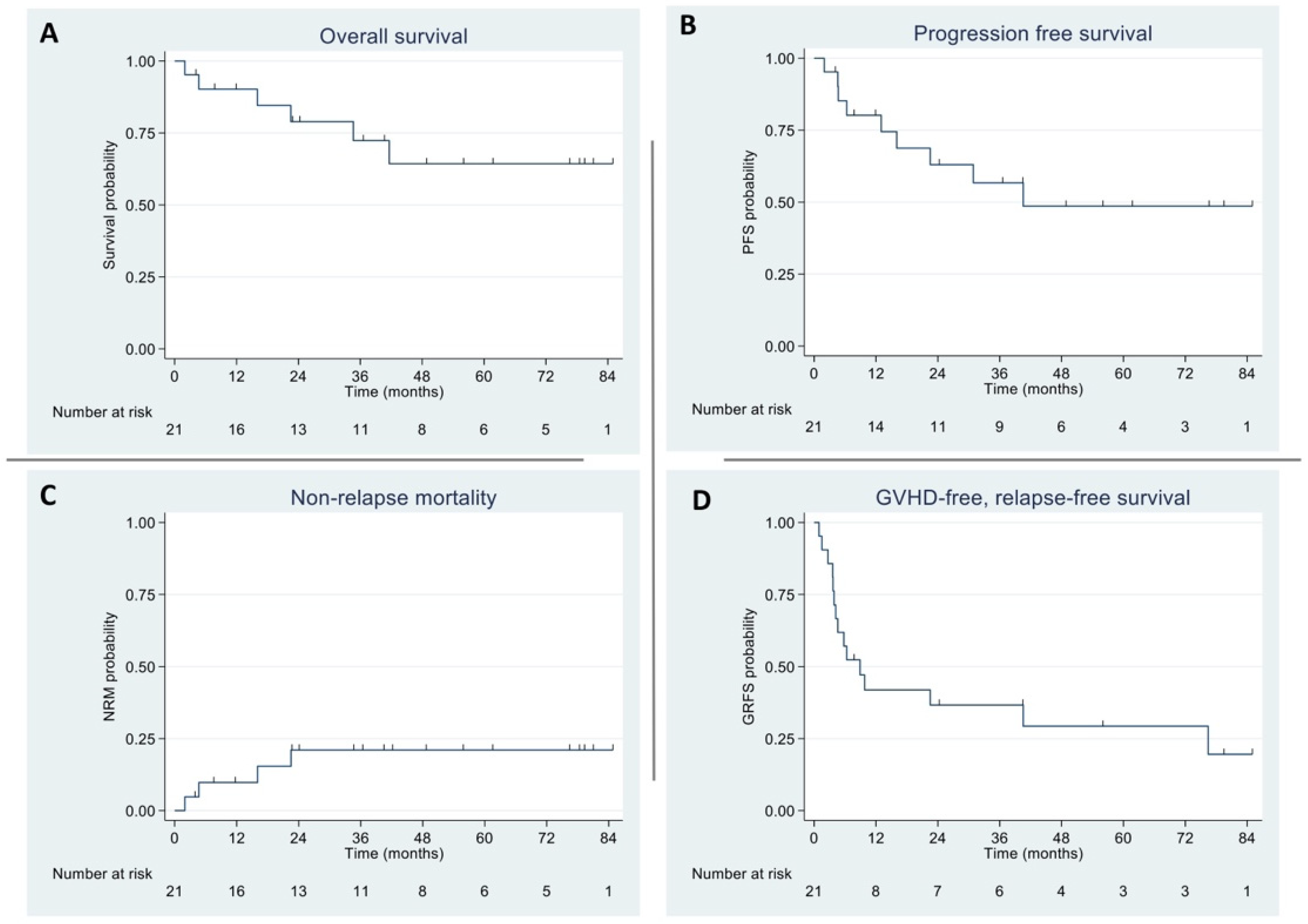
3.2. Overall and Progression-Free Survival
3.3. Non-Relapse Mortality and Graft-Versus-Host Disease/Relapse-Free Survival
3.4. Transplant Response and Complications
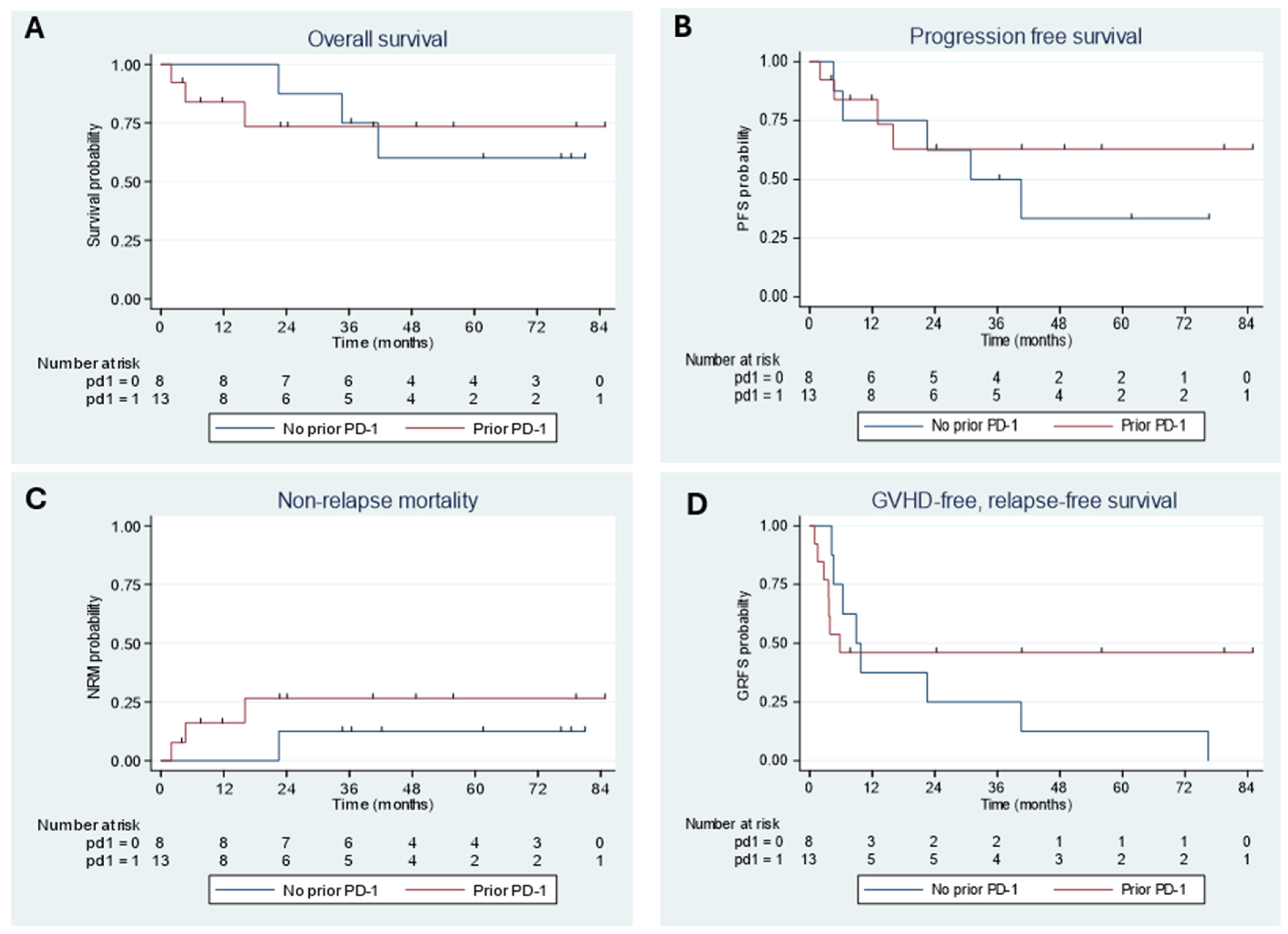
3.5. Outcomes by Prior PD-1 Inhibitor Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimm, B.; Goergen, H.; Fuchs, M.; von Tresckow, B.; Böll, B.; Meissner, J.; Glunz, A.; Diehl, V.; Eich, H.T.; Engert, A.; et al. Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: An analysis of international staging definitions. Ann. Oncol. 2013, 24, 3070–3076. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V.; Armitage, J.O.; Assouline, D.; Björkholm, M.; Brusamolino, E.; Canellos, G.P.; Carde, P.; Crowther, D.; Cunningham, D.; et al. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Hodgkin lymphoma: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2022, 97, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Josting, A.; Rueffer, U.; Franklin, J.; Sieber, M.; Diehl, V.; Engert, A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: A report from the German Hodgkin Lymphoma Study Group. Blood 2000, 96, 1280–1286. [Google Scholar] [CrossRef]
- Fermé, C.; Mounier, N.; Diviné, M.; Brice, P.; Stamatoullas, A.; Reman, O.; Voillat, L.; Jaubert, J.; Lederlin, P.; Colin, P.; et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: Results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J. Clin. Oncol. 2002, 20, 467–475. [Google Scholar] [CrossRef]
- Mei, M.G.; Lee, H.J.; Palmer, J.M.; Chen, R.; Tsai, N.-C.; Chen, L.; McBride, K.; Smith, D.L.; Melgar, I.; Song, J.Y.; et al. Response-adapted anti-PD-1–based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood 2022, 139, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.F.; LeBlanc, M.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Parsons, S.K.; Ahmed, S.; et al. Nivolumab-AVD in Advanced Stage Classic Hodgkin Lymphoma. N. Engl. J. Med. 2024, 391, 1379. [Google Scholar] [CrossRef] [PubMed]
- Kewalramani, T.; Nimer, S.D.; Zelenetz, A.D.; Malhotra, S.; Qin, J.; Yahalom, J.; Moskowitz, C.H. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transpl. 2003, 32, 673–679. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Gianni, A.M.; Carella, A.; et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 385, 1853–1862. [Google Scholar] [CrossRef]
- Peggs, K.S. Should all patients with Hodgkin lymphoma who relapse after autologous SCT be considered for allogeneic SCT? Blood Adv. 2018, 2, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Canals, C.; Arranz, R.; Caballero, D.; Ribera, J.M.; Brune, M.; Passweg, J.; Martino, R.; Valcárcel, D.; Besalduch, J.; et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study—A prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica 2012, 97, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Dulery, R.; Bazarbachi, A.H.; Savani, M.; Al Hamed, R.; Bazarbachi, A.; Mohty, M. Latest advances in the management of classical Hodgkin lymphoma: The era of novel therapies. Blood Cancer J. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Robinson, S.; Canals, C.; Carella, A.M.; Boogaerts, M.A.; Caballero, D.; Hunter, A.E.; Kanz, L.; Slavin, S.; Cornelissen, J.J.; et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: An analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J. Clin. Oncol. 2008, 26, 455–462. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.; Luznik, L.; Jones, R.; Vogelsang, G.; Leffell, M.; Phelps, M.; Rhubart, P.; Cowan, K.; Piantadosi, S.; Fuchs, E. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2002, 8, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Hunter, A.; Chopra, R.; Parker, A.; Mahendra, P.; Milligan, D.; Craddock, C.; Pettengell, R.; Dogan, A.; Thomson, K.J.; et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet 2005, 365, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Reyal, Y.; Kayani, I.; Bloor, A.J.; Fox, C.P.; Chakraverty, R.; Sjursen, A.-M.; Fielding, A.K.; Ben Taylor, M.; Bishton, M.J.; Morris, E.C.; et al. Impact of Pretransplantation (18)F-Fluorodeoxyglucose-Positron Emission Tomography on Survival Outcomes after T Cell-Depleted Allogeneic Transplantation for Hodgkin Lymphoma. Biol. Blood Marrow Transpl. 2016, 22, 1234–1241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gauthier, J.; Castagna, L.; Garnier, F.; Guillaume, T.; Socié, G.; Maury, S.; Maillard, N.; Tabrizi, R.; Marchand, T.; Malfuson, J.; et al. Reduced-intensity and non-myeloablative allogeneic stem cell transplantation from alternative HLA-mismatched donors for Hodgkin lymphoma: A study by the French Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transpl. 2017, 52, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Boumendil, A.; Finel, H.; Castagna, L.; Dominietto, A.; Blaise, D.; Diez-Martin, J.L.; Tischer, J.; Gülbas, Z.; Wallet, H.L.; et al. Influence of donor type, stem cell source and conditioning on outcomes after haploidentical transplant for lymphoma—A LWP-EBMT study. Br. J. Haematol. 2020, 188, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Castagna, L.; Busca, A.; Bramanti, S.; Anna, M.R.; Malagola, M.; Ciceri, F.; Arcese, W.; Vallisa, D.; Patriarca, F.; Specchia, G.; et al. Haploidentical related donor compared to HLA-identical donor transplantation for chemosensitive Hodgkin lymphoma patients. BMC Cancer 2020, 20, 1140. [Google Scholar] [CrossRef] [PubMed]
- Mussetti, A.; Kanate, A.S.; Wang, T.; He, M.; Hamadani, M.; Finel, H.; Boumendil, A.; Glass, B.; Castagna, L.; Dominietto, A.; et al. Haploidentical Versus Matched Unrelated Donor Transplants Using Post-Transplantation Cyclophosphamide for Lymphomas. Transpl. Cell. Ther. 2023, 29, 184.e1–184.e9. [Google Scholar] [CrossRef]
- Ahmed, S.; Kanakry, J.A.; Ahn, K.W.; Litovich, C.; Abdel-Azim, H.; Aljurf, M.; Bacher, V.U.; Bejanyan, N.; Cohen, J.B.; Farooq, U.; et al. Lower Graft-versus-Host Disease and Relapse Risk in Post-Transplant Cyclophosphamide-Based Haploidentical versus Matched Sibling Donor Reduced-Intensity Conditioning Transplant for Hodgkin Lymphoma. Biol. Blood Marrow Transpl. 2019, 25, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.-A.; Ahmed, S. When to use stem cell transplantation for classical Hodgkin lymphoma. Hematology 2024, 2024, 517–523. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Pintilie, M.; Stewart, D.; Lachance, S.; Power, M.; Couture, F.; Xenocostas, A.; Voralia, M.; Couban, S.; Foley, R. Outcomes of reduced-intensity conditioning allo-SCT for Hodgkins lymphoma: A national review by the Canadian Blood and Marrow Transplant Group. Bone Marrow Transpl. 2010, 45, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Koyun, D.; Şahin, U.; Seval, G.C.; Şahin, D.G.; Gökmen, A.; Bozdağ, S.C.; Toprak, S.K.; Topçuoğlu, P.; Yüksel, M.K.; Arslan, Ö.; et al. Allogeneic Stem Cell Transplantation for Relapsed and/or Refractory Hodgkin Lymphoma: A Multicenter Real World Experience. Blood 2023, 142 (Suppl. 1), 3617. [Google Scholar] [CrossRef]
- Chen, R.; Palmer, J.M.; Tsai, N.-C.; Thomas, S.H.; Siddiqi, T.; Popplewell, L.; Farol, L.; Nademanee, A.; Forman, S.J. Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol. Blood Marrow Transpl. 2014, 20, 1864. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Zinzani, P.L.; Lee, H.J.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; Tomita, A.; et al. Five-year follow-up of KEYNOTE-087: Pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma. Blood 2023, 142, 878–886. [Google Scholar] [CrossRef]
- Moskowitz, A.J. PD-1 blockade for untreated Hodgkin lymphoma. Blood 2021, 137, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. PD-1 Blockade in Classic Hodgkin Lymphoma. JCO Oncol. Pract. 2021, 17, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Merryman, R.W.; Armand, P. Immune Checkpoint Blockade and Hematopoietic Stem Cell Transplant. Curr. Hematol. Malig. Rep. 2017, 12, 44–50. [Google Scholar] [CrossRef]
- Merryman, R.W.; Kim, H.T.; Zinzani, P.L.; Carlo-Stella, C.; Ansell, S.M.; Perales, M.-A.; Avigdor, A.; Halwani, A.S.; Houot, R.; Marchand, T.; et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017, 129, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Herbaux, C.; Gauthier, J.; Brice, P.; Drumez, E.; Ysebaert, L.; Doyen, H.; Fornecker, L.; Bouabdallah, K.; Manson, G.; Ghesquières, H.; et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017, 129, 2471–2478. [Google Scholar] [CrossRef]
- de Philippis, C.; Legrand-Izadifar, F.; Bramanti, S.; Giordano, L.; de Oca, C.M.; Duléry, R.; Bouabdallah, R.; Granata, A.; Devillier, R.; Mariotti, J.; et al. Checkpoint inhibition before haploidentical transplantation with posttransplant cyclophosphamide in Hodgkin lymphoma. Blood Adv. 2020, 4, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Ebadi, M.; Cashen, A.F. Allogeneic hematopoietic stem cell transplantation in Hodgkin lymphoma: A systematic review and meta-analysis. Bone Marrow Transpl. 2016, 51, 521. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | N (Total = 21) | |
|---|---|---|
| Age, years, median (range) | 36 (20–66) | |
| Sex, n (%) | Male | 11 (52%) |
| Female | 10 (48%) | |
| HL subtype, n (%) | Classical (no subtype) | 4 (19%) |
| Nodular sclerosing | 13 (62%) | |
| Mixed cellularity | 4 (19%) | |
| HCT-CI, n (%) | 0 | 4 (19%) |
| 1–2 | 9 (43%) | |
| 3–4 | 5 (24%) | |
| >5 | 2 (9%) | |
| Missing | 1 (5%) | |
| Karnofsky Performance Status, n (%) | ≥90 | 14 (67%) |
| <90 | 7 (33%) | |
| Status at transplantation | CR | 7 (36%) |
| PR | 14 (67%) | |
| Number of prior treatment lines, median (range) | 4 (3–6) | |
| Prior ASCT, n (%) | 21 (100%) | |
| Prior BV exposure, n (%) | 19 (90%) | |
| Prior PD1 inhibitor exposure, n (%) | 13 (62%) | |
| Prior BV and PD1 inhibitor exposure, n (%) | 11 (52%) | |
| Time from diagnosis to transplant, years, median (range) | 3.1 (1.9–12.3) | |
| Donor type, n (%) | Related, haploidentical | 17 (81%) |
| Matched unrelated | 4 (19%) | |
| Stem cell source, n (%) | PBSC | 21 (100%) |
| Female donor to male recipient, n (%) | 4 (19%) | |
| Stem cell dose (CD34 × 106 cells/recipient kg), median (range) | 6.4 (3.9–11.8) | |
| Fresh vs. cryopreserved, n (%) | Fresh | 19 (90%) |
| Cryopreserved | 2 (10%) | |
| Conditioning regimen, n (%) | Flu/Cy/TBI 200 | 20 (95%) |
| Flu/Mel | 1 (5%) | |
| GVHD prophylaxis, n (%) | PTCy/tacro/MMF | 18 (86%) |
| ATG/CNI/MMF | 3 (14%) | |
| CMV status, n (%) | R+/D+ | 4 (19%) |
| R+/D− | 1 (5%) | |
| R−/D− | 11 (52%) | |
| R−/D+ | 5 (24%) | |
| ABO mismatch, n (%) | None | 9 (43%) |
| Major | 3 (14%) | |
| Minor | 7 (33%) | |
| Missing | 2 (10%) | |
| Complication | N (Total = 21) | |
|---|---|---|
| CMV reactivation, n (%) | No | 17 (81%) |
| Yes | 3 (14%) | |
| EBV reactivation, n (%) | No | 19 (90%) |
| Yes | 2 (10%) | |
| PTLD | 1 (5%) | |
| BK virus infection, n (%) | No | 20 (95%) |
| Yes | 1 (5%) | |
| Engraftment syndrome, n (%) | No | 19 (90%) |
| Yes | 2 (10%) | |
| VOD/SOS, n (%) | 1 (5%) | |
| aGVHD (all grades), n (%) | 16 (76%) | |
| aGVHD, grade III-IV, n (%) | 4 (19%) | |
| cGVHD, extensive, n (%) | 8 (38%) | |
| Deaths, n (%) | 6 (29%) | |
| Cause of death, n (%) | Bacterial sepsis | 1 (17%) |
| Pneumonia | 1 (17%) | |
| Fungal pneumonia | 1 (17%) | |
| aGVHD | 1 (17%) | |
| Hodgkin lymphoma | 2 (33%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, S.; Lepic, K.; Bhindi, R.; Berg, T.; Khalaf, D.; Leber, B.; Radford, M.; Walker, I.; Davies, G.; Garcia-Horton, A. Outcomes of Allogeneic Stem Cell Transplant in Patients with Relapsed/Refractory Hodgkin Lymphoma. Curr. Oncol. 2025, 32, 118. https://doi.org/10.3390/curroncol32020118
Ge S, Lepic K, Bhindi R, Berg T, Khalaf D, Leber B, Radford M, Walker I, Davies G, Garcia-Horton A. Outcomes of Allogeneic Stem Cell Transplant in Patients with Relapsed/Refractory Hodgkin Lymphoma. Current Oncology. 2025; 32(2):118. https://doi.org/10.3390/curroncol32020118
Chicago/Turabian StyleGe, Shiliang, Kylie Lepic, Ravi Bhindi, Tobias Berg, Dina Khalaf, Brian Leber, Michael Radford, Irwin Walker, Gwynivere Davies, and Alejandro Garcia-Horton. 2025. "Outcomes of Allogeneic Stem Cell Transplant in Patients with Relapsed/Refractory Hodgkin Lymphoma" Current Oncology 32, no. 2: 118. https://doi.org/10.3390/curroncol32020118
APA StyleGe, S., Lepic, K., Bhindi, R., Berg, T., Khalaf, D., Leber, B., Radford, M., Walker, I., Davies, G., & Garcia-Horton, A. (2025). Outcomes of Allogeneic Stem Cell Transplant in Patients with Relapsed/Refractory Hodgkin Lymphoma. Current Oncology, 32(2), 118. https://doi.org/10.3390/curroncol32020118





