Causative Genes of Homologous Recombination Deficiency (HRD)-Related Breast Cancer and Specific Strategies at Present
Abstract
:1. Introduction
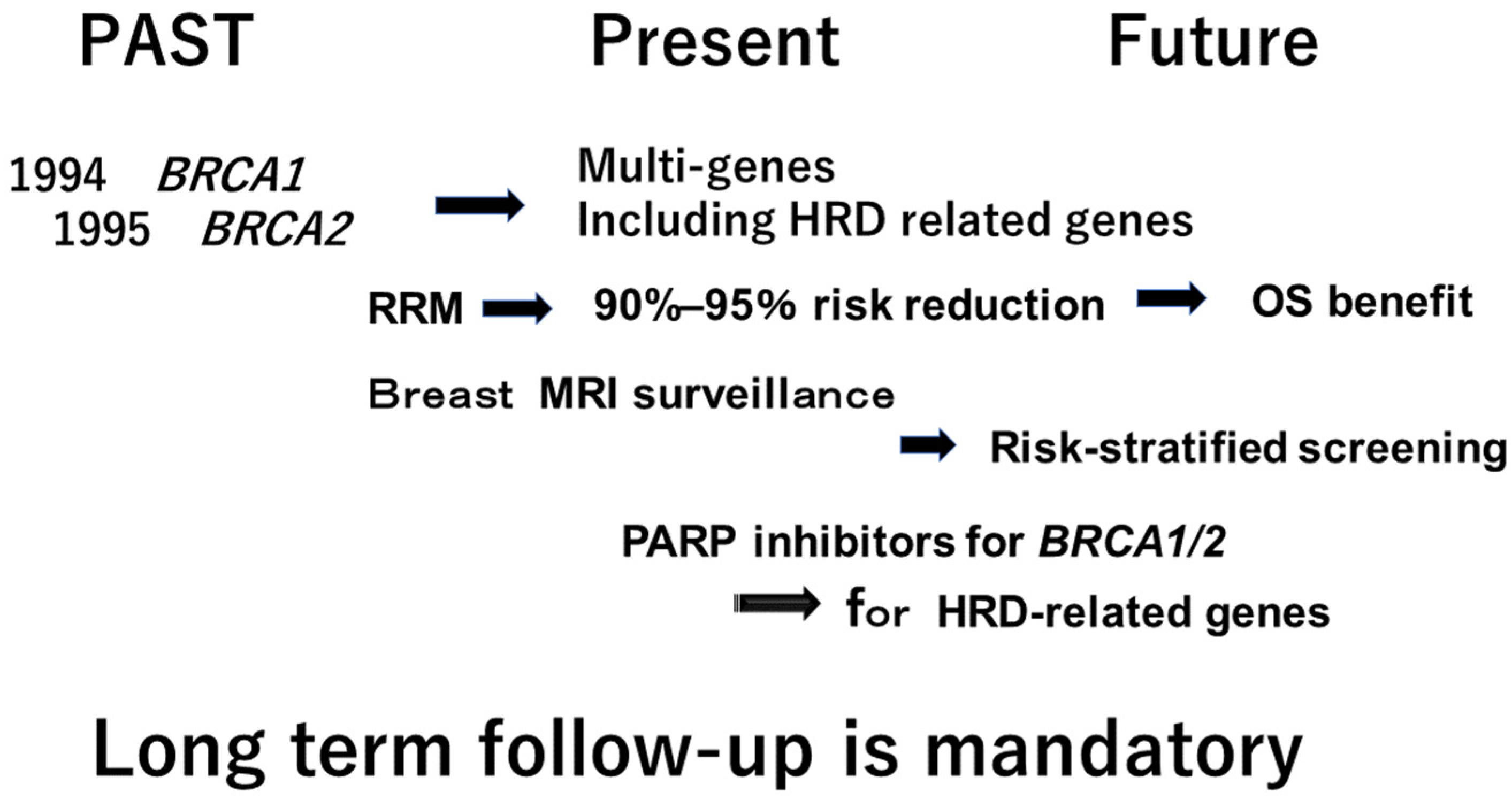
2. Multi-Gene Panel Testing for Hereditary Breast Cancer
- Offer BRCA1/2 testing to all patients diagnosed with breast cancer at or below age 65.
- Testing for other high penetrance genes, such as PALB2, TP53, PTEN, STK11, and CDH1, should be offered to appropriate patients, as mutations in these genes could inform medical treatment, influence surgical decision making, refine estimates of second primary cancer risks, and inform family risk assessment.
- Testing for moderate penetrance genes, such as ATM, CHEK2, RAD51C, RAD51D and BARD1, may be offered to appropriate patients who are undergoing BRCA1/2 testing.
- While mutations in these genes may inform the risks of second primary cancer or family risk assessment, they currently offer no treatment benefits for breast cancer patients.
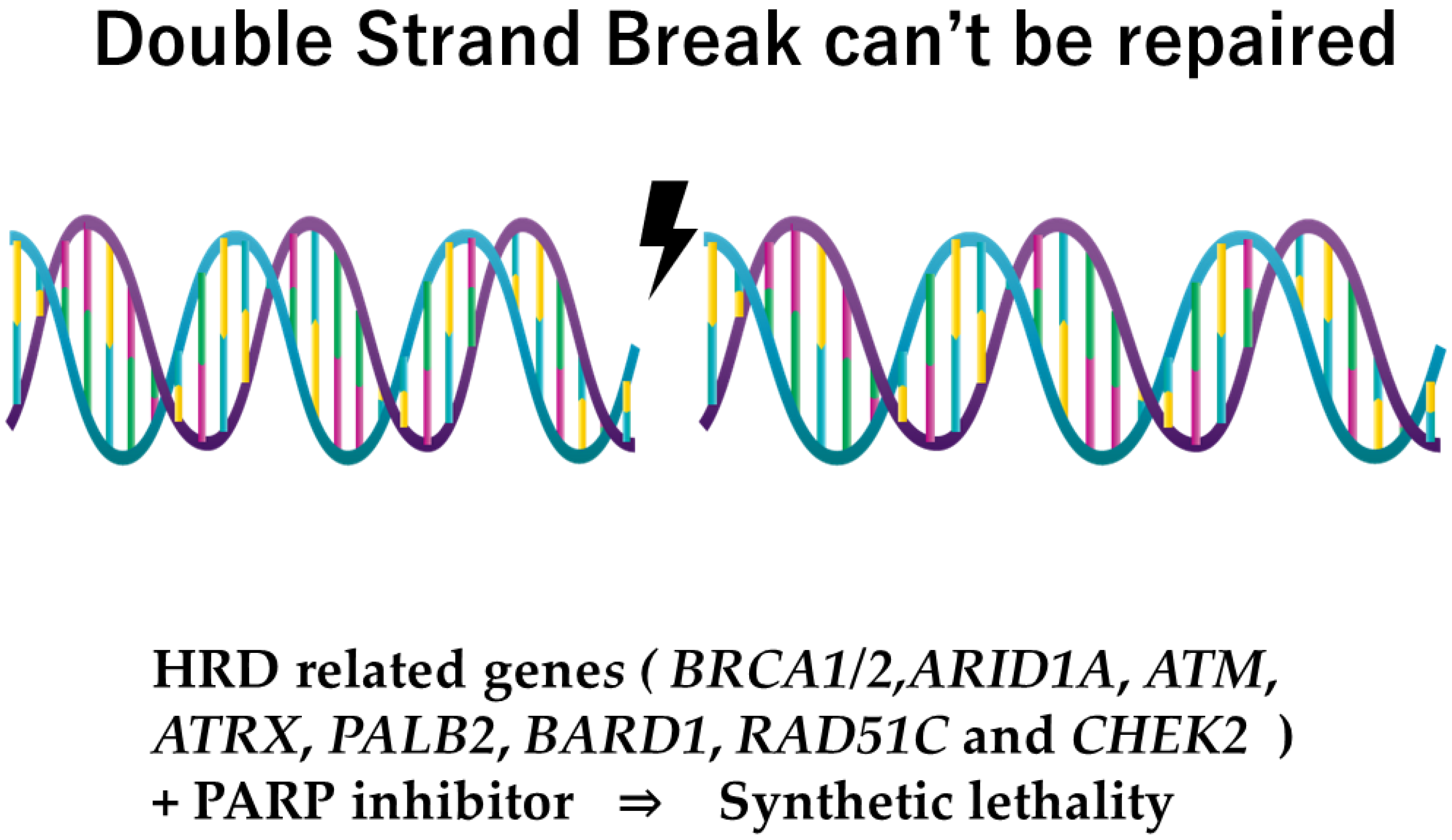
- Nowadays, the genes relating to homologous repair recombination (HRR) divide into one group, known as homologous repair deficiency (HRD), that refers to a dysfunction of HRR, or, in other words, to a group for which the PARP inhibitor will have the possibility of efficacy.
3. Poly (Adenosine Diphosphate-Ribose) Polymerase Inhibitors (PARP Inhibitor) for Hereditary Breast Cancer
4. Surveillance for Other Cancers
5. Risk -Stratified Screening for Breast Cancer in the Future
6. Significance of Multi-Disciplinary Approach
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G.; et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, Å.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pal, T.; Maxwell, K.N.; Churpek, J.; Kohlmann, W.; AlHilli, Z.; Arun, B.; Buys, S.S.; Cheng, H.; Domchek, S.M.; et al. NCCN Guidelines Genetic/Familial High-Risk Assessment: Breast, Ovarian, Pancreatic, and Prostate. 2.2024 ed. USA. J. Natl. Compr. Cancer Network 2023, 21, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Bismeijer, T.; van der Velden, B.H.M.; Canisius, S.; Lips, E.H.; Loo, C.E.; Viergever, M.A.; Wesseling, J.; Gilhuijs, K.G.A.; Wessels, L.F.A. Radiogenomic Analysis of Breast Cancer by Linking MRI Phenotypes with Tumor Gene Expression. Radiology 2020, 296, 277–287. [Google Scholar] [CrossRef]
- Geuzinge, H.A.; Obdeijn, I.-M.; Rutgers, E.J.T.; Saadatmand, S.; Mann, R.M.; Oosterwijk, J.C.; Tollenaar, R.A.E.M.; Zuidewijn, D.B.W.d.R.v.; Lobbes, M.B.I.; Riet, M.v.; et al. Cost-effectiveness of Breast Cancer Screening with Magnetic Resonance Imaging for Women at Familial Risk. JAMA Oncol. 2020, 6, 1381–1389. [Google Scholar] [CrossRef]
- Gonçalves, D.; Pires, A.S.; Marques, I.A.; Gomes, I.; Sousa, G.; Botelho, M.F.; Abrantes, A.M. An Overview on Radiation Sensitivity in Hereditary Breast and Ovarian Cancer Syndrome. Cancers 2022, 14, 3254. [Google Scholar] [CrossRef]
- Naranjo, I.D.; Sogani, J.; Saccarelli, C.; Horvat, J.V.; Sevilimedu, V.; Hughes, M.C.; Gullo, R.L.; Jochelson, M.S.; Reiner, J.; Pinker, K. MRI Screening of BRCA Mutation Carriers: Comparison of Standard Protocol and Abbreviated Protocols with and Without T2-Weighted Images. AJR Am. J. Roentgenol. 2022, 218, 810–820. [Google Scholar] [CrossRef]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, Cd012464. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.-M.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Dutton, B.; Weng, S.; Sheehan, C.; Chorley, W.; Robertson, J.F.R.; Kendrick, D.; Kai, J. Improving primary care identification of familial breast cancer risk using proactive invitation and decision support. Fam. Cancer 2021, 20, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Grossmith, S.; Cutts, D.; Mikami, S.A.; Suskin, J.A.; Graichen, M.K.; Rojas, N.A.; Pace, L.E.; Joyce, E.; Rhei, E.; et al. Customized breast cancer risk assessment in an ambulatory clinic: A portal for identifying women at risk. Breast Cancer Res. Treat. 2019, 175, 229–237. [Google Scholar] [CrossRef]
- Kaneyasu, T.; Mori, S.; Yamauchi, H.; Ohsumi, S.; Ohno, S.; Aoki, D.; Baba, S.; Kawano, J.; Miki, Y.; Matsumoto, N.; et al. Prevalence of disease-causing genes in Japanese patients with BRCA1/2-wildtype hereditary breast and ovarian cancer syndrome. NPJ Breast Cancer 2020, 6, 25. [Google Scholar] [CrossRef]
- Momozawa, Y.; Iwasaki, Y.; Parsons, M.T.; Kamatani, Y.; Takahashi, A.; Tamura, C.; Katagiri, T.; Yoshida, T.; Nakamura, S.; Sugano, K.; et al. Germline pathogenic variants of 11 breast cancer genes in 7051 Japanese patients and 11,241 controls. Nat. Commun. 2018, 9, 4083. [Google Scholar] [CrossRef]
- Bellhouse, S.; Hawkes, R.E.; Howell, S.J.; Gorman, L.; French, D.P. Breast Cancer Risk Assessment and Primary Prevention Advice in Primary Care: A Systematic Review of Provider Attitudes and Routine Behaviours. Cancers 2021, 13, 4150. [Google Scholar] [CrossRef]
- Kurian, A.W.; Hughes, E.; Simmons, T.; Bernhisel, R.; Probst, B.; Meek, S.; Caswell-Jin, J.L.; John, E.M.; Lanchbury, J.S.; Slavin, T.P.; et al. Performance of the IBIS/Tyrer-Cuzick model of breast cancer risk by race and ethnicity in the Women’s Health Initiative. Cancer 2021, 127, 3742–3750. [Google Scholar] [CrossRef]
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef]
- Nakamura, S.; Kwong, A.; Kim, S.-W.; Iau, P.; Patmasiriwat, P.; Dofitas, R.; Aryandono, T.; Hu, Z.; Huang, C.-S.; Ginsburg, O.; et al. Current Status of the Management of Hereditary Breast and Ovarian Cancer in Asia: First Report by the Asian BRCA Consortium. Public Health Genom. 2016, 19, 53–60. [Google Scholar] [CrossRef]
- Nakamura, S.; Takahashi, M.; Tozaki, M.; Nakayama, T.; Nomizu, T.; Miki, Y.; Murakami, Y.; Aoki, D.; Iwase, T.; Nishimura, S.; et al. Prevalence and differentiation of hereditary breast and ovarian cancers in Japan. Breast Cancer 2015, 22, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Hill, K.; Causer, P.; Plewes, D.; Jong, R.; Yaffe, M.; Foulkes, W.D.; Ghadirian, P.; Lynch, H.; Couch, F.; et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J. Clin. Oncol. 2011, 29, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.Y.; Shekar, S.; Chan, C.H.T.; Hong, L.Z.; Poon, S.-Y.; Silla, T.; Lin, C.; Kumar, V.; Davila, S.; Voorhoeve, M.; et al. Predictive Factors for BRCA1 and BRCA2 Genetic Testing in an Asian Clinic-Based Population. PLoS ONE 2015, 10, e0134408. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Watanabe, C.; Yokoyama, S.; Inuzuka, M.; Yotsumoto, J.; Arai, M.; Nakamura, S.; The Registration Committee of the Japanese HBOC consortium. Analysis of clinical characteristics of breast cancer patients with the Japanese founder mutation BRCA1 L63X. Oncotarget 2019, 10, 3276–3284. [Google Scholar] [CrossRef] [PubMed]
- Bick, U.; Engel, C.; Krug, B.; Heindel, W.; Fallenberg, E.M.; Rhiem, K.; Maintz, D.; Golatta, M.; Speiser, D.; Rjosk-Dendorfer, D.; et al. High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res. Treat. 2019, 175, 217–228. [Google Scholar] [CrossRef]
- You, C.; Xiao, Q.; Zhu, X.; Sun, Y.; Di, G.; Liu, G.; Hou, Y.; Chen, C.; Wu, J.; Shao, Z.; et al. The clinicopathological and MRI features of patients with BRCA1/2 mutations in familial breast cancer. Gland. Surg. 2021, 10, 262–272. [Google Scholar] [CrossRef]
- de Andrade, K.C.; Khincha, P.P.; Hatton, J.N.; Frone, M.N.; Wegman-Ostrosky, T.; Mai, P.L.; Best, A.F.; Savage, S.A. Cancer incidence, patterns, and genotype-phenotype associations in individuals with pathogenic or likely pathogenic germline TP53 variants: An observational cohort study. Lancet Oncol. 2021, 22, 1787–1798. [Google Scholar] [CrossRef]
- Pîrlog, L.M.; Pătrășcanu, A.A.; Militaru, M.S.; Cătană, A. Insights into Clinical Disorders in Cowden Syndrome: A Comprehensive Review. Medicina 2024, 60, 767. [Google Scholar] [CrossRef]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 16, 24. [Google Scholar] [CrossRef]
- Fabi, A.; Metro, G.; Di Benedetto, A.; Nisticò, C.; Vici, P.; Melucci, E.; Antoniani, B.; Perracchio, L.; Sperduti, I.; Milella, M.; et al. Clinical significance of PTEN and p-Akt co-expression in HER2-positive metastatic breast cancer patients treated with trastuzumab-based therapies. Oncology 2010, 78, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kole, C.; Charalampakis, N.; Sakellariou, S.; Papaxoinis, G.; Apostolou, K.G.; Machairas, N.; Papanikolaou, I.S.; Schizas, D. Hereditary Diffuse Gastric Cancer: A 2022 Update. J. Pers. Med. 2022, 12, 2032. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Marino, E.; Zanzottera, C.; Oliveira, C.; Bernard, L.; Macis, D.; Figueiredo, J.; Pereira, J.; Carneiro, P.; Massari, G.; et al. CDH1 Genotype Exploration in Women With Hereditary Lobular Breast Cancer Phenotype. JAMA Netw. Open 2024, 7, e247862. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated with Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef]
- Bergstrom, C.; Pence, C.; Berg, J.; Partain, N.; Sadeghi, N.; Mauer, C.; Pirzadeh-Miller, S.; Gao, A.; Li, H.; Unni, N.; et al. Clinicopathological Features and Outcomes in Individuals with Breast Cancer and ATM, CHEK2, or PALB2 Mutations. Ann. Surg. Oncol. 2021, 28, 3383–3393. [Google Scholar] [CrossRef]
- Dorling, L.; Carvalho, S.; Allen, J.; Parsons, M.T.; Fortuno, C.; González-Neira, A.; Heijl, S.M.; Adank, M.A.; Ahearn, T.U.; Andrulis, I.L.; et al. Breast cancer risks associated with missense variants in breast cancer susceptibility genes. Genome Med. 2022, 14, 51. [Google Scholar] [CrossRef]
- Lowry, K.P.; Geuzinge, H.A.; Stout, N.K.; Alagoz, O.; Hampton, J.M.; Kerlikowske, K.; de Koning, H.J.; Miglioretti, D.L.; van Ravesteyn, N.T.; Schecter, C.; et al. Breast Cancer Screening Strategies for Women With ATM, CHEK2, and PALB2 Pathogenic Variants: A Comparative Modeling Analysis. JAMA Oncol. 2022, 8, 587–596. [Google Scholar] [CrossRef]
- Cragun, D.; Weidner, A.; Tezak, A.; Clouse, K.; Pal, T. Cancer risk management among female BRCA1/2, PALB2, CHEK2, and ATM carriers. Breast Cancer Res. Treat. 2020, 182, 421–428. [Google Scholar] [CrossRef]
- Vysotskaia, V.; Kaseniit, K.E.; Bucheit, L.; Ready, K.; Price, K.; Johansen Taber, K. Clinical utility of hereditary cancer panel testing: Impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations. Cancer 2020, 126, 549–558. [Google Scholar] [CrossRef]
- Weber-Lassalle, N.; Hauke, J.; Ramser, J.; Richters, L.; Groß, E.; Blümcke, B.; Gehrig, A.; Kahlert, A.-K.; Müller, C.R.; Hackmann, K.; et al. BRIP1 loss-of-function mutations confer high risk for familial ovarian cancer, but not familial breast cancer. Breast Cancer Res. 2018, 20, 7. [Google Scholar] [CrossRef]
- Crawford, B.; Adams, S.B.; Sittler, T.; Akker, J.v.D.; Chan, S.; Leitner, O.; Ryan, L.; Gil, E.; Veer, L.v. Multi-gene panel testing for hereditary cancer predisposition in unsolved high-risk breast and ovarian cancer patients. Breast Cancer Res. Treat. 2017, 163, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.S.; Curcio, L.D.; Blakemore, C.A.; Bremner, A.K.; McFarland, R.E.; West, J.G.; Banks, K.C. Multigene Panel Testing Detects Equal Rates of Pathogenic BRCA1/2 Mutations and has a Higher Diagnostic Yield Compared to Limited BRCA1/2 Analysis Alone in Patients at Risk for Hereditary Breast Cancer. Ann. Surg. Oncol. 2015, 22, 3282–3288. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc). Lancet Oncol. 2019, 20, e465. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, S.; Geuzinge, H.A.; Rutgers, E.J.T.; Mann, R.M.; Zuidewijn, D.B.W.d.R.v.; Zonderland, H.M.; Tollenaar, R.A.E.M.; Lobbes, M.B.I.; Ausems, M.G.E.M.; Riet, M.v.; et al. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019, 20, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Rhodes, D.; Wick, M.; Okuno, S.; Kunze, K.L.; Golafshar, M.; Uson, P.L.S.; Mountjoy, L.; et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients with Hereditary Cancer Syndrome. JAMA Oncol. 2021, 7, 230–237. [Google Scholar] [CrossRef]
- Elsayegh, N.; Webster, R.D.; Barrera, A.M.G.; Lin, H.; Kuerer, H.M.; Litton, J.K.; Bedrosian, I.; Arun, B.K. Contralateral prophylactic mastectomy rate and predictive factors among patients with breast cancer who underwent multigene panel testing for hereditary cancer. Cancer Med. 2018, 7, 2718–2726. [Google Scholar] [CrossRef]
- Chang, J.; Seng, S.; Yoo, J.; Equivel, P.; Lum, S.S. Clinical Management of Patients at Risk for Hereditary Breast Cancer with Variants of Uncertain Significance in the Era of Multigene Panel Testing. Ann. Surg. Oncol. 2019, 26, 3389–3396. [Google Scholar] [CrossRef]
- Kwong, A.; Ho, C.Y.S.; Shin, V.Y.; Au, C.H.; Chan, T.L.; Ma, E.S.K. How does re-classification of variants of unknown significance (VUS) impact the management of patients at risk for hereditary breast cancer? BMC Med. Genom. 2022, 15, 122. [Google Scholar] [CrossRef]
- Cragun, D.; Dean, M.; Baker, D.; Kelley, M.; Hooker, G.; Weidner, A.; Hunt, P.; Pal, T. The Development and Evaluation of Novel Patient Educational Material for a Variant of Uncertain Significance (VUS) Result in Hereditary Cancer Genes. Curr. Oncol. 2024, 31, 3361–3378. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, M.D.; Lee, M.C.; Niell, B.L. The Breast Cancer Screening and Timing of Breast MRI-Experience in a Genetic High-Risk Screening Clinic in a Comprehensive Cancer Center. Curr. Oncol. 2022, 29, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef]
- Tutt, A.N.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Tozaki, M.; Nakamura, S. Current status of breast cancer screening in high-risk women in Japan. Breast Cancer 2021, 28, 1181–1187. [Google Scholar] [CrossRef]
- Hodgson, D.; Lai, Z.; Dearden, S.; Barrett, J.C.; Harrington, E.A.; Timms, K.; Lanchbury, J.; Wu, W.; Allen, A.; Senkus, E.; et al. Analysis of mutation status and homologous recombination deficiency in tumors of patients with germline BRCA1 or BRCA2 mutations and metastatic breast cancer: OlympiAD. Ann. Oncol. 2021, 32, 1582–1589. [Google Scholar] [CrossRef]
- Llop-Guevara, A.; Loibl, S.; Villacampa, G.; Vladimirova, V.; Schneeweiss, A.; Karn, T.; Zahm, D.M.; Herencia-Ropero, A.; Jank, P.; van Mackelenbergh, M.; et al. Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): Analysis of the GeparSixto randomized clinical trial. Ann. Oncol. 2021, 32, 1590–1596. [Google Scholar] [CrossRef]
- Nakamura, S. Homologous Recombination Deficiency in Triple-Negative Breast Cancer: Potential Benefit of a New Target. Ann. Surg. Oncol. 2023, 30, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Chu, C.; Badve, S.S.; Vinayak, S.; Silver, D.P.; Isakoff, S.J.; Kaklamani, V.; Gradishar, W.; Stearns, V.; Connolly, R.M.; et al. Association of Tumor-Infiltrating Lymphocytes with Homologous Recombination Deficiency and BRCA1/2 Status in Patients with Early Triple-Negative Breast Cancer: A Pooled Analysis. Clin. Cancer Res. 2020, 26, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.-J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Matis, T.S.; Zayed, N.; Labraki, B.; de Ladurantaye, M.; Matis, T.A.; Camacho Valenzuela, J.; Hamel, N.; Atayan, A.; Rivera, B.; Tabach, Y.; et al. Current gene panels account for nearly all homologous recombination repair-associated multiple-case breast cancer families. NPJ Breast Cancer 2021, 7, 109. [Google Scholar] [CrossRef]
- Creeden, J.F.; Nanavaty, N.S.; Einloth, K.R.; Gillman, C.E.; Stanbery, L.; Hamouda, D.M.; Dworkin, L.; Nemunaitis, J. Homologous recombination proficiency in ovarian and breast cancer patients. BMC Cancer 2021, 21, 1154. [Google Scholar] [CrossRef]
- Min, A.; Kim, K.; Jeong, K.; Choi, S.; Kim, S.; Suh, K.J.; Lee, K.-H.; Kim, S.; Im, S.-A. Homologous repair deficiency score for identifying breast cancers with defective DNA damage response. Sci. Rep. 2020, 10, 12506. [Google Scholar] [CrossRef]
- Nguyen, L.; Martens, J.W.M.; Van Hoeck, A.; Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 2020, 11, 5584. [Google Scholar] [CrossRef]
- Wood, M.E.; McKinnon, W.; Garber, J. Risk for breast cancer and management of unaffected individuals with non-BRCA hereditary breast cancer. Breast J. 2020, 26, 1528–1534. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Schram, A.M.; Rosen, E.Y.; Won, H.; Gong, Y.; Noronha, A.M.; Janjigian, Y.Y.; Stadler, Z.K.; Chang, J.C.; Yang, S.R.; et al. Reversion mutations in germline BRCA1/2-mutant tumors reveal a BRCA-mediated phenotype in non-canonical histologies. Nat. Commun. 2022, 13, 7182. [Google Scholar] [CrossRef]
- Nakamura, K.; Hayashi, H.; Kawano, R.; Ishikawa, M.; Aimono, E.; Mizuno, T.; Kuroda, H.; Kojima, Y.; Niikura, N.; Kawanishi, A.; et al. BRCA1/2 reversion mutations in a pan-cancer cohort. Cancer Sci. 2024, 115, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Page, E.C.; Bancroft, E.K.; Brook, M.N.; Assel, M.; Hassan Al Battat, M.; Thomas, S.; Taylor, N.; Chamberlain, A.; Pope, J.; Ni Raghallaigh, H.; et al. Interim Results from the IMPACT Study: Evidence for Prostate-specific Antigen Screening in BRCA2 Mutation Carriers. Eur. Urol. 2019, 76, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Corrias, G.; Raeside, M.C.; Agostini, A.; Huicochea-Castellanos, S.; Aramburu-Nunez, D.; Paudyal, R.; Shukla-Dave, A.; Smelianskaia, O.; Capanu, M.; Zheng, J.; et al. Pilot study of rapid MR pancreas screening for patients with BRCA mutation. Eur. Radiol. 2019, 29, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Raufi, A.G.; Safyan, R.A.; Bates, S.E.; Manji, G.A. BRCA Mutations in Pancreas Cancer: Spectrum, Current Management, Challenges and Future Prospects. Cancer Manag. Res. 2020, 12, 2731–2742. [Google Scholar] [CrossRef]
- Momozawa, Y.; Sasai, R.; Usui, Y.; Shiraishi, K.; Iwasaki, Y.; Taniyama, Y.; Parsons, M.T.; Mizukami, K.; Sekine, Y.; Hirata, M.; et al. Expansion of Cancer Risk Profile for BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2022, 8, 871–878. [Google Scholar] [CrossRef]
- Uson, P.L.; Riegert-Johnson, D.; Boardman, L.; Kisiel, J.; Mountjoy, L.; Patel, N.; Lizaola-Mayo, B.; Borad, M.J.; Ahn, D.; Sonbol, M.B.; et al. Germline Cancer Susceptibility Gene Testing in Unselected Patients with Colorectal Adenocarcinoma: A Multicenter Prospective Study. Clin. Gastroenterol. Hepatol. 2022, 20, e508–e528. [Google Scholar] [CrossRef]
- Arun, B.K.; Peterson, S.K.; Sweeney, L.E.; Bluebond, R.D.; Tidwell, R.S.S.; Makhnoon, S.; Kushwaha, A.C. Increasing referral of at-risk women for genetic counseling and BRCA testing using a screening tool in a community breast imaging center. Cancer 2022, 128, 94–102. [Google Scholar] [CrossRef]
- Berliner, J.L.; Cummings, S.A.; Boldt Burnett, B.; Ricker, C.N. Risk assessment and genetic counseling for hereditary breast and ovarian cancer syndromes-Practice resource of the National Society of Genetic Counselors. J. Genet. Couns. 2021, 30, 342–360. [Google Scholar] [CrossRef]
- Kramer, I.; Hooning, M.J.; Mavaddat, N.; Hauptmann, M.; Keeman, R.; Steyerberg, E.W.; Giardiello, D.; Antoniou, A.C.; Pharoah, P.D.; Canisius, S.; et al. Breast Cancer Polygenic Risk Score and Contralateral Breast Cancer Risk. Am. J. Hum. Genet. 2020, 107, 837–848. [Google Scholar] [CrossRef]
- Liu, C.; Zeinomar, N.; Chung, W.K.; Kiryluk, K.; Gharavi, A.G.; Hripcsak, G.; Crew, K.D.; Shang, N.; Khan, A.; Fasel, D.; et al. Generalizability of Polygenic Risk Scores for Breast Cancer Among Women with European, African, and Latinx Ancestry. JAMA Netw. Open 2021, 4, e2119084. [Google Scholar] [CrossRef]
- Mars, N.; Widén, E.; Kerminen, S.; Meretoja, T.; Pirinen, M.; Parolo, P.d.B.; Palta, P.; Palotie, A.; Kaprio, J.; Joensuu, H.; et al. The role of polygenic risk and susceptibility genes in breast cancer over the course of life. Nat. Commun. 2020, 11, 6383. [Google Scholar] [CrossRef] [PubMed]
- Clift, A.K.; Dodwell, D.; Lord, S.; Petrou, S.; Brady, S.M.; Collins, G.S.; Hippisley-Cox, J. The current status of risk-stratified breast screening. Br. J. Cancer 2022, 126, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Choudhury, P.P.; García-Closas, M. Toward Risk-Stratified Breast Cancer Screening: Considerations for Changes in Screening Guidelines. JAMA Oncol. 2020, 6, 31–33. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, L.; Woof, V.G.; Donnelly, L.S.; Howell, A.; Evans, D.G.; French, D.P. Risk stratified breast cancer screening: UK healthcare policy decision-making stakeholders’ views on a low-risk breast screening pathway. BMC Cancer 2020, 20, 680. [Google Scholar] [CrossRef]
- Bhardwaj, P.V.; Mason, H.; Kaufman, S.A.; Visintainer, P.; Makari-Judson, G. Outcomes of a Multidisciplinary Team in the Management of Patients with Early-Stage Breast Cancer Undergoing Neoadjuvant Chemotherapy at a Community Cancer Center. Curr. Oncol. 2023, 30, 4861–4870. [Google Scholar] [CrossRef]
- Kurniasih, D.A.A.; Setiawati, E.P.; Pradipta, I.S.; Subarnas, A. Interprofessional collaboration in the breast cancer unit: How do healthcare workers see it? BMC Womens Health 2022, 22, 227. [Google Scholar] [CrossRef]
- Milana, F.; Famularo, S.; Luberto, A.; Rimassa, L.; Scorsetti, M.; Comito, T.; Pressiani, T.; Franzese, C.; Poretti, D.; Di Tommaso, L.; et al. Multidisciplinary Tumor Board in the Management of Patients with Colorectal Liver Metastases: A Single-Center Review of 847 Patients. Cancers 2022, 14, 3952. [Google Scholar] [CrossRef]
- Pangarsa, E.A.; Rizky, D.; Tandarto, K.; Setiawan, B.; Santosa, D.; Hadiyanto, J.N.; Kyana, S.; Suharti, C. The effect of multidisciplinary team on survival rates of women with breast cancer: A systematic review and meta-analysis. Ann. Med Surg. 2023, 85, 2940–2948. [Google Scholar] [CrossRef]
- Selby, P.; Gillis, C.; Haward, R. Benefits from specialised cancer care. Lancet 1996, 348, 313–318. [Google Scholar] [CrossRef]
- Stirling, R.G.; Harrison, A.; Huang, J.; Lee, V.; Taverner, J.; Barnes, H. Multidisciplinary meeting review in nonsmall cell lung cancer: A systematic review and meta-analysis. Eur. Respir. Rev. 2024, 33, 230157. [Google Scholar] [CrossRef]
- Walraven, J.E.; Ripping, T.M.; Oddens, J.R.; van Rhijn, B.W.; Goossens-Laan, C.A.; Hulshof, M.C.; BlaZIB Study Group; Kiemeney, L.A.; Witjes, J.A.; Lemmens, V.E.; et al. The influence of multidisciplinary team meetings on treatment decisions in advanced bladder cancer. BJU Int. 2023, 131, 244–252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Kojima, Y.; Takeuchi, S. Causative Genes of Homologous Recombination Deficiency (HRD)-Related Breast Cancer and Specific Strategies at Present. Curr. Oncol. 2025, 32, 90. https://doi.org/10.3390/curroncol32020090
Nakamura S, Kojima Y, Takeuchi S. Causative Genes of Homologous Recombination Deficiency (HRD)-Related Breast Cancer and Specific Strategies at Present. Current Oncology. 2025; 32(2):90. https://doi.org/10.3390/curroncol32020090
Chicago/Turabian StyleNakamura, Seigo, Yasuyuki Kojima, and Sayoko Takeuchi. 2025. "Causative Genes of Homologous Recombination Deficiency (HRD)-Related Breast Cancer and Specific Strategies at Present" Current Oncology 32, no. 2: 90. https://doi.org/10.3390/curroncol32020090
APA StyleNakamura, S., Kojima, Y., & Takeuchi, S. (2025). Causative Genes of Homologous Recombination Deficiency (HRD)-Related Breast Cancer and Specific Strategies at Present. Current Oncology, 32(2), 90. https://doi.org/10.3390/curroncol32020090





