Real-World Clinical Outcomes of Trilaciclib for the Prevention of Myelosuppression in Patients with Esophageal Cancer Undergoing Chemotherapy
Abstract
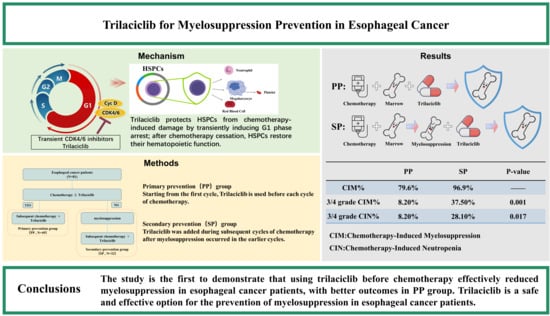
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Regimens
2.3. Study Endpoints
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Patient Characteristics
3.2. Hematological Toxicity
3.3. Safety
3.4. Efficacy and Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIA | Chemotherapy-induced anemia |
| CIM | Chemotherapy-induced myelosuppression |
| CIN | Chemotherapy-induced neutropenia |
| CIT | Chemotherapy-induced thrombocytopenia |
| CR | Complete response |
| CRA | Chemotherapy-induced Anemia |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DCR | Disease control rate |
| ESCC | Esophageal squamous cell carcinoma |
| ES-SCLC | Extensive-stage small-cell lung cancer |
| FN | Febrile neutropenia |
| G-CSFs | Granulocyte colony-stimulating factors |
| HSPCs | Hematopoietic stem and progenitor cells |
| IMRT | Intensity-modulated radiation therapy |
| ORR | Objective response rate |
| OS | Overall survival |
| PD | Progressive disease |
| PP | Primary prevention |
| PR | Partial response |
| Q3W | Every three weeks |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SCLC | Small-cell lung cancer |
| SD | Stable disease |
| SP | Secondary prevention |
| UICC | Union for International Cancer Control |
References
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kwong, D.L.; Cao, T.; Hu, Q.; Zhang, L.; Ming, X.; Chen, J.; Fu, L.; Guan, X. Esophageal squamous cell carcinoma (ESCC): Advance in genomics and molecular genetics. Dis. Esophagus 2015, 28, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Bie, F.; Zang, R.; Zhang, M.; Song, P.; Liu, L.; Peng, Y.; Bai, G.; Huai, Q.; Li, Y.; et al. Global burden and temporal trends in incidence and mortality of oesophageal cancer. J. Adv. Res. 2023, 50, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 855–883. [Google Scholar] [CrossRef]
- Barsouk, A.; Rawla, P.; Hadjinicolaou, A.V.; Aluru, J.S.; Barsouk, A. Targeted Therapies and Immunotherapies in the Treatment of Esophageal Cancers. Med. Sci. 2019, 7, 100. [Google Scholar] [CrossRef]
- Tsuji, T.; Matsuda, S.; Sato, Y.; Tanaka, K.; Sasaki, K.; Watanabe, M.; Hamai, Y.; Nasu, M.; Saze, Z.; Nakashima, Y.; et al. Safety and Efficacy of Conversion Therapy After Systemic Chemotherapy in Advanced Esophageal Cancer with Distant Metastases: A Multicenter Retrospective Observational Study. Ann. Surg. Oncol. 2025, 32, 274–283. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Liu, T.; Song, Y.; Hua, P.; Chen, S.; Li, J.; Liu, Y.; Zhao, Y. Efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy in locally advanced esophageal cancer: A meta-analysis. Front. Oncol. 2022, 12, 974684. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Zuo, F.; Liu, J.; Yang, J. Immune-based combination therapy for esophageal cancer. Front. Immunol. 2022, 13, 1020290. [Google Scholar] [CrossRef]
- Shen, B.; Han, Z. Survey Report on the Current Status of Myelosuppression and Clinical Management in Chinese Cancer Chemotherapy; Southeast University Press: Nanjing, China, 2023; pp. 10–15. [Google Scholar]
- Epstein, R.S.; Aapro, M.S.; Basu Roy, U.K.; Salimi, T.; Krenitsky, J.; Leone-Perkins, M.L.; Girman, C.; Schlusser, C.; Crawford, J. Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors. Adv. Ther. 2020, 37, 3606–3618. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Q.; Han, Y.; Wu, T.; Zhang, Y. Predictive Model of Chemotherapy-Induced Myelosuppression for Patients with Esophageal Cancer. Cancer Control 2022, 29, 10732748221126929. [Google Scholar] [CrossRef]
- Daniel, D.; Crawford, J. Myelotoxicity from chemotherapy. Semin. Oncol. 2006, 33, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, J.; Sun, Z.; Liu, C.; Li, Z.; Xiao, Y.; Yu, J.; Du, F.; Shi, Y.; Sun, J.; et al. Polymorphism of FGD4 and myelosuppression in patients with esophageal squamous cell carcinoma. Future Oncol. 2021, 17, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Goldschmidt, J.; Andric, Z.; Dragnev, K.H.; Gwaltney, C.; Skaltsa, K.; Pritchett, Y.; Antal, J.M.; Morris, S.R.; Daniel, D. Effects of Trilaciclib on Chemotherapy-Induced Myelosuppression and Patient-Reported Outcomes in Patients with Extensive-Stage Small Cell Lung Cancer: Pooled Results from Three Phase II Randomized, Double-Blind, Placebo-Controlled Studies. Clin. Lung Cancer 2021, 22, 449–460. [Google Scholar] [CrossRef]
- Bisi, J.E.; Sorrentino, J.A.; Roberts, P.J.; Tavares, F.X.; Strum, J.C. Preclinical Characterization of G1T28: A Novel CDK4/6 Inhibitor for Reduction of Chemotherapy-Induced Myelosuppression. Mol. Cancer Ther. 2016, 15, 783–793. [Google Scholar] [CrossRef]
- Dhillon, S. Trilaciclib: First Approval. Drugs 2021, 81, 867–874. [Google Scholar] [CrossRef]
- Lai, A.Y.; Sorrentino, J.A.; Dragnev, K.H.; Weiss, J.M.; Owonikoko, T.K.; Rytlewski, J.A.; Hood, J.; Yang, Z.; Malik, R.K.; Strum, J.C.; et al. CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J. Immunother. Cancer 2020, 8, e000847. [Google Scholar] [CrossRef]
- Goel, S.; Tan, A.R.; Rugo, H.S.; Aftimos, P.; Andrić, Z.; Beelen, A.; Zhang, J.; Yi, J.S.; Malik, R.; O’Shaughnessy, J. Trilaciclib prior to gemcitabine plus carboplatin for metastatic triple-negative breast cancer: Phase III PRESERVE 2. Future Oncol. 2022, 18, 3701–3711. [Google Scholar] [CrossRef]
- Tan, A.R.; Wright, G.S.; Thummala, A.R.; Danso, M.A.; Popovic, L.; Pluard, T.J.; Han, H.S.; Vojnović, Ž.; Vasev, N.; Ma, L.; et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019, 20, 1587–1601. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Wang, Z.X.; Cui, C.; Yao, J.; Zhang, Y.; Li, M.; Feng, J.; Yang, S.; Fan, Y.; Shi, J.; Zhang, X.; et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022, 40, 277–288.E3. [Google Scholar] [CrossRef]
- Expert Committee of Integrated Chinese and Western Medicine. Expert consensus on the diagnosis and treatment of bone marrow suppression caused by antitumor drugs with integrated traditional Chinese and Western medicine. Chin. J. Clin. Oncol. 2021, 26, 1020–1027. [Google Scholar]
- Rapoport, B.L.; Cooksley, T.; Johnson, D.B.; Anderson, R.; Shannon, V.R. Treatment of infections in cancer patients: An update from the neutropenia, infection and myelosuppression study group of the Multinational Association for Supportive Care in Cancer (MASCC). Expert. Rev. Clin. Pharmacol. 2021, 14, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, J.; Monnette, A.; Shi, P.; Venkatasetty, D.; Lopez-Gonzalez, L.; Huang, H. Burden of chemotherapy-induced myelosuppression among patients with ES-SCLC in US community oncology settings. Future Oncol. 2022, 18, 3881–3894. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.S.; Bohlius, J.; Cameron, D.A.; Dal Lago, L.; Donnelly, J.P.; Kearney, N.; Lyman, G.H.; Pettengell, R.; Tjan-Heijnen, V.C.; Walewski, J.; et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer 2011, 47, 8–32. [Google Scholar] [CrossRef]
- Weiss, J.M.; Csoszi, T.; Maglakelidze, M.; Hoyer, R.J.; Beck, J.T.; Domine Gomez, M.; Lowczak, A.; Aljumaily, R.; Rocha Lima, C.M.; Boccia, R.V.; et al. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: A phase Ib/randomized phase II trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1613–1621. [Google Scholar] [CrossRef]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef]
- Daniel, D.; Kuchava, V.; Bondarenko, I.; Ivashchuk, O.; Reddy, S.; Jaal, J.; Kudaba, I.; Hart, L.; Matitashvili, A.; Pritchett, Y.; et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: A multicentre, randomised, double-blind, placebo-controlled Phase II trial. Int. J. Cancer 2021, 148, 2557–2570. [Google Scholar] [CrossRef]
- Tan, A.R.; Wright, G.S.; Thummala, A.R.; Danso, M.A.; Popovic, L.; Pluard, T.J.; Han, H.S.; Vojnović, Ž.; Vasev, N.; Ma, L.; et al. Trilaciclib Prior to Chemotherapy in Patients with Metastatic Triple-Negative Breast Cancer: Final Efficacy and Subgroup Analysis from a Randomized Phase II Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 629–636. [Google Scholar] [CrossRef]
- Dai, H.R.; Yang, Y.; Wang, C.Y.; Chen, Y.T.; Cui, Y.F.; Li, P.J.; Chen, J.; Yang, C.; Jiao, Z. Trilaciclib dosage in Chinese patients with extensive-stage small cell lung cancer: A pooled pharmacometrics analysis. Acta Pharmacol. Sin. 2024, 45, 2212–2225. [Google Scholar] [CrossRef]




| Total (n = 81) | PP (n = 49) | SP (n = 32) | p | ||
|---|---|---|---|---|---|
| Gender | Male | 65 (80.2%) | 40 (81.63%) | 25 (78.13%) | 0.698 |
| Female | 16 (19.8%) | 9 (18.37%) | 7 (21.88%) | ||
| Age | <65 | 19 (23.5%) | 11 (22.45%) | 8 (25.00%) | 0.791 |
| ≥65 | 62 (76.5%) | 38 (77.55%) | 24 (75.00%) | ||
| BMI | <18.5 | 13 (16.0%) | 8 (16.33%) | 5 (15.63%) | 0.98 |
| 18.5–24 | 47 (58.0%) | 28 (57.14%) | 19 (59.38%) | ||
| ≥24 | 21 (26.0%) | 13 (26.53%) | 8 (25.00%) | ||
| Smoking | Yes | 35 (43.2%) | 23 (46.94%) | 12 (37.50%) | 0.402 |
| No | 46 (56.8%) | 26 (53.06%) | 20 (62.50%) | ||
| Alcohol abuse | Yes | 38 (46.9%) | 25 (51.02%) | 13 (40.63%) | 0.359 |
| No | 43 (53.1%) | 24 (48.98%) | 19 (59.38%) | ||
| Nutritional management mode | Soft diet | 19 (23.5%) | 11 (22.45%) | 8 (25.00%) | 0.897 |
| Semi-liquid diet | 41 (50.6%) | 24 (48.98%) | 17 (53.13%) | ||
| Liquid diet | 17 (21.0%) | 11 (22.45%) | 6 (18.75%) | ||
| Naso-intestinal tube/gastrostomy tube | 4 (4.9%) | 3 (6.12%) | 1 (3.13%) | ||
| PS score | 0 | 17 (21.0%) | 13 (26.53%) | 4 (12.50%) | 0.13 |
| 1 | 64 (79.0%) | 36 (73.47%) | 28 (87.50%) | ||
| Clinical stage | I | 3 (3.7%) | 3 (6.12%) | 0 (0.00%) | 0.502 |
| II | 19 (23.5%) | 11 (22.45%) | 8 (25.00%) | ||
| III | 24 (29.6%) | 15 (30.61%) | 9 (28.13%) | ||
| IV | 35 (43.2%) | 20 (40.82%) | 15 (46.88%) | ||
| Grade | G1 | 4 (4.9%) | 1 (2.04%) | 3 (9.38%) | 0.242 |
| G2 | 38 (46.9%) | 22 (44.90%) | 16 (50.00%) | ||
| G3 | 39 (48.1%) | 26 (53.06%) | 13 (40.63%) | ||
| Tumor size | <5 cm | 25 (30.9%) | 15 (30.61%) | 10 (31.25%) | 0.952 |
| ≥5 cm | 56 (69.1%) | 34 (69.39%) | 22 (68.75%) | ||
| Tumor location | Cervical | 11 (13.6%) | 8 (16.33%) | 3 (9.38%) | 0.819 |
| Upper chest | 16 (19.8%) | 10 (20.41%) | 6 (18.75%) | ||
| Middle chest | 16 (19.8%) | 9 (18.37%) | 7 (21.88%) | ||
| Lower chest | 17 (21.0%) | 11 (22.45%) | 6 (18.75%) | ||
| Overlapping | 21 (26.0%) | 11 (22.45%) | 10 (31.25%) | ||
| Therapy | RT + CT | 12 (14.8) | 6 (12.24%) | 6 (18.75%) | 0.319 |
| RT + CT + TT | 1 (1.2%) | 1 (2.04%) | 0 (0.00%) | ||
| RT + CT + IT | 59 (72.8%) | 36 (73.47%) | 23 (71.88%) | ||
| RT + CT + IT + TT | 5 (6.2%) | 2 (4.08%) | 3 (9.38%) | ||
| CT + IT | 4 (4.9%) | 4 (8.16%) | 0 (0.00%) | ||
| Prevention | Primary | 49 (60.5%) | 49 (100.00%) | 0 (0.00%) | 1.00 |
| Secondary | 32 (39.5%) | 0 (0.00%) | 32 (100.00%) | ||
| CIM | % | CIN | % | CIT | % | CRA | % |
|---|---|---|---|---|---|---|---|
| I | 21 (25.9%) | I | 13 (16.0%) | I | 22 (27.2%) | I | 38 (46.9%) |
| II | 33 (40.7%) | II | 22 (27.2%) | II | 13 (16.0%) | II | 15 (18.5%) |
| III | 14 (17.3%) | III | 10 (12.3%) | III | 6 (7.4%) | III | 3 (3.7%) |
| IV | 2 (2.5%) | IV | 3 (3.7%) | IV | 0 (0%) | IV | 0 (0%) |
| ALL | 70 (86.4%) | ALL | 48 (59.3%) | ALL | 41 (50.6%) | ALL | 56 (69.1%) |
| CIM | CIN | CIT | CRA | |||||
|---|---|---|---|---|---|---|---|---|
| PP | SP | PP | SP | PP | SP | PP | SP | |
| I | 17 (34.7%) | 4 (12.5%) | 8 (16.3%) | 5 (15.6%) | 15 (30.6%) | 7 (21.9%) | 21 (42.9%) | 17 (53.1) |
| II | 18 (36.7%) | 15 (46.9%) | 13 (26.5%) | 9 (28.1%) | 3 (6.1%) | 10 (31.3%) | 8 (16.3%) | 7 (21.9%) |
| III | 4 (8.2%) | 10 (31.3%) | 4 (8.2%) | 6 (18.8%) | 2 (4.1%) | 4 (12.5%) | 1 (2.0%) | 2 (6.3%) |
| IV | 0 (0%) | 2 (6.3%) | 0 (0%) | 3 (9.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ALL | 39 (79.6%) | 31 (96.9%) | 25 (51.0%) | 23 (71.9%) | 20 (40.8%) | 21 (65.6%) | 30 (61.2%) | 26 (81.3%) |
| TRAE | PP (n = 49) | SP (n = 32) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2 | 3 | 4 | 0–1 | 2 | 3 | 4 | ||
| Fatigue | 42 (85.7%) | 7 (14.3%) | 0 | 0 | 31 (96.9%) | 1 (3.1%) | 0 | 0 | 0.182 |
| Decreased appetite | 41 (83.7%) | 8 (16.3%) | 0 | 0 | 28 (87.5%) | 2 (6.3%) | 2 (6.3%) | 0 | 0.134 |
| Hepatic function abnormal | 43 (87.8%) | 3 (6.1%) | 3 (6.1%) | 0 | 26 (81.3%) | 4 (12.5%) | 2 (6.3%) | 0 | 0.793 |
| Renal function abnormal | 48 (97.9%) | 1 (2.1%) | 0 | 0 | 31 (96.9%) | 1 (3.1%) | 0 | 0 | 0.401 |
| Vomiting | 46 (93.9%) | 3 (6.1%) | 0 | 0 | 32 (100%) | 0 | 0 | 0 | 0.06 |
| Hiccups | 49 (100%) | 0 | 0 | 0 | 32 (100%) | 0 | 0 | 0 | 0.645 |
| Stomatitis | 40 (81.6%) | 9 (18.4%) | 0 | 0 | 28 (87.5%) | 4 (12.5%) | 0 | 0 | 0.554 |
| Cough | 49 (100%) | 0 | 0 | 0 | 31 (96.9%) | 1 (3.1%) | 0 | 0 | 0.245 |
| Constipation | 49 (100%) | 0 | 0 | 0 | 32 (100%) | 0 | 0 | 0 | 0.129 |
| Diarrhea | 49 (100%) | 0 | 0 | 0 | 31 (96.9%) | 1 (3.1%) | 0 | 0 | 0.414 |
| Radiation dermatitis | 48 (97.9%) | 0 | 1 (2.1%) | 0 | 31 (96.9%) | 1 (3.1%) | 0 | 0 | 0.209 |
| Radiation pneumonitis | 48 (97.9%) | 1 (2.1%) | 0 | 0 | 32 (100%) | 0 | 0 | 0 | 0.589 |
| Hypocalcemia | 47 (96.0%) | 1 (2.1%) | 1 (2.1%) | 0 | 29 (90.6%) | 3 (9.4%) | 0 | 0 | 0.053 |
| Hyponatremia | 47 (96.0%) | 2 (4.1%) | 0 | 0 | 31 (96.9%) | 1 (3.1%) | 0 | 0 | 0.926 |
| Hyperkalemia | 47 (96.0%) | 2 (4.1%) | 0 | 0 | 32 (100%) | 0 | 0 | 0 | 0.492 |
| Hypokalemia | 46 (93.9%) | 3 (6.1%) | 0 | 0 | 31 (96.9%) | 0 | 0 | 0 | 0.662 |
| PP Group | SP Group | p-Value | |
|---|---|---|---|
| CR | 22 (44.9%) | 13 (40.6%) | 0.704 |
| PR | 23 (46.9%) | 14 (43.8%) | 0.778 |
| SD | 2 (4.1%) | 3 (9.4%) | 0.333 |
| PD | 2 (4.1%) | 2 (6.3%) | 0.66 |
| ORR | 91.80% | 84.40% | 0.161 |
| DCR | 95.90% | 93.80% | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yan, J.; Liu, Z.; Ge, X.; Sun, X.; Xia, X. Real-World Clinical Outcomes of Trilaciclib for the Prevention of Myelosuppression in Patients with Esophageal Cancer Undergoing Chemotherapy. Curr. Oncol. 2025, 32, 189. https://doi.org/10.3390/curroncol32040189
Chen H, Yan J, Liu Z, Ge X, Sun X, Xia X. Real-World Clinical Outcomes of Trilaciclib for the Prevention of Myelosuppression in Patients with Esophageal Cancer Undergoing Chemotherapy. Current Oncology. 2025; 32(4):189. https://doi.org/10.3390/curroncol32040189
Chicago/Turabian StyleChen, Hui, Jingze Yan, Zeyuan Liu, Xiaolin Ge, Xinchen Sun, and Xiaojie Xia. 2025. "Real-World Clinical Outcomes of Trilaciclib for the Prevention of Myelosuppression in Patients with Esophageal Cancer Undergoing Chemotherapy" Current Oncology 32, no. 4: 189. https://doi.org/10.3390/curroncol32040189
APA StyleChen, H., Yan, J., Liu, Z., Ge, X., Sun, X., & Xia, X. (2025). Real-World Clinical Outcomes of Trilaciclib for the Prevention of Myelosuppression in Patients with Esophageal Cancer Undergoing Chemotherapy. Current Oncology, 32(4), 189. https://doi.org/10.3390/curroncol32040189