Symptoms, Symptom Profiles, and Healthcare Utilization in Patients with Hematologic Malignancies: A Retrospective Observational Cohort Study and Latent Class Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Data Sources
2.1.2. Edmonton Symptom Assessment System (ESAS)
2.1.3. Sociodemographic, Disease, and Treatment-Specific Variables
2.1.4. Acute Care Utilization Outcomes
2.2. Ethical Considerations
2.3. Data Analysis
2.3.1. Symptom Prevalence and Severity
2.3.2. Latent Class Analysis
3. Results
3.1. Demographics
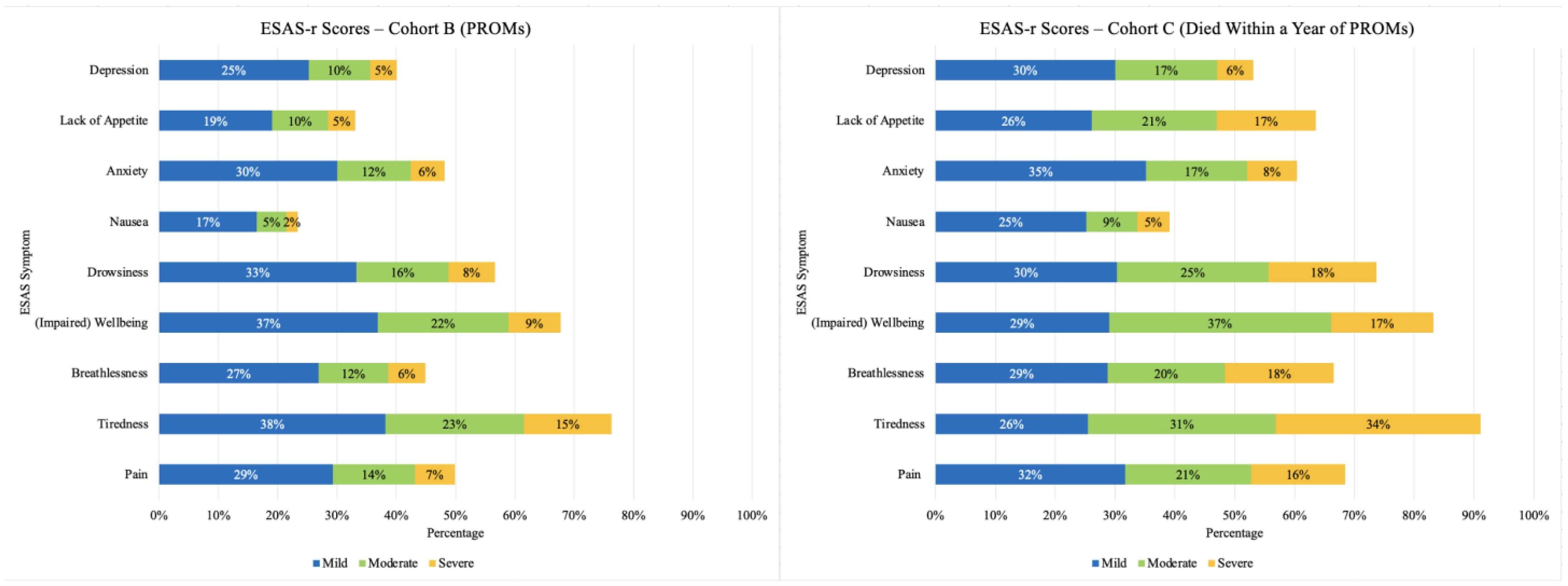
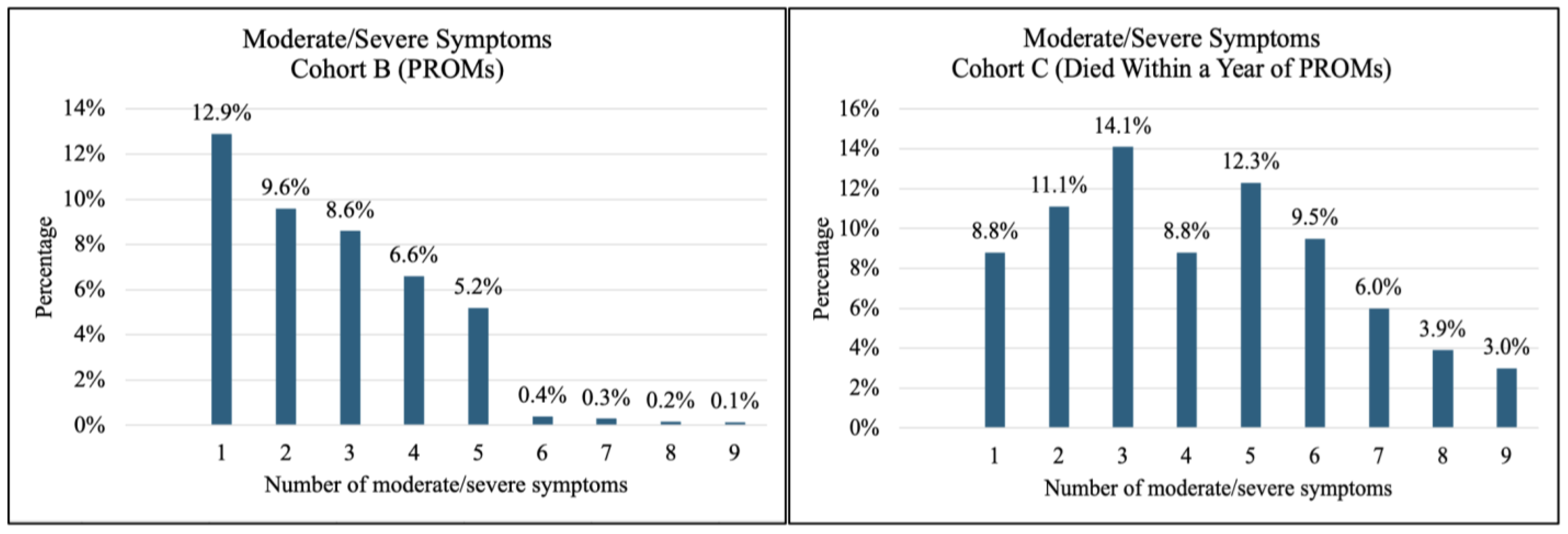
3.2. Prevalence and Severity of Symptoms
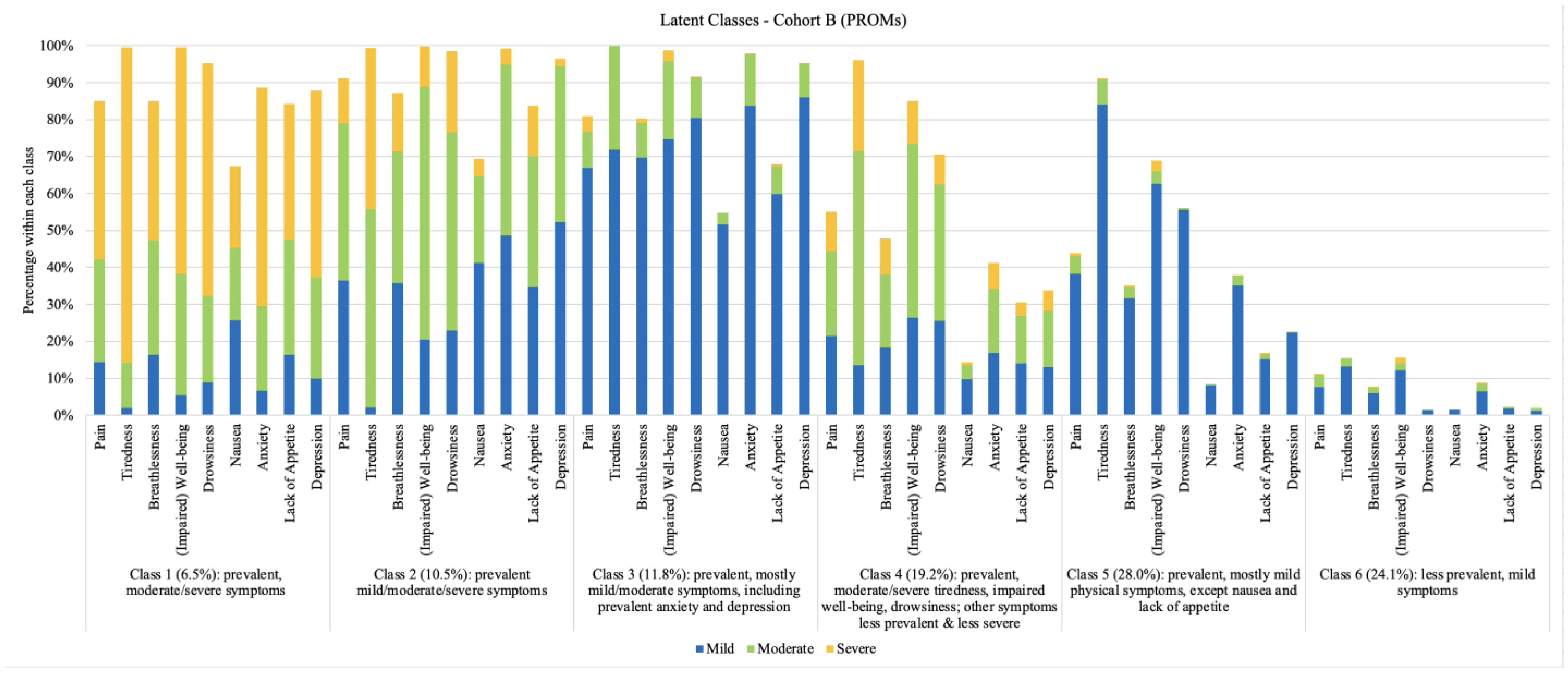
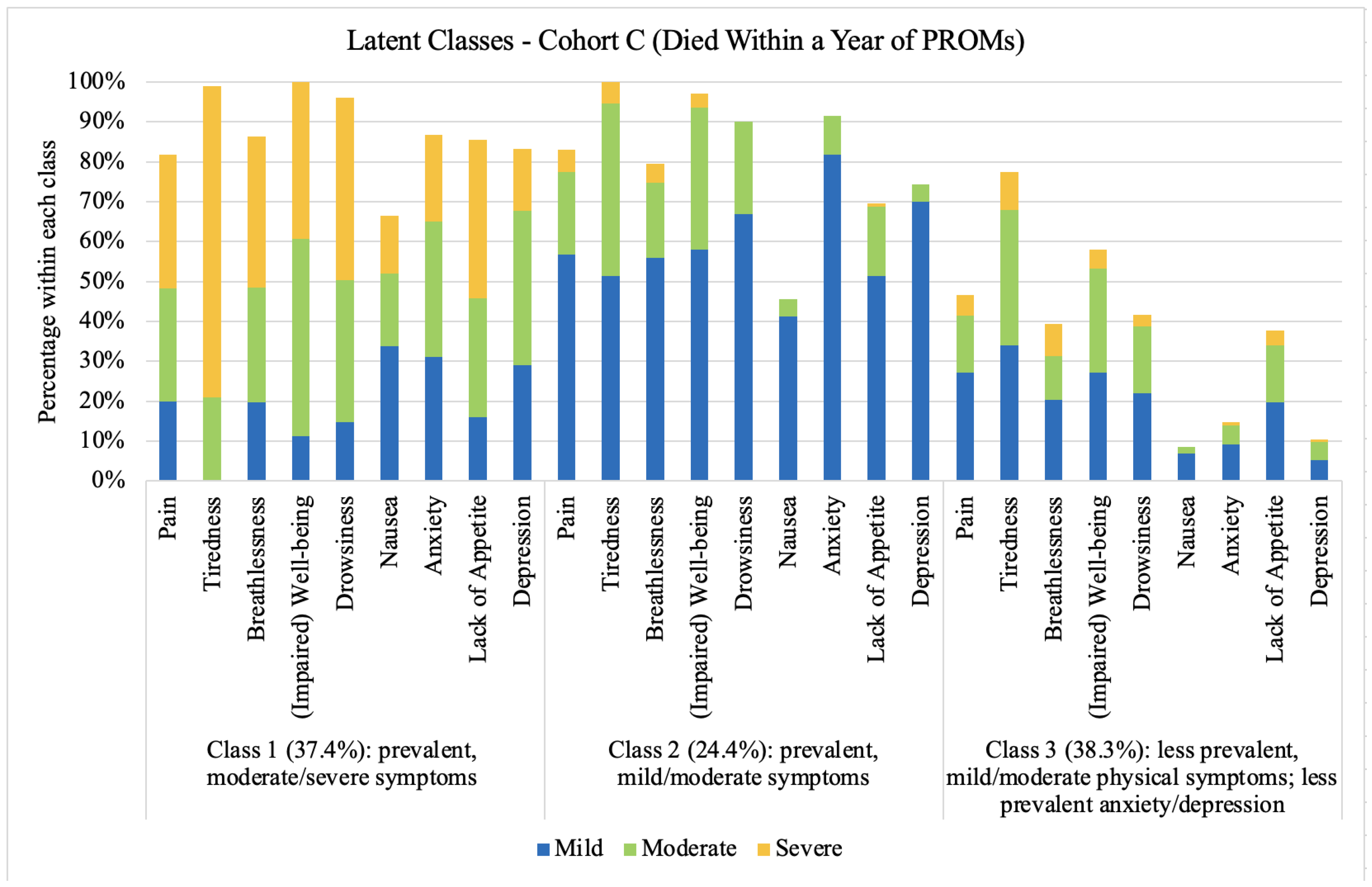
3.3. Symptom Profiles
Correlates of Latent Class Membership
4. Discussion
4.1. Patient-Reported Outcomes Measures
4.2. Rural and Remote Challenges
4.3. Sex Differences and Symptoms
4.4. Age and Symptoms
4.5. Symptoms and Healthcare Utilization
4.6. Symptoms in the Last Year of Life
4.7. Implications for Practice, Education, and Research
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Jawahri, A.; Webb, J.A.; Breffni, H.; Zimmermann, C. Integrating palliative care and hematologic malignancies: Bridging the gaps for our patients and their caregivers. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432196. [Google Scholar] [CrossRef] [PubMed]
- El-Jawahri, A.; Nelson, A.M.; Gray, T.F.; Lee, S.J.; LeBlanc, T.W. Palliative and end-of-life care for patients with hematologic malignancies. J. Clin. Oncol. 2020, 38, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Gebel, C.; Ditscheid, B.; Meissner, F.; Slotina, E.; Kruschel, I.; Marschall, U.; Wedding, U.; Freytag, A. Utilization and quality of palliative care in patients with hematological and solid cancers: A population-based study. J. Cancer Res. Clin. Oncol. 2024, 150, 191. [Google Scholar] [CrossRef] [PubMed]
- Robbins-Welty, G.A.; Webb, J.A.; Shalev, D.; El-Jawahri, A.; Jackson, V.; Mitchell, C.; LeBlanc, T.W. Advancing palliative care integration in hematology: Building upon existing evidence. Curr. Treat. Options Oncol. 2023, 24, 542–564. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S. Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. J. Natl. Cancer Inst. 2007, 37, 16–21. [Google Scholar] [CrossRef]
- Gapstur, R.L. Symptom burden: A concept analysis and implications for oncology nurses. Oncol. Nurs. Forum 2007, 34, 673–680. [Google Scholar] [CrossRef]
- Hochman, M.J.; Yu, Y.; Wolf, S.P.; Samsa, G.P.; Kamal, A.H.; LeBlanc, T.W. Comparing the palliative care needs of patients with hematologic and solid malignancies. J. Pain Symptom Manag. 2018, 55, 82–88.e1. [Google Scholar] [CrossRef]
- Manitta, V.; Zordan, R.; Cole-Sinclair, M.; Nandurkar, H.; Philip, J. The symptom burden of patients with hematological malignancy: A cross-sectional observational study. J. Pain Symptom Manag. 2011, 42, 432–442. [Google Scholar] [CrossRef]
- Kuczmarski, T.M.; Roemer, L.; Odejide, O.O. Depression in patients with hematologic malignancies: The current landscape and future directions. Blood Rev. 2024, 65, 101182. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Howell, D.A.; McCaughan, D.; Smith, A.G.; Patmore, R.; Roman, E. Incurable but treatable: Understanding, uncertainty and impact in chronic blood cancers—A qualitative study from the UK’s Haematological Malignancy Research Network. PLoS ONE 2022, 17, e0263672. [Google Scholar] [CrossRef] [PubMed]
- Simao, D.; Barata, P.C.; Alves, M.; Papoila, A.L.; Oliveira, S.; Lawlor, P. Symptom clusters in patients with advanced cancer: A prospective longitudinal cohort study to examine their stability and prognostic significance. Oncologist 2024, 29, e152–e163. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Yuen, D.; Mischitelle, A.; Minden, M.D.; Brandwein, J.M.; Schimmer, A.; Gagliese, L.; Lo, C.; Rydall, A.; Rodin, G. Symptom burden and supportive care in patients with acute leukemia. Leuk. Res. 2013, 37, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Chan, T.S.Y.; Gill, H.; Chan, T.C.W.; Li, C.W.; Au, H.Y.; Wong, C.Y.; Tsang, K.W.; Lo, R.S.K.; Cheng, B.H.W.; et al. Supportive care and symptom management in patients with advanced hematological malignancies: A literature review. Ann. Palliat. Med. 2022, 11, 3273–3291. [Google Scholar] [CrossRef]
- Schatz, F.; Mehnert-Theuerkauf, A.; Platzbecker, U.; Springer, F.; Götze, H. Health-related quality of life in older hematological cancer survivors (70+) compared to older general population—A German cancer-register-based cross-sectional comparative study. Eur. J. Haematol. 2024, 113, 693–703. [Google Scholar] [CrossRef]
- Ferraz, L.F.M.; Ferreira, A.P.S.; Guimarães, T.V.V.; de Melo Campos, P. Early integration of palliative care in hematology: An urgency for patients, a challenge for physicians. Hematol. Transfus. Cell Ther. 2022, 44, 567–573. [Google Scholar] [CrossRef]
- Goswami, P.; Oliva, E.N.; Ionova, T.; Else, R.; Kell, J.; Fielding, A.K.; Jennings, D.M.; Karakantza, M.; Al-Ismail, S.; Collins, G.P.; et al. Quality-of-life issues and symptoms reported by patients living with haematological malignancy: A qualitative study. Ther. Adv. Hematol. 2020, 11, 2040620720955002. [Google Scholar] [CrossRef]
- Mian, H.; Sutradhar, R.; Pond, G.R.; Sivapathasundaram, B.; Sussman, J.; Balitsky, A.; D’Souza, A.; Wildes, T.M.; Seow, H. Patient-reported outcome measures are associated with health care utilization in patients with transplant ineligible multiple myeloma: A population-based study. Blood Cancer J. 2022, 12, 17. [Google Scholar] [CrossRef]
- Shaulov, A.; Aviv, A.; Alcalde, J.; Zimmermann, C. Early integration of palliative care for patients with haematological malignancies. Br. J. Haematol. 2022, 199, 14–30. [Google Scholar] [CrossRef]
- Sommer, M.; Nielsen, L.K.; Nielsen, L.B.; Brondum, R.F.; Nielsen, M.M.; Rytter, A.S.; Vesteghem, C.; Severinsen, M.T.; El-Galaly, T.C.; Bøgsted, M.; et al. Patient-reported outcomes in patients with hematological relapse or progressive disease: A longitudinal observational study. Health Qual. Life Outcomes 2021, 19, 251. [Google Scholar] [CrossRef]
- Dodd, M.J.; Miaskowski, C.; Paul, S.M. Symptom clusters and their effect on the functional status of patients with cancer. Oncol. Nurs. Forum 2001, 28, 465–470. [Google Scholar] [PubMed]
- Miaskowski, C.; Barsevick, A.; Berger, A.; Casagrande, R.; Grady, P.A.; Jacobsen, P.; Kutner, J.; Patrick, D.; Zimmerman, L.; Xiao, C.; et al. Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. J. Natl. Cancer Inst. 2017, 109, djw253. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Han, C.J.; Saligan, L.; Wallen, G.R. Comparing symptom clusters in cancer survivors by cancer diagnosis: A latent class profile analysis. Support. Care Cancer 2024, 32, 308. [Google Scholar] [CrossRef] [PubMed]
- Lynch Kelly, D.; Dickinson, K.; Hsiao, C.P.; Lukkahatai, N.; Gonzalez-Marrero, V.; McCabe, M.; Saligan, L.N. Biological basis for the clustering of symptoms. Semin. Oncol. Nurs. 2016, 32, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.T.; Butow, P.N.; Costa, D.S.; Lovell, M.R.; Agar, M. Symptom clusters in patients with advanced cancer: A systematic review of observational studies. J. Pain Symptom Manag. 2014, 48, 411–450. [Google Scholar] [CrossRef]
- Wu, C.J.; Bai, L.Y.; Chen, Y.C.; Wu, C.F.; Lin, K.C.; Wang, Y.J. Symptom clusters in lymphoma survivors before, during, and after chemotherapy: A prospective study. Oncol. Nurs. Forum 2023, 50, 361–371. [Google Scholar] [CrossRef]
- Fang, J.; Wong, C.L.; Liu, C.Q.; Huang, H.Y.; Qi, Y.S.; Xu, L.L.; Wang, M.X.; Lin, Y. Identifying central symptom clusters and correlates in children with acute leukemia undergoing chemotherapy: A network analysis. Front. Oncol. 2023, 13, 1236129. [Google Scholar] [CrossRef]
- Lin, D.M.; Yin, X.X.; Wang, N.; Zheng, W.; Wen, Y.P.; Meng, L.M.; Zhang, L.L. Consensus in identification and stability of symptom clusters using different symptom dimensions in newly diagnosed acute myeloid leukemia patients undergoing induction therapy. J. Pain Symptom Manag. 2019, 57, 783–792. [Google Scholar] [CrossRef]
- Brazauskas, R.; Flynn, K.; Krishnan, A.; Landau, H.; Giralt, S.; Pasquini, M.C.; Stadtmauer, E.A.; D’Souza, A. Symptom clusters and their impact on quality of life in multiple myeloma survivors: Secondary analysis of BMT CTN 0702 trial. Br. J. Haematol. 2024, 204, 1429–1438. [Google Scholar] [CrossRef]
- Chen, F.; Leng, Y.; Ni, J.; Niu, T.; Zhang, L.; Li, J.; Zheng, Y. Symptom clusters and quality of life in ambulatory patients with multiple myeloma. Support. Care Cancer 2022, 30, 4961–4970. [Google Scholar] [CrossRef]
- Zeng, L.; Huang, H.; Qirong, C.; Ruan, C.; Liu, Y.; An, W.; Guo, Q.; Zhou, J. Multiple myeloma patients undergoing chemotherapy: Which symptom clusters impact quality of life? J. Clin. Nurs. 2023, 32, 7247–7259. [Google Scholar] [CrossRef] [PubMed]
- Boyes, A.W.; Clinton-McHarg, T.; Waller, A.E.; Steele, A.; D’Este, C.A.; Sanson-Fisher, R.W. Prevalence and correlates of the unmet supportive care needs of individuals diagnosed with a haematological malignancy. Acta Oncol. 2015, 54, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, S.; Mols, F.; Nijziel, M.R.; Lybeert, M.; van de Poll-Franse, L.V. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin’s and non-Hodgkin’s lymphoma survivors: A systematic review. Ann. Hematol. 2011, 90, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Shreders, A.J.; Niazi, S.K.; Hodge, D.O.; Chimato, N.T.; Kureti, M.; Kirla, N.; Agrawal, A.; Swaika, A.; Gustetic, E.; Foster, R.; et al. Correlation of sociodemographic and clinical parameters with depression and distress in patients with hematologic malignancies. Ann. Hematol. 2018, 97, 519–528. [Google Scholar] [CrossRef]
- Pulgar, A.; Alcala, A.; Reyes Del Paso, G.A. Psychosocial predictors of quality of life in hematological cancer. Behav. Med. 2015, 41, 1–8. [Google Scholar] [CrossRef]
- Tulk, J.; Wurz, A.; Hou, S.H.J.; Bender, J.; Schulte, F.S.M.; Eaton, G.; Chalifour, K.; Garland, S.N. Rural-urban differences in distress, quality of life, and social support among Canadian young adult cancer survivors: A Young Adults with Cancer in Their Prime (YACPRIME) study. J. Rural Health 2024, 40, 121–127. [Google Scholar] [CrossRef]
- Cerni, J.; Rhee, J.; Hosseinzadeh, H. End-of-life cancer care resource utilisation in rural versus urban settings: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 4955. [Google Scholar] [CrossRef]
- Bhatia, S.; Landier, W.; Paskett, E.D.; Peters, K.B.; Merrill, J.K.; Phillips, J.; Osarogiagbon, R.U. Rural-urban disparities in cancer outcomes: Opportunities for future research. J. Natl. Cancer Inst. 2022, 114, 940–952. [Google Scholar] [CrossRef]
- Loughery, J.; Woodgate, R.L. Supportive care needs of rural individuals living with cancer: A literature review. Can. Oncol. Nurs. J. 2015, 25, 157–178. [Google Scholar] [CrossRef]
- Watson, L.; Qi, S.; Link, C.; DeIure, A.; Afzal, A.; Barbera, L. Patient-reported symptom complexity and acute care utilization among patients with cancer: A population-based study using a novel symptom complexity algorithm and observational data. J. Natl. Compr. Canc. Netw. 2023, 21, 173–180. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information. Discharge Abstract Database Metadata. 2024. Available online: www.cihi.ca/en/discharge-abstract-database-dad-metadata (accessed on 21 October 2024).
- Canadian Institute for Health Information. National Ambulatory Care Reporting System Metadata. Available online: www.cihi.ca/en/national-ambulatory-care-reporting-system-nacrs-metadata (accessed on 21 October 2024).
- Ashbury, F.D.; Findlay, H.; Reynolds, B.; McKerracher, K. A Canadian survey of cancer patients’ experiences: Are their needs being met? J. Pain Symptom Manag. 1998, 16, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Bultz, B.D.; Groff, S.L.; Fitch, M.; Blais, M.C.; Howes, J.; Levy, K.; Mayer, C. Implementing screening for distress, the 6th vital sign: A Canadian strategy for changing practice. Psycho-Oncology 2011, 20, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, C.A.; Watson, L.; Xu, Y.; Boyne, D.J.; Hemmelgarn, B.R.; Cheung, W.Y. Patient-reported outcomes in Alberta: Rationale, scope, and design of a database initiative. Curr. Oncol. 2019, 26, e503–e509. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 years later: Past, present, and future developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Watanabe, S.M.; Nekolaichuk, C.L.; Beaumont, C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: Development and refinement. Psycho-Oncology 2012, 21, 977–985. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- World Health Organization. Ageing and Health. Available online: www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 21 October 2024).
- Varian Medical Services. ARIA Oncology Information System. Available online: www.varian.com/products/software/digital-oncology/oncology-management-systems/aria-oncology-information-system (accessed on 21 October 2024).
- Muthen, L.K.; Muthen, B.O. MPlus Statistical Analysis with Latent Variables. User’s Guide. Available online: www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf (accessed on 21 October 2024).
- Barbera, L.; Atzema, C.; Sutradhar, R.; Seow, H.; Howell, D.; Husain, A.; Sussman, J.; Earle, C.; Liu, Y.; Dudgeon, D. Do patient-reported symptoms predict emergency department visits in cancer patients? A population-based analysis. Ann. Emerg. Med. 2013, 61, 427–437.e5. [Google Scholar] [CrossRef]
- IBM SPSS Statistics 29. Available online: www.ibm.com/spss (accessed on 25 October 2024).
- Oldenmenger, W.H.; de Raaf, P.J.; de Klerk, C.; van der Rijt, C.C. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: A systematic review. J. Pain Symptom Manag. 2013, 45, 1083–1093. [Google Scholar] [CrossRef]
- Sutradhar, R.; Rostami, M.; Barbera, L. Patient-reported symptoms improve performance of risk prediction models for emergency department visits among patients with cancer: A population-wide study in Ontario using administrative data. J. Pain Symptom Manag. 2019, 58, 745–755. [Google Scholar] [CrossRef]
- Hammer, M.J.; Cooper, B.; Paul, S.M.; Kober, K.M.; Cartwright, F.; Conley, Y.P.; Wright, F.; Levine, J.D.; Miaskowski, C. Identification of distinct symptom profiles in cancer patients using a pre-specified symptom cluster. J. Pain Symptom Manag. 2022, 64, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C.; Dunn, L.; Ritchie, C.; Paul, S.M.; Cooper, B.; Aouizerat, B.E.; Alexander, K.; Skerman, H.; Yates, P. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J. Pain Symptom Manag. 2015, 50, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Cazzaniga, S. Research techniques made simple: Latent class analysis. J. Investig. Dermatol. 2020, 140, 1676–1680.e1. [Google Scholar] [CrossRef] [PubMed]
- Weller, B.E.; Bowen, N.K.; Faubert, S.J. Latent class analysis: A guide to best practice. J. Black Psychol. 2020, 46, 287–311. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S.; Delucchi, K.L. Practitioner’s guide to latent class analysis: Methodological considerations and common pitfalls. Crit. Care Med. 2021, 49, e63–e79. [Google Scholar] [CrossRef]
- Asparouhov, T.; Muthén, B. Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Struct. Equ. Model. 2014, 21, 329–341. [Google Scholar] [CrossRef]
- Nylund-Gibson, K.; Grimm, R.P.; Masyn, K.E. Prediction from latent classes: A demonstration of different approaches to include distal outcomes in mixture models. Struct. Equ. Model. 2019, 26, 967–985. [Google Scholar] [CrossRef]
- Lanza, S.T.; Tan, X.; Bray, B.C. Latent class analysis with distal outcomes: A flexible model-based approach. Struct. Equ. Model. 2013, 20, 1–26. [Google Scholar] [CrossRef]
- LeBlanc, T.W.; Smith, J.M.; Currow, D.C. Symptom burden of haematological malignancies as death approaches in a community palliative care service: A retrospective cohort study of a consecutive case series. Lancet Haematol. 2015, 2, e334–e338. [Google Scholar] [CrossRef]
- Button, E.; Chan, R.; Chambers, S.; Butler, J.; Yates, P. Signs, symptoms, and characteristics associated with end of life in people with a hematologic malignancy: A review of the literature. Oncol. Nurs. Forum 2016, 43, E178–E187. [Google Scholar] [CrossRef]
- Barbera, L.; Sutradhar, R.; Seow, H.; Mittmann, N.; Howell, D.; Earle, C.C.; Li, Q.; Thiruchelvam, D. The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: Results of a population-based retrospective matched cohort analysis. Cancer Med. 2020, 9, 7107–7115. [Google Scholar] [CrossRef] [PubMed]
- Mierzynska, J.; Piccinin, C.; Pe, M.; Martinelli, F.; Gotay, C.; Coens, C.; Mauer, M.; Eggermont, A.; Groenvold, M.; Bjordal, K.; et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: A systematic review. Lancet Oncol. 2019, 20, e685–e698. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, C.; Maglietta, G.; Diodati, F.; Puntoni, M.; Marcomini, B.; Lazzarelli, S.; Pinto, C.; Perrone, F. The effects of patient-reported outcome screening on the survival of people with cancer: A systematic review and meta-analysis. Cancers 2022, 14, 5470. [Google Scholar] [CrossRef] [PubMed]
- Lizan, L.; Perez-Carbonell, L.; Comellas, M. Additional value of patient-reported symptom monitoring in cancer care: A systematic review of the literature. Cancers 2021, 13, 4615. [Google Scholar] [CrossRef]
- Basch, E.; Barbera, L.; Kerrigan, C.L.; Velikova, G. Implementation of patient-reported outcomes in routine medical care. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 122–134. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef]
- Chan, J.; Polo, A.; Zubizarreta, E.; Bourque, J.M.; Hanna, T.P.; Gaudet, M.; Dennis, K.; Brundage, M.; Slotman, B.; Abdel-Wahab, M. Access to radiotherapy and its association with cancer outcomes in a high-income country: Addressing the inequity in Canada. Radiother. Oncol. 2019, 141, 48–55. [Google Scholar] [CrossRef]
- Henley, S.J.; Jemal, A. Rural cancer control: Bridging the chasm in geographic health inequity. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1248–1251. [Google Scholar] [CrossRef]
- Levit, L.A.; Byatt, L.; Lyss, A.P.; Paskett, E.D.; Levit, K.; Kirkwood, K.; Schenkel, C.; Schilsky, R.L. Closing the rural cancer care gap: Three institutional approaches. JCO Oncol. Pract. 2020, 16, 422–430. [Google Scholar] [CrossRef]
- Canadian Medical Association. Medical Oncology Profile. Available online: www.cma.ca/sites/default/files/2019-01/medical-oncology-e.pdf (accessed on 29 December 2024).
- Chih, M.Y.; McCowan, A.; Whittaker, S.; Krakow, M.; Ahern, D.K.; Aronoff-Spencer, E.; Hesse, B.W.; Mullett, T.W.; Vanderpool, R.C. The landscape of connected cancer symptom management in rural America: A narrative review of opportunities for launching connected health interventions. J. Appalach. Health 2020, 2, 64–81. [Google Scholar]
- Ream, E.; Hughes, A.E.; Cox, A.; Skarparis, K.; Richardson, A.; Pedersen, V.H.; Wiseman, T.; Forbes, A.; Bryant, A. Telephone interventions for symptom management in adults with cancer. Cochrane Database Syst. Rev. 2020, 6, CD007568. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, R.; Sabesan, S.; Thota, R.; Merrill, J.; Hensold, J.O. Revolutionizing rural oncology: Innovative models and global perspectives. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432078. [Google Scholar] [CrossRef] [PubMed]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Han, X.; Zhao, J.; Nogueira, L.; Jemal, A. Rural cancer disparities in the United States: A multilevel framework to improve access to care and patient outcomes. JCO Oncol. Pract. 2020, 16, 409–413. [Google Scholar] [CrossRef]
- Baldwin-Medsker, A.; Holland, J.; Rodriguez, E.S. Access to care: Using eHealth to limit location-based barriers for patients with cancer. Clin. J. Oncol. Nurs. 2020, 24, 16–23. [Google Scholar] [CrossRef]
- Booker, R.; Haase, K.R. Virtual cancer care equity in Canada: Lessons from COVID-19. Clin. J. Oncol. Nurs. 2022, 26, 224–227. [Google Scholar] [CrossRef]
- Sanders, J.J.; Temin, S.; Ghoshal, A.; Alesi, E.R.; Ali, Z.V.; Chauhan, C.; Cleary, J.F.; Epstein, A.S.; Firn, J.I.; Jones, J.A.; et al. Palliative care for patients with cancer: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 2336–2357. [Google Scholar] [CrossRef]
- Franzoi, M.A.; Ferreira, A.R.; Lemaire, A.; Rodriguez, J.; Grosjean, J.; Ribeiro, J.M.; Polastro, L.; Grellety, T.; Artignan, X.; Le Du, K.; et al. Implementation of a remote symptom monitoring pathway in oncology care: Analysis of real-world experience across 33 cancer centres in France and Belgium. Lancet Reg. Health Eur. 2024, 44, 101005. [Google Scholar] [CrossRef]
- Maguire, R.; McCann, L.; Kotronoulas, G.; Kearney, N.; Ream, E.; Armes, J.; Patiraki, E.; Furlong, E.; Fox, P.; Gaiger, A.; et al. Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ 2021, 374, n1647. [Google Scholar] [CrossRef]
- Mooney, K.; Iacob, E.; Wilson, C.M.; Lloyd, J.; Nielson, H.; Ward, J.H. Randomized trial of remote cancer symptom monitoring during COVID-19: Impact on symptoms, QoL, and unplanned health care utilization. J. Clin. Oncol. 2021, 39, 12000. [Google Scholar] [CrossRef]
- Chen, L.; Bartel, C.; Cai, X.; Cheng, Y.; Perer, A.; McClaine, S.; Kairis, E.; Durica, K.; Huang, W.; Low, C.A. Patient and provider perspectives on symptom monitoring during outpatient chemotherapy: Interview study. JMIR Form. Res. 2023, 7, e46001. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.S.; Henderson, N.L.; Hendrix, E.K.; Padalkar, T.; Huang, C.S.; Dent, D.N.; Ingram, S.A.; McGowan, C.; Odom, J.N.; Kaufmann, T.; et al. Patient-perceived benefits and limitations of standard of care remote symptom monitoring during cancer treatment. Cancer Control 2024, 31, 10732748241297368. [Google Scholar] [CrossRef] [PubMed]
- Tinsley-Vance, S.M.; Durosier Mertilus, D.S.; Nodzon, L.; Lengacher, C.A. An integrative review of sex differences in quality of life and symptoms among survivors of hematologic malignancies. Oncol. Nurs. Forum 2023, 50, 299–312. [Google Scholar] [PubMed]
- Ebraheem, M.S.; Seow, H.; Balitsky, A.K.; Pond, G.R.; Wildes, T.M.; Sivapathasundaram, B.; Sussman, J.; Mian, H. Trajectory of symptoms in patients undergoing autologous stem cell transplant for multiple myeloma: A population-based cohort study of patient-reported outcomes. Clin. Lymphoma Myeloma Leuk. 2021, 21, e714–e721. [Google Scholar] [CrossRef]
- Morse, L.; Cooper, B.A.; Ritchie, C.S.; Wong, M.L.; Kober, K.M.; Harris, C.; Shin, J.; Oppegaard, K.; Hammer, M.J.; Schimmel, A.C.; et al. Stability and consistency of symptom clusters in younger versus older patients receiving chemotherapy. BMC Geriatr. 2024, 24, 164. [Google Scholar] [CrossRef]
- Flannery, M.A.; Culakova, E.; Canin, B.E.; Peppone, L.; Ramsdale, E.; Mohile, S.G. Understanding treatment tolerability in older adults with cancer. J. Clin. Oncol. 2021, 39, 2150–2163. [Google Scholar] [CrossRef]
- Gaudernack, H.E.; Hareide, M.M.; Miaskowski, C.; Ritchie, C.; Løyland, B.; Grov, E.K.; Paul, S.M.; Torstveit, A.H.; Utne, I. Symptom experience of older oncology patients with low versus high levels of multimorbidity prior to chemotherapy. Eur. J. Oncol. Nurs. 2021, 54, 102029. [Google Scholar] [CrossRef]
- Cataldo, J.K.; Paul, S.; Cooper, B.; Skerman, H.; Alexander, K.; Aouizerat, B.; Blackman, V.; Merriman, J.; Dunn, L.; Ritchie, C.; et al. Differences in the symptom experience of older versus younger oncology outpatients: A cross-sectional study. BMC Cancer 2013, 13, 6. [Google Scholar] [CrossRef]
- Goerling, U.; Ernst, J.; Esser, P.; Haering, C.; Hermann, M.; Hornemann, B.; Hovel, P.; Keilholz, U.; Kissane, D.; von dem Knesebeck, O.; et al. Estimating the prevalence of mental disorders in patients with newly diagnosed cancer in relation to socioeconomic status: A multicenter prospective observational study. ESMO Open 2024, 9, 103655. [Google Scholar] [CrossRef]
- Wu, H.S.; Harden, J.K. Symptom burden and quality of life in survivorship: A review of the literature. Cancer Nurs. 2015, 38, E29–E54. [Google Scholar] [CrossRef]
- Nipp, R.D.; El-Jawahri, A.; Moran, S.M.; D’Arpino, S.M.; Johnson, P.C.; Lage, D.E.; Wong, R.L.; Pirl, W.F.; Traeger, L.; Lennes, I.T.; et al. The relationship between physical and psychological symptoms and health care utilization in hospitalized patients with advanced cancer. Cancer 2017, 123, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Didwaniya, N.; Vidal, M.; Shin, S.H.; Chisholm, G.; Roquemore, J.; Bruera, E. Quality of end-of-life care in patients with hematologic malignancies: A retrospective cohort study. Cancer 2014, 120, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.; Parker, C.; Opat, S.; Shortt, J.; Poon, P. Hematologic malignancy: Who cares in the end? A retrospective cohort study of markers of quality end-of-life care. J. Natl. Compr. Canc. Netw. 2023, 21, 813–820.e1. [Google Scholar] [CrossRef] [PubMed]
- Phung, Q.H.; Masel, R.H.; Bijlani, S.; Tanzer, J.R.; Hsu, A.; Almhanna, K. Characterizing after-hours hematology/oncology clinic calls. Ann. Palliat. Med. 2024, 13, 93–100. [Google Scholar] [CrossRef]
- Grewal, K.; Krzyzanowska, M.K.; McLeod, S.; Borgundvaag, B.; Atzema, C.L. Outcomes after emergency department use in patients with cancer receiving chemotherapy in Ontario, Canada: A population-based cohort study. CMAJ Open 2020, 8, E496–E505. [Google Scholar] [CrossRef]
- Cui, J.; Fang, P.; Bai, J.; Tan, L.; Wan, C.; Yu, L. Meta-analysis of effects of early palliative care on health-related outcomes among advanced cancer patients. Nurs. Res. 2023, 72, E180–E190. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.L.; Perry, L.M.; Hoerger, M. Summarizing the evidence base for palliative oncology care: A critical evaluation of the meta-analyses. Clin. Med. Insights Oncol. 2020, 14, 1179554920915722. [Google Scholar] [CrossRef]
- Elliott, E.; Watson, T.; Singh, D.; Wong, C.; Lo, S.S. Outcomes of specialty palliative care interventions for patients with hematologic malignancies: A systematic review. J. Pain Symptom Manag. 2021, 62, 863–875. [Google Scholar] [CrossRef]
- Fulton, J.J.; LeBlanc, T.W.; Cutson, T.M.; Porter Starr, K.N.; Kamal, A.; Ramos, K.; Freiermuth, C.E.; McDuffie, J.R.; Kosinski, A.; Adam, S.; et al. Integrated outpatient palliative care for patients with advanced cancer: A systematic review and meta-analysis. Palliat. Med. 2019, 33, 123–134. [Google Scholar] [CrossRef]
- Seow, H.; Barbera, L.C.; McGrail, K.; Burge, F.; Guthrie, D.M.; Lawson, B.; Chan, K.K.W.; Peacock, S.J.; Sutradhar, R. Effect of early palliative care on end-of-life health care costs: A population-based, propensity score-matched cohort study. JCO Oncol. Pract. 2022, 18, e183–e192. [Google Scholar] [CrossRef]
- Tsang, M.; Bischoff, K.; Schoenbeck, K.L.; Berry, K.; O’Riordan, D.; Fakhri, B.; Wong, S.W.; Shah, N.; Olin, R.; Andreadis, C.; et al. Value of embedded palliative care: Outpatient palliative care and health care utilization for patients with hematologic malignancies. Blood Adv. 2023, 7, 3146–3149. [Google Scholar] [CrossRef] [PubMed]
- Arenare, L.; Di Liello, R.; De Placido, P.; Gridelli, C.; Morabito, A.; Pignata, S.; Nuzzo, F.; Avallone, A.; Maiello, E.; Gargiulo, P.; et al. Under-reporting of subjective symptoms and its prognostic value: A pooled analysis of 12 cancer clinical trials. ESMO Open 2024, 9, 102941. [Google Scholar] [CrossRef] [PubMed]
- Hannon, B.; Dyck, M.; Pope, A.; Swami, N.; Banerjee, S.; Mak, E.; Bryson, J.; Rodin, G.; Ridley, J.; Lo, C.; et al. Modified Edmonton Symptom Assessment System including constipation and sleep: Validation in outpatients with cancer. J. Pain Symptom Manag. 2015, 49, 945–952. [Google Scholar] [CrossRef]
- Martin, M.L.; Chung, H.; Rydén, A. Willingness to report treatment-related symptoms of immunotherapy among patients with non-small cell lung cancer. Qual. Life Res. 2022, 31, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Booker, R.; McLennan, A.I.G.; Beattie, S.; Stajduhar, K.I.; Sawatzky, R. Integrating palliative care in hematopoietic stem cell transplantation: A qualitative study exploring patient, caregiver, and clinician perspectives. Oncol. Nurs. Forum 2023, 50, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, M.; Basch, E.; Bryce, J.; Perrone, F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 319–325. [Google Scholar] [CrossRef]
- Harsanyi, H.; Cuthbert, C.; Schulte, F. The stigma surrounding opioid use as a barrier to cancer-pain management: An overview of experiences with fear, shame, and poorly controlled pain in the context of advanced cancer. Curr. Oncol. 2023, 30, 5835–5848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makhlouf, S.M.; Pini, S.; Ahmed, S.; Bennett, M.I. Managing pain in people with cancer-a systematic review of the attitudes and knowledge of professionals, patients, caregivers and public. J. Cancer Educ. 2020, 35, 214–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, B.L.; Lacchetti, C.; Ashing, K.; Berek, J.S.; Berman, B.S.; Bolte, S.; Dizon, D.S.; Given, B.; Nekhlyudov, L.; Pirl, W.; et al. Management of anxiety and depression in adult survivors of cancer: ASCO guideline update. J. Clin. Oncol. 2023, 41, 3426–3453. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Hansen, C.H.; Martin, P.; Symeonides, S.; Ramessu, R.; Murray, G.; Sharpe, M. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: A cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014, 1, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fisch, M.J.; Zhao, F.; Manola, J.; Miller, A.H.; Pirl, W.F.; Wagner, L.I. Patterns and predictors of antidepressant use in ambulatory cancer patients with common solid tumors. Psychooncology 2015, 24, 523–532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed, F.; Uvais, N.A.; Moideen, S.; Cp, R.R.; Saif, M. Psychotropic medication prescriptions for home-based palliative care oncology patients. Prim. Care Companion CNS Disord. 2024, 26, 23m03668. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Caruso, R.; Riba, M.B.; Lloyd-Williams, M.; Kissane, D.; Rodin, G.; McFarland, D.; Campos-Ródenas, R.; Zachariae, R.; Santini, D.; et al. Anxiety and depression in adult cancer patients: ESMO clinical practice guideline. ESMO Open 2023, 8, 101155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perna, G.; Pinto, E.; Spiti, A.; Torti, T.; Cucchi, M.; Caldirola, D. Foundations for a personalized psycho-oncology: The state of the art. J. Pers. Med. 2024, 14, 892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basch, E.; Schrag, D.; Henson, S.; Jansen, J.; Ginos, B.; Stover, A.M.; Carr, P.; Spears, P.A.; Jonsson, M.; Deal, A.M.; et al. Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer: A randomized clinical trial. JAMA 2020, 327, 2413–2422. [Google Scholar] [CrossRef]
- Emery, J.; Butow, P.; Lai-Kwon, J.; Nekhlyudov, L.; Rynderman, M.; Jefford, M. Management of common clinical problems experienced by survivors of cancer. Lancet 2022, 399, 1537–1550. [Google Scholar] [CrossRef]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Prochnicki, M.; Rudzki, S.; Laskowska, B.; Brudniak, J. Quality of life of cancer patients treated with chemotherapy. Int. J. Environ. Res. Public Health 2020, 17, 6938. [Google Scholar] [CrossRef]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C.I.; ESMO Guidelines Committee. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef]
- Balitsky, A.K.; Rayner, D.; Britto, J.; Lionel, A.C.; Ginsberg, L.; Cho, W.; Wilfred, A.M.; Sardar, H.; Cantor, N.; Mian, H.; et al. Patient-reported outcome measures in cancer care: An updated systematic review and meta-analysis. JAMA Netw. Open 2024, 7, e2424793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Higgins, C.M.; Brady, B.; O’Connor, B.; Walsh, D.; Reilly, R.B. The pathophysiology of cancer-related fatigue: Current controversies. Support. Care Cancer 2018, 10, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, K.L.; Tostrud, L.; Costanzo, E.; Coe, C.L.; Serlin, R.C.; Ward, S.E.; Zhang, Y. The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. J. Pain Symptom Manag. 2018, 55, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Nilsberth, C.; Sackmann, V.; Fransson, K.; Jakobsson, M.; Karlsson, M.; Milberg, A. Symptom clusters in palliative-stage cancer correlate with proinflammatory cytokine cluster. Ann. Palliat. Med. 2023, 12, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front. Endocrinol. 2019, 8, 788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Booker, R.; Olson, K.; Pilarski, L.M.; Noon, J.P.; Bahlis, N.J. The relationships among physiologic variables, quality of life, and fatigue in patients with multiple myeloma. Oncol. Nurs. Forum 2009, 36, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, A.I.; Jim, H.S.L.; Gonzalez, B.D.; Small, B.J.; Gilvary, D.; Breen, E.C.; Bower, J.E.; Fishman, M.; Zachariah, B.; Jacobsen, P.B. Systemic inflammation and symptomatology in patients with prostate cancer treated with androgen deprivation therapy: Preliminary findings. Cancer 2021, 127, 1476–1482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, L.; Murata, K.; Holder, H.; Marcello, L.; Cho, S.; Hoover, E.; Chung, D.J.; Bahi, P.B.; Lahoud, O.B.; Scordo, M.; et al. Improved quality of life with interleukin-6 (IL-6) blockade with siltuximab peri-autologous hematopoietic stem cell transplantation (AHCT) in older patients with multiple myeloma (MM). Biol. Blood Marrow Transplant. 2020, 26, S129–S130. [Google Scholar] [CrossRef]
- Patton, R.; Paval, D.R.; McDonald, J.J.; Brown, D.; Gallagher, I.J.; Skipworth, R.J.E.; McMillan, D.C.; Dolan, R.D.; Fallon, M.; Laird, B.J.A. Relationship between cytokines and symptoms in people with incurable cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 159, 103222. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Walton, R.; Kataria, S.P. Chemotherapy-induced nausea and vomiting: Pathogenesis, recommendations, and new trends. Cancer Treat. Res. Commun. 2021, 26, 100278. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M. Nausea and vomiting in advanced cancer. Curr. Treat. Options Oncol. 2020, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Karstens-Smith, B. A Timeline of COVID-19 in Alberta. Global News. 28 December 2020. Available online: https://globalnews.ca/news/7538547/covid-19-alberta-health-timeline/ (accessed on 21 October 2024).
- Watson, L.; Qi, S.; Delure, A.; Link, C.; Photitai, E.; Chmielewski, L.; Hildebrand, A.; Ruether, D.; Rawson, K. Virtual cancer care during the COVID-19 pandemic in Alberta: Evidence from a mixed methods evaluation and key learnings. JCO Oncol. Pract. 2021, 17, e1354–e1361. [Google Scholar] [CrossRef]
| Variable | Cohort A: No PROMS | Cohort B: PROMs | p | |
|---|---|---|---|---|
| N = 944 (%) | N = 6136 (%) | |||
| Age | ≤60 | 352 (37.3) | 2084 (34.0) | 0.045 |
| >60 | 592 (62.7) | 4052 (66.0) | ||
| Sex | Male | 513 (54.3) | 3542 (57.7) | 0.051 |
| Female | 431 (45.7) | 2594 (42.3) | ||
| Type of HM | Leukemia | 261 (27.6) | 1605 (26.2) | <0.001 |
| HL | 56 (5.9) | 373 (6.1) | ||
| NHL | 357 (37.8) | 2260 (36.8) | ||
| MM | 84 (8.9) | 1022 (16.7) | ||
| IP diseases | 48 (5.1) | 340 (5.5) | ||
| Other | 138 (14.6) | 535 (8.7) | ||
| CCI a | 0 | 732 (77.5) | 5200 (84.7) | <0.001 |
| 1 | 99 (10.5) | 524 (8.5) | ||
| ≥2 | 113 (12.0) | 412 (6.7) | ||
| Rurality | Rural | 237 (25.2) | 1297 (21.2) | 0.006 |
| Urban | 705 (74.8) | 4828 (78.8) | ||
| ED Visits | None | 499 (52.9) | 3632 (59.2) | <0.001 |
| Number of ED Visits a | 1–3 | 342 (36.2) | 2044 (33.3) | |
| 4–6 | 59 (6.3) | 302 (4.9) | ||
| 7+ | 44 (4.7) | 158 (2.6) | ||
| Hospital Admissions | None | 680 (72.0) | 4691 (76.5) | <0.001 |
| Number of HAs a | 1–3 | 228 (24.2) | 1283 (20.9) | |
| 4–6 | 27 (2.9) | 144 (2.3) | ||
| 7+ | 9 (1.0) | 18 (0.3) | ||
| Deceased | No | 829 (87.8) | 5691 (92.7) | <0.001 |
| Yes | 115 (12.2) | 445 (7.3) | ||
| Variable | N = 432 | % | |
|---|---|---|---|
| Age | ≤60 | 57 | 13.2 |
| >60 | 375 | 86.8 | |
| Sex | Male | 273 | 63.2 |
| Female | 159 | 36.8 | |
| Type of HM | Acute leukemia | 61 | 14.1 |
| Chronic leukemia | 39 | 9.0 | |
| Lymphoma | 177 | 41.0 | |
| Multiple myeloma | 107 | 24.8 | |
| Myelodysplastic syndrome | 34 | 7.9 | |
| Other | 14 | 3.2 | |
| CCI | 0 | 269 | 62.3 |
| 1 | 90 | 20.8 | |
| ≥2 | 73 | 16.9 | |
| Rurality | Urban | 332 | 76.9 |
| Rural | 100 | 23.1 | |
| Marital Status | Not married | 148 | 34.3 |
| Married | 284 | 65.7 | |
| ED Visits | None | 45 | 10.4 |
| Number of ED Visits a | 1–3 | 256 | 59.3 |
| 4–6 | 87 | 20.1 | |
| 7+ | 44 | 10.2 | |
| Reasons for ED Visits | Symptom management | 207 | 47.9 |
| Disease/treatment | 33 | 7.6 | |
| Complications | 111 | 25.7 | |
| Other | 35 | 8.1 | |
| n/a | 46 | 10.6 | |
| Hospital Admissions | None | 43 | 10.0 |
| Number of HAs a | 1–3 | 326 | 75.5 |
| 4–6 | 56 | 13.0 | |
| 7+ | 7 | 1.6 | |
| Reasons for HAs | Symptom management | 50 | 11.6 |
| Disease/treatment | 9 | 2.1 | |
| Complications | 292 | 67.6 | |
| Other | 38 | 8.8 | |
| n/a | 43 | 10.0 | |
| Bone Marrow/Stem Cell Transplant | No | 358 | 82.9 |
| Yes | 74 | 17.1 | |
| Lines of Treatment a | None | 54 | 12.5 |
| 1–3 | 247 | 57.2 | |
| 4–6 | 95 | 22.0 | |
| 7+ | 36 | 8.3 | |
| Supportive Care Medications | No | 171 | 39.6 |
| Yes | 261 | 60.4 | |
| Opioid pain medication | 187 b | 43.3 | |
| Non-opioid pain medication | 97 | 22.5 | |
| Anxiolytic | 29 | 6.7 | |
| Antidepressant | 103 | 23.8 | |
| Progressive Disease c | No | 253 | 58.6 |
| Yes | 179 | 41.4 | |
| Place of Death | Hospital | 278 | 64.4 |
| Home | 82 | 19.0 | |
| Hospice | 51 | 11.8 | |
| Other | 12 | 2.8 | |
| Unknown | 9 | 2.1 | |
| k | BIC | Entropy | VLMR | BLRT | P: Class 1 | P: Class 2 | P: Class 3 | P: Class 4 | P: Class 5 | P: Class 6 | P: Class 7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 118,983.50 | n/a | n/a | n/a | 1 | ||||||
| 2 | 104,160.70 | 0.86 | p < 0.01 | p < 0.01 | 0.44 | 0.56 | |||||
| 3 | 99,493.40 | 0.83 | p < 0.01 | p < 0.01 | 0.26 | 0.40 | 0.34 | ||||
| 4 | 97,730.51 | 0.84 | p < 0.01 | p < 0.01 | 0.14 | 0.25 | 0.38 | 0.23 | |||
| 5 | 96,279.90 | 0.83 | p < 0.01 | p < 0.01 | 0.14 | 0.25 | 0.29 | 0.13 | 0.20 | ||
| 6 | 95,792.97 | 0.83 | p < 0.01 | p < 0.01 | 0.12 | 0.24 | 0.19 | 0.07 | 0.11 | 0.28 | |
| 7 | 95,402.23 | 0.82 | p = 0.70 | p < 0.01 | 0.08 | 0.11 | 0.25 | 0.08 | 0.12 | 0.21 | 0.15 |
| k | BIC | Entropy | VLMR | BLRT | P: Class 1 | P: Class 2 | P: Class 3 | P: Class 4 |
|---|---|---|---|---|---|---|---|---|
| 1 | 9798.99 | n/a | n/a | n/a | 1 | |||
| 2 | 9014.76 | 0.87 | p < 0.01 | p < 0.01 | 0.44 | 0.56 | ||
| 3 | 8842.29 | 0.86 | p = 0.01 | p < 0.01 | 0.24 | 0.37 | 0.38 | |
| 4 | 8835.93 | 0.86 | p = 0.78 | p < 0.01 | 0.32 | 0.24 | 0.21 | 0.23 |
| Variable | Class 1 Prevalent, Moderate/ Severe Symptoms | Class 2 Prevalent, Mild/ Moderate Symptoms | Class 3 Prevalent, Mostly Mild/Moderate Symptoms, Including Anxiety/ Depression | Class 4 Prevalent, Moderate/ Severe Tiredness, Impaired Well-Being, Drowsiness | Class 5 Prevalent, Mostly Mild Physical Symptoms Except Nausea, Lack of Appetite | Class 6 Less Prevalent, Mild Symptoms | x2 |
|---|---|---|---|---|---|---|---|
| n = 397 (6.5%) % | n = 639 (10.5%) % | n = 720 (11.8%) % | n = 1174 (19.2%) % | n = 1707 (28.0%) % | n = 1470 (24.1%) % | p | |
| Sex | |||||||
| Male | 39.7 | 54.8 | 62.5 | 51.5 | 58.0 | 66.4 | 101.66 |
| Female | 60.3 | 45.2 | 37.5 | 48.5 | 42.0 | 33.6 | p < 0.001 |
| Age | |||||||
| ≤60 | 30.3 | 32.2 | 35.4 | 28.9 | 34.6 | 38.6 | 25.11 |
| >60 | 69.7 | 67.8 | 64.6 | 71.1 | 65.4 | 61.4 | p < 0.001 |
| Type of HM | |||||||
| Leukemia | 4.4 | 3.9 | 4.1 | 5.2 | 6.4 | 8.7 | 90.32 |
| HL | 35.5 | 35.3 | 35.1 | 36.4 | 37.4 | 38.4 | p < 0.001 |
| NHL | 24.6 | 24.4 | 22.9 | 27.6 | 26.0 | 27.9 | |
| MM | 16.5 | 19.9 | 24.0 | 14.8 | 18.0 | 11.6 | |
| IP | 6.8 | 6.6 | 5.6 | 6.4 | 4.4 | 5.5 | |
| Other | 12.2 | 10.0 | 8.3 | 9.6 | 7.7 | 7.8 | |
| CCI | |||||||
| 0–1 | 86.4 | 87.2 | 94.0 | 92.2 | 95.5 | 95.9 | 64.72 |
| 2+ | 13.6 | 12.8 | 6.0 | 7.8 | 4.5 | 4.1 | p < 0.001 |
| Rurality | |||||||
| Urban | 78.3 | 80.0 | 81.4 | 78.0 | 78.2 | 78.5 | 3.56 |
| Rural | 21.7 | 20.0 | 18.6 | 22.0 | 21.8 | 21.5 | p = 0.62 |
| ED Visits | |||||||
| No | 43.9 | 43.0 | 55.8 | 51.5 | 65.3 | 71.7 | 235.28 |
| Yes | 56.1 | 57.0 | 44.2 | 48.5 | 34.7 | 28.3 | p < 0.001 |
| Number of ED Visits | |||||||
| None | 44.0 | 43.0 | 55.8 | 51.4 | 65.3 | 71.7 | 252.70 |
| 1–3 | 41.2 | 43.6 | 37.1 | 38.8 | 29.5 | 24.5 | p < 0.001 |
| 4–6 | 9.2 | 8.4 | 5.2 | 6.4 | 3.6 | 2.4 | |
| 7+ | 5.6 | 5.0 | 1.9 | 3.3 | 1.6 | 1.4 | |
| Hospital Admissions | |||||||
| No | 60.6 | 61.9 | 72.5 | 73.6 | 80.1 | 87.5 | 223.42 |
| Yes | 39.4 | 38.1 | 27.5 | 26.4 | 19.9 | 12.5 | p < 0.001 |
| Number of HAs | |||||||
| None | 60.5 | 62.0 | 72.5 | 73.7 | 80.0 | 87.5 | 230.43 |
| 1–3 | 31.9 | 33.1 | 25.1 | 23.1 | 18.3 | 11.5 | p < 0.001 |
| 4–6 | 6.8 | 4.7 | 2.2 | 2.8 | 1.5 | 0.8 | |
| 7+ | 0.9 | 0.3 | 0.3 | 0.4 | 0.2 | 0.2 | |
| Deceased | |||||||
| No | 94.1 | 91.6 | 93.2 | 91.6 | 92.6 | 93.7 | 5.01 |
| Yes | 5.9 | 8.4 | 6.8 | 8.4 | 7.4 | 6.3 | p = 0.415 |
| Variable | Class 1 Prevalent, Moderate/ Severe Symptoms | Class 2 Prevalent, Mild/ Moderate Symptoms | Class 3 Less Prevalent Mild/Moderate Physical Symptoms; Less Prevalent Anxiety, Depression | x2 |
|---|---|---|---|---|
| n = 161 (37.4%) % | n = 105 (24.4%) % | N = 165 (38.3%) % | p | |
| Sex | ||||
| Male | 58.3 | 69.0 | 63.7 | 2.10 |
| Female | 41.7 | 31.0 | 36.3 | p = 0.350 |
| Age | ||||
| ≤60 | 15.9 | 8.8 | 13.5 | 1.35 |
| >60 | 84.1 | 91.2 | 86.5 | p = 0.510 |
| Type of HM | ||||
| Acute Leukemia | 10.9 | 16.1 | 16.4 | 11.95 |
| Chronic Leukemia | 11.6 | 6.8 | 7.8 | p = 0.289 |
| Lymphoma | 37.5 | 36.5 | 47.2 | |
| Multiple Myeloma | 25.3 | 32.8 | 19.6 | |
| Myelodysplastic Syndrome | 11.0 | 7.0 | 4.7 | |
| Other | 3.0 | 0.7 | 4.3 | |
| Rurality | ||||
| Urban | 75.6 | 76.1 | 78.2 | 0.28 |
| Rural | 24.4 | 23.9 | 21.8 | p = 0.870 |
| Marital Status | ||||
| Not Married | 36.6 | 26.1 | 36.8 | 2.03 |
| Married | 63.4 | 73.9 | 63.2 | p = 0.363 |
| ED Visits | ||||
| No | 10.5 | 12.0 | 8.3 | 0.78 |
| Yes | 89.5 | 88.0 | 91.7 | p = 0.677 |
| Number of ED Visits | ||||
| None | 10.4 | 13.0 | 8.3 | 5.99 |
| 1–3 | 61.0 | 55.9 | 60.4 | p = 0.424 |
| 4–6 | 16.0 | 19.4 | 24.3 | |
| 7+ | 12.6 | 11.7 | 7.0 | |
| HAs | ||||
| No | 10.6 | 7.8 | 10.8 | 0.47 |
| Yes | 89.4 | 92.2 | 89.2 | p = 0.791 |
| Number of HAs | ||||
| None | 10.6 | 7.8 | 10.8 | 1.32 |
| 1–3 | 72.8 | 80.4 | 74.6 | p = 0.970 |
| 4–6 | 15.0 | 9.8 | 13.1 | |
| 7+ | 1.6 | 1.9 | 1.4 | |
| Supportive Care Medications | ||||
| No | 37.3 | 55.6 | 61.7 | 17.74 |
| Yes | 62.7 | 44.4 | 38.3 | p < 0.001 |
| Progressive Disease | ||||
| No | 60.8 | 62.5 | 54.5 | 1.40 |
| Yes | 39.2 | 37.5 | 45.5 | p = 0.496 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Booker, R.; Sawatzky, R.; Sinnarajah, A.; Qi, S.; Link, C.; Watson, L.; Stajduhar, K. Symptoms, Symptom Profiles, and Healthcare Utilization in Patients with Hematologic Malignancies: A Retrospective Observational Cohort Study and Latent Class Analysis. Curr. Oncol. 2025, 32, 62. https://doi.org/10.3390/curroncol32020062
Booker R, Sawatzky R, Sinnarajah A, Qi S, Link C, Watson L, Stajduhar K. Symptoms, Symptom Profiles, and Healthcare Utilization in Patients with Hematologic Malignancies: A Retrospective Observational Cohort Study and Latent Class Analysis. Current Oncology. 2025; 32(2):62. https://doi.org/10.3390/curroncol32020062
Chicago/Turabian StyleBooker, Reanne, Richard Sawatzky, Aynharan Sinnarajah, Siwei Qi, Claire Link, Linda Watson, and Kelli Stajduhar. 2025. "Symptoms, Symptom Profiles, and Healthcare Utilization in Patients with Hematologic Malignancies: A Retrospective Observational Cohort Study and Latent Class Analysis" Current Oncology 32, no. 2: 62. https://doi.org/10.3390/curroncol32020062
APA StyleBooker, R., Sawatzky, R., Sinnarajah, A., Qi, S., Link, C., Watson, L., & Stajduhar, K. (2025). Symptoms, Symptom Profiles, and Healthcare Utilization in Patients with Hematologic Malignancies: A Retrospective Observational Cohort Study and Latent Class Analysis. Current Oncology, 32(2), 62. https://doi.org/10.3390/curroncol32020062





