Sex and Gender in Myeloid and Lymphoblastic Leukemias and Multiple Myeloma: From Molecular Mechanisms to Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
3. Defining Sex and Gender: A Crucial Distinction
Addressing the Challenges in Data Collection and Reporting
4. Biological Sex and Hematologic Malignancies: Mechanisms and Manifestations
4.1. Genetic and Hormonal Influences
4.2. Immune System Variations
4.3. Pharmacogenomics
5. Sex Differences in Hematologic Malignancies: A Disease-Specific Review
5.1. Leukemia
| Category | AML | ALL | CML |
|---|---|---|---|
| Epidemiology | • AML hospitalizations peaked in the 60–79 age group (males: 33.8, females: 23.3 per 10,000) with a significantly higher rate in males aged 18–39 (23.6 vs. 7.7 per 10,000, p < 0.01) [27]. • Upward trend for 1990–2017 (from 63.84 × 103 in 1990 to 119.57 × 103 cases in 2017, increasing by 87.3%, EAPC = 0.56, 95% CI 0.49~0.62) [42]. | • >90% survival in high-income countries [43]. • Boys with B-cell acute lymphoblastic leukemia (ALL) exhibited inferior 5-year event-free survival (84.6% vs. 86.0%, p = 0.009) and overall survival (91.3% vs. 92.5%, p = 0.02) compared to girls, primarily due to increased central nervous system relapses [44]. • B-cell (24 subtypes) and T-cell (10 subtypes) acute lymphoblastic leukemia (ALL) demonstrate a sex disparity, with T-ALL more common in males (63%) and B-ALL more common in females (58%) [45]. | • Higher male incidence: Studies indicate a higher risk of CML in males, observed across age groups and supported by data from both general populations and specific cohorts like A-bomb survivors [46]. • Sex-specific transcript distribution: e13a2 (b2a2) is more frequent in males (39.2% vs. 36.2% in females); rare transcripts are more frequent in females (2.27% vs. 1.69% in males) [47]. • e14a2 (b3a2) and treatment response: Patients with e14a2 (b3a2) showed significantly better complete cytogenetic response at 12 months (78.6% vs. 21.4% for e13a2/b2a2) [48]. |
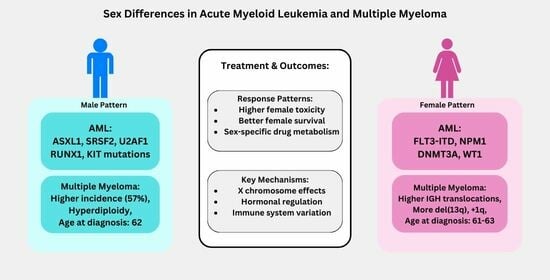
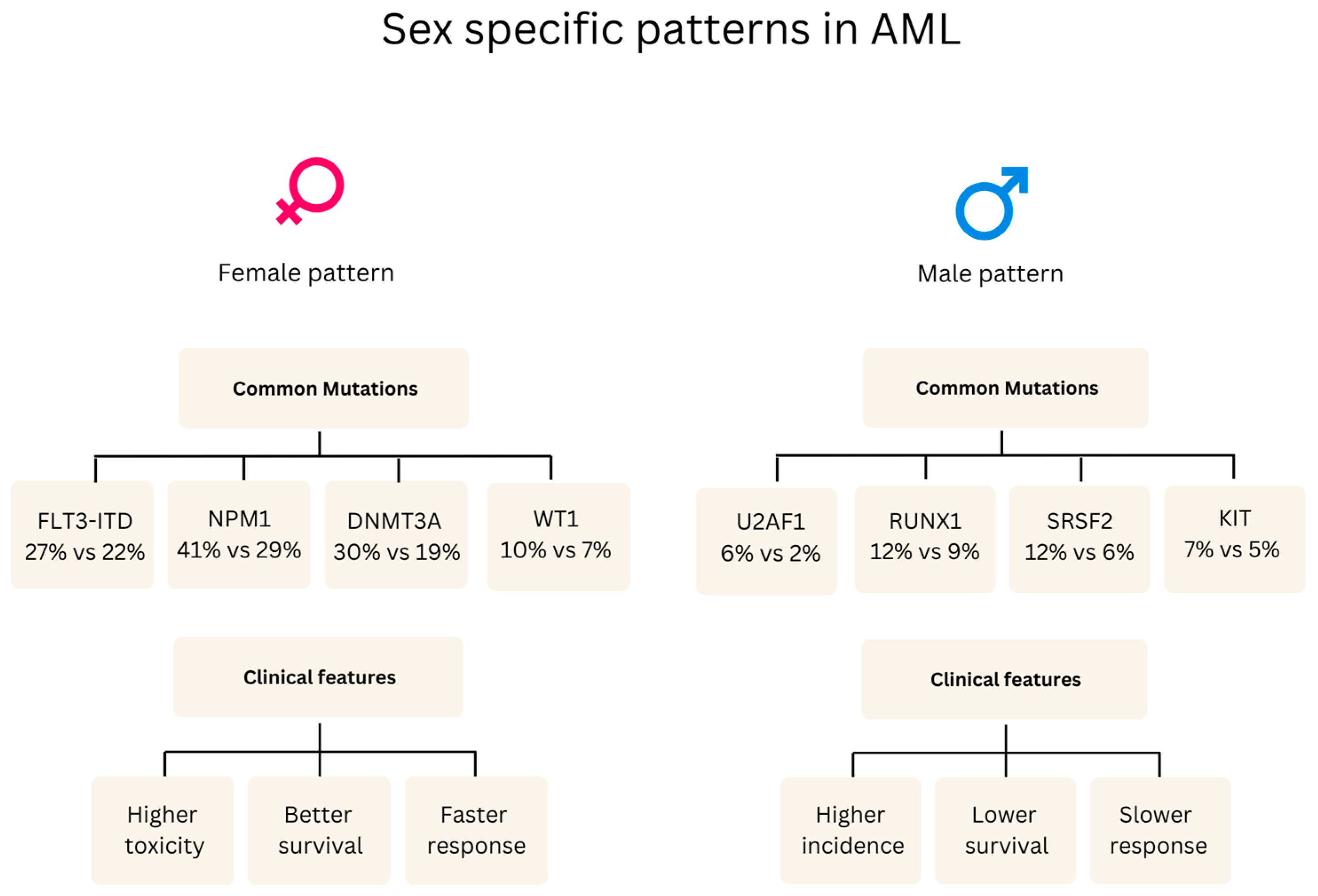
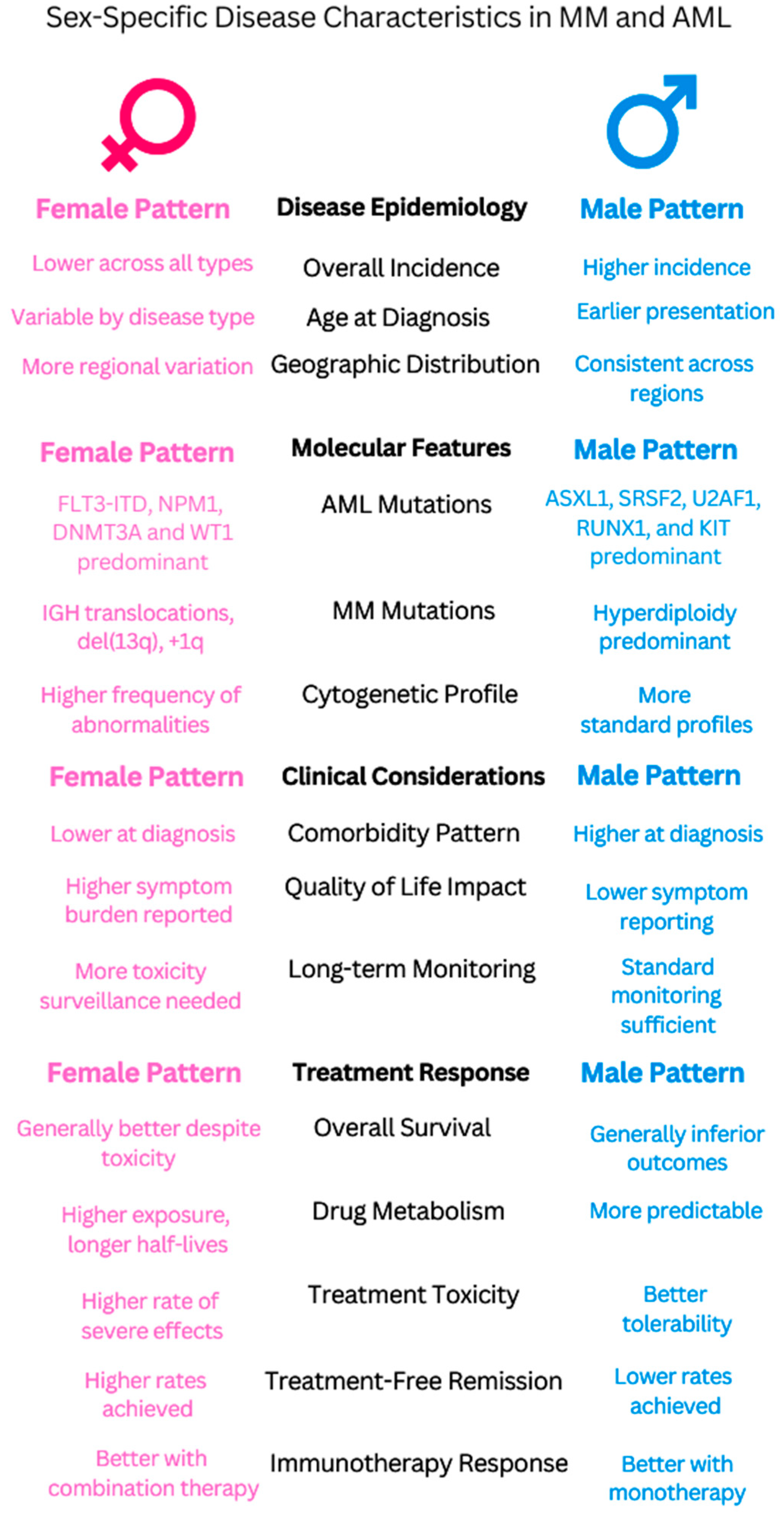
| Clinical manifestations | • Males: ASXL1, SRSF2, U2AF1, RUNX1, and KIT mutations; aggressive features [49]. • Female-predominant mutations: FLT3-ITD, DNMT3A, NPM1, and WT1 [49]. | • Males exhibit a higher prevalence of T-ALL (83%) and B-ALL (89%) and increased central nervous system involvement, particularly in B-ALL (22% of cases, mostly in males) compared to T-ALL (4% of cases) [50]. • Females show a higher frequency of early B-phenotype ALL associated with elevated cell counts [51], which contrasts with the general findings in [50] of a higher overall B-ALL prevalence in males (89%). • RASSF2 SNP (rs7704443): The minor allele (G) of this SNP was found to be significantly more frequent in males with childhood ALL, with an odds ratio (OR) of 1.7 (95% CI = 1.3–2.2) [52]. | • Males: Higher WBC counts, larger spleen, earlier diagnosis [53]. • Females: Higher platelets, lower hemoglobin, later diagnosis [54]. • The disease predominately affected children who were older than 10 years (67% of the patients), with a higher prevalence in boys than girls (gender ratio: 1.5) [55]. |
| Prognostic factors | • Inferior survival in males: Males with myeloid malignancies showed a lower 5-year relative survival rate of 48.8% (95% CI 46.5–51.2) compared to females, who had a rate of 60.4% (95% CI 57.7–62.9) [41]. • Black adolescent and young adult patients with AML, particularly those aged 18–29 years, experienced worse outcomes compared to White patients, including higher early death rates (16% vs. 3%), lower complete remission rates (66% vs. 83%), and decreased 5-year overall survival (22% vs. 51%). These disparities persisted even within specific cytogenetic groups [56]. | • While historically, boys with ALL have experienced inferior survival, this study of pediatric B-ALL patients treated on Children’s Oncology Group trials between 2004 and 2014 did not find statistically significant differences in 5-year event-free survival (EFS: 85.3% for boys vs. 86.4% for girls, p = 0.07) or overall survival (OS: 89.8% for boys vs. 90.4% for girls, p = 0.17) between sexes [44]. | • In low- and intermediate-risk CML patients (according to the Sokal score), females had a statistically significant higher cumulative incidence of major molecular response (MMR) at 12 months compared to males (70.3% vs. 52.9%, p = 0.037) [32]. • Females with CML in the chronic phase were more likely to achieve an early complete cytogenetic response (CCyR) at 3 months (HR 1.38 [95% CI 1.02–1.87], p = 0.03) and a major molecular response (MMR) at 6 months (HR 1.34 [95% CI 1.03–1.74], p = 0.03) compared to males. Females also had a higher cumulative incidence of MMR at 12 months (66.1% vs. 56.7%, p = 0.03) [57]. • Female sex has been identified as a predictor of durable deep molecular response, which is often a prerequisite for attempting treatment-free remission [57,58]. |
| Treatment Response | Anthracycline cardiotoxicity varies by sex/life stage: Prepubertal females: Higher risk (RR 1.89, CI 1.28–2.78) Premenopausal females: Lower risk (16.5% vs. 7.3% hospitalization) Postmenopausal: No significant difference. Dexrazoxane protection is stronger in females (p = 0.019) [59]. | • Clinical trials show females have better survival outcomes (42% vs. 16% of trials) but more toxicity than males (13 vs. 22 trials) [60]. • Sex-specific drug effects: - Vincristine increased non-void contractions by 700% in females compared to only 180% in males [61] - Asparaginase: males are more likely than females to develop severe hypertriglyceridemia, with 66% of grade 4 cases occurring in males compared to 34% in females [62]. • Female survivors showed significantly better overall metacognition (p = 0.024) compared to males following cisplatin/carboplatin treatment [63]. | TKI therapy: Women have 23% higher dose-normalized plasma imatinib concentrations than men (0.0043 vs. 0.0035 L−1). Women achieve higher MMR rates (80% vs. 45%, p = 0.018). Women experience 12% more samples with high (>2mg/L−1) imatinib levels (34.9% vs. 22.6%) [32,33]. Age-related treatment adequacy: Elderly women receive 24% fewer doses (62 vs. 81 doses/quarter) than elderly men [64]. |
5.2. Multiple Myeloma (MM)
5.2.1. Epidemiology
5.2.2. Clinical Manifestations
5.2.3. Impact on Prognosis
5.2.4. Treatment Considerations
5.3. Clinical Implications
5.3.1. Common Patterns
5.3.2. Clinical Applications
6. Gender and Hematologic Malignancies: Sociocultural and Clinical Implications
7. Translating Biology to Clinical Practice: The Future of Precision Medicine
7.1. Integrating Sex and Gender into Clinical Trials
7.2. Development of Sex- and Gender-Sensitive Clinical Guidelines
7.3. Eliminating Gender Disparity in Healthcare
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute Myeloid Leukemia |
| ALL | Acute Lymphoblastic Leukemia |
| CML | Chronic Myeloid Leukemia |
| MM | Multiple Myeloma |
| B-ALL | B-cell Acute Lymphoblastic Leukemia |
| T-ALL | T-cell Acute Lymphoblastic Leukemia |
| BCR-ABL | Breakpoint Cluster Region-Abelson |
| FLT3-ITD | FMS-like Tyrosine Kinase 3 Internal Tandem Duplication |
| DNMT3A | DNA Methyltransferase 3 Alpha |
| NPM1 | Nucleophosmin 1 |
| WT1 | Wilms Tumor 1 |
| ASXL1 | Additional Sex Combs Like 1 |
| SRSF2 | Serine/Arginine-Rich Splicing Factor 2 |
| U2AF1 | U2 Small Nuclear RNA Auxiliary Factor 1 |
| RUNX1 | Runt Related Transcription Factor 1 |
| IGH | Immunoglobulin Heavy Chain |
| del(13q) | Deletion of chromosome 13q |
| +1q | Addition of chromosome 1q |
| SNPs | Single Nucleotide Polymorphisms |
| TKI | Tyrosine Kinase Inhibitor |
| CNS | Central Nervous System |
| WBC | White Blood Cell |
| FISH | Fluorescence In Situ Hybridization |
| NIH | National Institutes of Health |
| PTSD | Post-Traumatic Stress Disorder |
| BSA | Body Surface Area |
| ESMO | European Society for Medical Oncology |
| COG | Children’s Oncology Group |
| AMLCG | AML Cooperative Group |
| SLA | Service Level Agreement |
| DNA | Deoxyribonucleic Acid |
| RNA | Ribonucleic Acid |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| MMR | Major Molecular Response |
| CCyR | Complete Cytogenetic Response |
| SEER | Surveillance, Epidemiology, and End Results |
| MMRF | Multiple Myeloma Research Foundation |
| ASCT | Autologous Stem Cell Transplantation |
| EBMT | European Group for Blood and Marrow Transplantation |
| HDCT | High-Dose Chemotherapy |
| TreoMel | Treosulfan and Melphalan |
| AUC | Area Under the Curve |
| RRMM | Relapsed/Refractory Multiple Myeloma |
| EFS | Event-Free Survival |
| EAPC | Estimated Annual Percentage Change |
| LOY | Loss of Y Chromosome |
| HSPCs | Hematopoietic Stem and Progenitor Cells |
| A:N | Abnormal-to-Normal (ratio) |
| CI | Confidence Interval |
| HR | Hazard Ratio |
| REL | Relapse Rate |
References
- Wästerlid, T.; Cavelier, L.; Haferlach, C.; Konopleva, M.; Fröhling, S.; Östling, P.; Bullinger, L.; Fioretos, T.; Smedby, K.E. Application of precision medicine in clinical routine in haematology—Challenges and opportunities. J. Intern. Med. 2022, 292, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T. Genomics-based precision medicine in hematology/oncology. [Rinsho Ketsueki] Jpn. J. Clin. Hematol. 2021, 62, 1004–1011. [Google Scholar]
- Rosenquist, R.; Bernard, E.; Erkers, T.; Scott, D.W.; Itzykson, R.; Rousselot, P.; Soulier, J.; Hutchings, M.; Östling, P.; Cavelier, L.; et al. Novel precision medicine approaches and treatment strategies in hematological malignancies. J. Intern. Med. 2023, 294, 413–436. [Google Scholar]
- Wagner, A.D.; Oertelt-Prigione, S.; Adjei, A.A.; Buclin, T.; Cristina, V.; Csajka, C.; Coukos, G.; Dafni, U.; Dafni, U.; Dotto, G.; et al. Gender Medicine and Oncology: Report and consensus of an ESMO Workshop. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1914–1924. [Google Scholar] [CrossRef]
- Bartz, D.; Chitnis, T.; Kaiser, U.B.; Rich-Edwards, J.W.; Rexrode, K.M.; Pennell, P.B.; Goldstein, J.M.; O’Neal, M.A.; LeBoff, M.S.; Behn, M.; et al. Clinical Advances in Sex- and Gender-Informed Medicine to Improve the Health of All: A Review. JAMA Intern. Med. 2020, 180, 574–583. [Google Scholar] [PubMed]
- Derman, B.A.; Langerman, S.S.; Maric, M.; Jakubowiak, A.J.; Zhang, W.; Chiu, B.C.-H. Sex differences in outcomes in multiple myeloma. Br. J. Haematol. 2020, 192, e66–e69. [Google Scholar]
- Ebert, B.L. Introduction to a review series on precision hematology. Blood 2017, 130, 408–409. [Google Scholar] [CrossRef]
- Radkiewicz, C.; Bruchfeld, J.B.; Weibull, C.; Lambe, M.; Jakobsen, L.H.; El-Galaly, T.C.; Smedby, K.E.; Wästerlid, T. Sex Differences in Lymphoma Incidence and Excess Mortality By Subtype: A Comprehensive National Study. Blood 2021, 138, 2534. [Google Scholar]
- Quintana, G.R.; Pfaus, J.G. Do Sex and Gender Have Separate Identities? Arch. Sex. Behav. 2024, 53, 2957–2975. [Google Scholar]
- Malik, M. The distinction between the terms sex and gender. In Sex and Cardiac Electrophysiology; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- vom Steeg, L.G.; Klein, S.L. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar]
- Bucher, M.L.; Anderson, F.L.; Lai, Y.; Dicent, J.; Miller, G.W.; Zota, A.R. Exposomics as a tool to investigate differences in health and disease by sex and gender. Exposome 2023, 3, osad003. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.M. Why are sex and gender important to basic physiology and translational and individualized medicine? Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H781–H788. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.W.; Stefanick, M.L.; Peragine, D.; Neilands, T.B.; Ioannidis, J.P.A.; Pilote, L.; Prochaska, J.J.; Cullen, M.R.; Einstein, G.; Klinge, I.; et al. Gender-related variables for health research. Biol. Sex Differ. 2020, 12, 23. [Google Scholar] [CrossRef]
- Becker, J.B.; McClellan, M.L.; Reed, B.G. Sex differences, gender and addiction. J. Neurosci. Res. 2017, 95, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Fattori, M.R.; Honeyborne, I.R.; Ritz, S.A. Considering hormones as sex- and gender-related factors in biomedical research: Challenging false dichotomies and embracing complexity. Horm. Behav. 2023, 156, 105442. [Google Scholar] [CrossRef]
- Edwards, D.A. Competition and testosterone. Horm. Behav. 2006, 50, 681–683. [Google Scholar] [CrossRef]
- Weng, S.; Stoner, S.A.; Zhang, D.-e. Sex chromosome loss and the pseudoautosomal region genes in hematological malignancies. Oncotarget 2016, 7, 72356–72372. [Google Scholar] [CrossRef]
- Shahrabi, S.; Khodadi, E.; Saba, F.; Shahjahani, M.; Saki, N. Sex chromosome changes in leukemia: Cytogenetics and molecular aspects. Hematology 2018, 23, 139–147. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Liang, Y. Sex differences in normal and malignant hematopoiesis. Blood Sci. 2022, 4, 185–191. [Google Scholar] [CrossRef]
- Ratajczak, M.Z. Why are hematopoietic stem cells so ‘sexy’? on a search for developmental explanation. Leukemia 2017, 31, 1671–1677. [Google Scholar]
- Feurstein, S.K.; Drazer, M.W.; Godley, L.A. Genetic predisposition to leukemia and other hematologic malignancies. Semin. Oncol. 2016, 43, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Crispino, J.D.; Horwitz, M.S. GATA factor mutations in hematologic disease. Blood 2017, 129, 2103–2110. [Google Scholar]
- Sood, R.; Kamikubo, Y.; Liu, P.P. Role of RUNX1 in hematological malignancies. Blood 2017, 129, 2070–2082. [Google Scholar]
- Anesi, N.; Miquel, C.-H.; Laffont, S.; Guéry, J.C. The Influence of Sex Hormones and X Chromosome in Immune Responses. Curr. Top. Microbiol. Immunol. 2023, 441, 21–59. [Google Scholar]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423. [Google Scholar] [PubMed]
- Dongarwar, D.; Moriel, K.; Wiafe, K.A.; Salihu, H.M. Sex and Age-Based Differences in Adult Acute Myeloid Leukemia Hospitalizations in the United States, 2009–2018. Int. J. Transl. Med. Res. Public Health 2022, 6, 1–4. [Google Scholar]
- Feng, Z.; Liao, M.; Zhang, L. Sex differences in disease: Sex chromosome and immunity. J. Transl. Med. 2024, 22, 1150. [Google Scholar]
- Evelyn, M.K. Sex Disparities in Cancer Immunotherapy: A Review. Res. Output J. Public Health Med. 2024, 4, 19–24. [Google Scholar]
- Khaldoyanidi, S.K.; Nagorsen, D.; Stein, A.S.; Ossenkoppele, G.J.; Subklewe, M. Immune Biology of Acute Myeloid Leukemia: Implications for Immunotherapy. J. Clin. Oncol. 2021, 39, 419–432. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar]
- Nachi, M.; Kihel, I.; Guella, D.; Dali-Ali, A.; Abed, A.; Boukhatmi, Y.; Enta-Soltane, B.; Belmir, I.; Bekadja, M.A.; Houari, T.E.; et al. Sex and Major Molecular Response to Imatinib Treatment for Patients with Chronic Myeloid Leukemia. Biochem. Pharmacol. Open Access 2019, 8, 2167-0501. [Google Scholar]
- Belsey, S.L.; Ireland, R.M.; Lang, K.; Kizilors, A.; Ho, A.Y.L.; Mufti, G.J.; Bisquera, A.; de Lavallade, H.; Flanagan, R.J. Women Administered Standard Dose Imatinib for Chronic Myeloid Leukemia Have Higher Dose-Adjusted Plasma Imatinib and Norimatinib Concentrations Than Men. Ther. Drug Monit. 2017, 39, 499–504. [Google Scholar]
- Kim, H.-I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Rakshith, H.T.; Lohita, S.; Rebello, A.P.; Goudanavar, P.S.; Raghavendra Naveen, N. Sex differences in drug effects and/or toxicity in oncology. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100152. [Google Scholar] [CrossRef]
- Schmetzer, O.; Flörcken, A. Sex differences in the drug therapy for oncologic diseases. Handb. Exp. Pharmacol. 2012, 214, 411–442. [Google Scholar]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Herrero, M.J.; Aliño, S.F.; Poveda, J.L.; Sanz, M.A.; Montesinos, P. Pharmacogenetics of Metabolic Genes of Anthracyclines in Acute Myeloid Leukemia. Curr. Drug Metab. 2017, 19, 55–74. [Google Scholar] [CrossRef]
- Darphin, X.; Moor, J.; da Silva, C.E.; Richters, A.; Özdemir, B.C. Awareness of the impact of sex and gender in the disease risk and outcomes in hematology and medical oncology—A survey of Swiss clinicians. Cancer Rep. 2024, 7, e1961. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D. Sex differences in cancer chemotherapy effects, and why we need to reconsider BSA-based dosing of chemotherapy. ESMO Open 2020, 5, e000770. [Google Scholar] [CrossRef] [PubMed]
- Delahousse, J.; Wagner, A.D.; Borchmann, S.; Adjei, A.A.; Haanen, J.G.; Burgers, F.; Letsch, A.; Quaas, A.; Oertelt-Prigione, S.; Oezdemir, B.C.; et al. Sex differences in the pharmacokinetics of anticancer drugs: A systematic review. ESMO Open 2024, 9, 104002. [Google Scholar]
- Roman, E.; Smith, A.; Appleton, S.; Crouch, S.; Kelly, R.J.; Kinsey, S.E.; Cargo, C.; Patmore, R. Myeloid malignancies in the real-world: Occurrence, progression and survival in the UK’s population-based Haematological Malignancy Research Network 2004–15. Cancer Epidemiol. 2016, 42, 186–198. [Google Scholar]
- Yi, M.; Li, A.; Zhou, L.; Chu, Q.; Song, Y.; Wu, K. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: Estimates based on the global burden of disease study 2017. J. Hematol. Oncol. 2020, 13, 72. [Google Scholar]
- Inaba, H.; Pui, C.-H. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J. Clin. Med. 2021, 10, 1926. [Google Scholar] [CrossRef]
- Gupta, S.; Teachey, D.T.; Devidas, M.; Chen, Z.; Dunsmore, K.P.; Larsen, E.C.; Maloney, K.W.; Mattano, L.A.; Winter, S.S.; Carroll, A.J.; et al. Sex-Based Disparities in Outcome in Childhood Acute Lymphoblastic Leukemia (ALL): A Children’s Oncology Group (COG) Report. Blood 2020, 136, 38–39. [Google Scholar]
- Brady, S.W.; Roberts, K.G.; Gu, Z.; Shi, L.; Pounds, S.B.; Pei, D.; Cheng, C.; Dai, Y.; Devidas, M.; Qu, C.; et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat. Genet. 2022, 54, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Radivoyevitch, T.; Janković, G.M.; Tiu, R.V.; Saunthararajah, Y.; Jackson, R.C.; Hlatky, L.; Gale, R.P.; Sachs, R.K. Sex differences in the incidence of chronic myeloid leukemia. Radiat. Environ. Biophys. 2014, 53, 55–63. [Google Scholar] [PubMed]
- Baccarani, M.; Castagnetti, F.; Gugliotta, G.; Rosti, G.; Soverini, S.; Albeer, A.; Pfirrmann, M.; International BCR-ABL Study Group. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia 2019, 33, 1173–1183. [Google Scholar]
- Laabidi, B.; Slama, N.; Ouahchi, I.; Boufrikha, W.; Laatiri, M.A. Chronic-phase chronic myeloid leukemia: Incidence of BCR/ABL transcript and its correlation with presenting features, response to treatment, and survival. Leuk. Res. Rep. 2023, 20, 100373. [Google Scholar] [CrossRef]
- Ozga, M.; Nicolet, D.; Mrózek, K.; Yilmaz, A.S.; Kohlschmidt, J.; Larkin, K.T.; Blachly, J.S.; Oakes, C.C.; Buss, J.; Walker, C.J.; et al. Sex-associated differences in frequencies and prognostic impact of recurrent genetic alterations in adult acute myeloid leukemia (Alliance, AMLCG). Leukemia 2023, 38, 45–57. [Google Scholar]
- Garand, R.; Vannier, J.-p.; Béné, M.C.; Faure, G.; Bernard, A. Correlations between acute lymphoid leukemia (ALL) immunophenotype and clinical and laboratory data at presentation. A study of 350 patients. Cancer 1989, 64, 1437–1446. [Google Scholar]
- Faderl, S.; Kantarjian, H.M.; Talpaz, M.; Estrov, Z.E. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood 1998, 91, 3995–4019. [Google Scholar]
- Singh, S.K.; Lupo, P.J.; Scheurer, M.E.; Saxena, A.; Kennedy, A.E.; Ibrahimou, B.; Barbieri, M.A.; Mills, K.I.; McCauley, J.L.; Okcu, M.F.; et al. A childhood acute lymphoblastic leukemia genome-wide association study identifies novel sex-specific risk variants. Medicine 2016, 95, e5300. [Google Scholar] [CrossRef]
- Savage, D.G.; Szydlo, R.; Goldman, J.M. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br. J. Haematol. 1997, 96, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Berger, U.; Maywald, O.; Pfirrmann, M.; Lahaye, T.; Hochhaus, A.; Reiter, A.; Hasford, J.; Heimpel, H.; Hossfeld, D.K.; Kolb, H.-J.; et al. Gender aspects in chronic myeloid leukemia: Long-term results from randomized studies. Leukemia 2005, 19, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Traore, P.; Guilhot, J.; Nelken, B.; Leblanc, T.M.; Leverger, G.; Plantaz, D.; Bertrand, Y.; Bordigoni, P.; Guilhot, F. Clinical and Biological Features at Diagnosis in 40 Children With Chronic Myeloid Leukemia. Pediatrics 2005, 116, 140–143. [Google Scholar] [CrossRef]
- Larkin, K.T.; Nicolet, D.; Kelly, B.J.; Mrózek, K.; LaHaye, S.; Miller, K.E.; Wijeratne, S.; Wheeler, G.L.; Kohlschmidt, J.; Blachly, J.S.; et al. High early death rates, treatment resistance, and short survival of Black adolescents and young adults with AML. Blood Adv. 2022, 6, 5570–5581. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, M.-E.; Szuber, N.; Moussa, H.; Harnois, M.; Assouline, S.E.; Busque, L. Sex-Related Differences in CML Outcomes in a Real-World Prospective Registry (GQR LMC-NMP). Blood 2024, 144, 533. [Google Scholar] [CrossRef]
- Shivani, U.; Shetty, R.A.; Shetty, V.V.; Krishna, R.; Shetty, P.D. Haematological and Clinical Characterization of Chronic Myeloid Leukemia. Res. J. Biotechnol. 2023, 19, 49–54. [Google Scholar] [CrossRef]
- Wilcox, N.S.; Rotz, S.J.; Mullen, M.; Song, E.J.; Ky Hamilton, B.; Moslehi, J.J.; Armenian, S.H.; Wu, J.C.; Rhee, J.-W.; Ky, B. Sex-Specific Cardiovascular Risks of Cancer and Its Therapies. Circ. Res. 2022, 130, 632–651. [Google Scholar] [CrossRef]
- Kammula, A.V.; Schäffer, A.A.; Rajagopal, P.S.; Kurzrock, R.; Ruppin, E. Outcome differences by sex in oncology clinical trials. Nat. Commun. 2024, 15, 2608. [Google Scholar] [CrossRef]
- Iguchi, N.; Hecht, S.L.; Gao, D.; Wilcox, D.T.; Malykhina, A.P.; Cost, N.G. Sexual dimorphic impacts of systemic vincristine on lower urinary tract function. Sci. Rep. 2022, 12, 5113. [Google Scholar] [CrossRef]
- Finch, E.R.; Smith, C.A.; Yang, W.; Liu, Y.; Kornegay, N.M.; Panetta, J.C.; Crews, K.R.; Molinelli, A.R.; Cheng, C.; Pei, D.; et al. Asparaginase formulation impacts hypertriglyceridemia during therapy for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2020, 67, e28040. [Google Scholar]
- Gandy, K.C.; Scoggins, M.A.; Phillips, N.S.; van der Plas, E.; Fellah, S.; Jacola, L.M.; Pui, C.-H.; Hudson, M.M.; Reddick, W.E.; Sitaram, R.; et al. Sex-Based Differences in Functional Brain Activity During Working Memory in Survivors of Pediatric Acute Lymphoblastic Leukemia. JNCI Cancer Spectr. 2022, 6, pkac026. [Google Scholar] [PubMed]
- Lauseker, M.; Gerlach, R.; Worseg, W.; Haferlach, T.; Tauscher, M.; Hasford, J.; Hoffmann, V.S. Differences in treatment and monitoring of chronic myeloid leukemia with regard to age, but not sex: Results from a population-based study. Eur. J. Haematol. 2019, 103, 362–369. [Google Scholar] [PubMed]
- Bird, S.A.; Cairns, D.A.; Menzies, T.; Boyd, K.D.; Davies, F.E.; Cook, G.; Drayson, M.T.; Gregory, W.A.; Jenner, M.W.; Jones, J.R.; et al. Sex Differences in Multiple Myeloma Biology but not Clinical Outcomes: Results from 3894 Patients in the Myeloma XI Trial. Clin. Lymphoma Myeloma Leuk. 2021, 21, 667–675. [Google Scholar]
- Cantú, E.S.; Szymańska, J.A.; Watson, A.; Trevizo, S.L.; Rostami, S. The Frequency of Cytogenetic and FISH Abnormalities Is Higher in Female Than in Male Multiple Myeloma (MM) Patients. Blood 2019, 134, 5514. [Google Scholar]
- Brioli, A.; Nägler, T.M.; Yomade, O.; Rüthrich, M.M.; Scholl, S.; Frietsch, J.J.; Hilgendorf, I.; Ernst, T.; Sayer, H.G.; Hochhaus, A.; et al. Sex-Disaggregated Analysis of Biology, Treatment Tolerability, and Outcome of Multiple Myeloma in a German Cohort. Oncol. Res. Treat. 2022, 45, 494–503. [Google Scholar]
- Dweik, A.; Dweik, H.; Mian, H.S.; Mohan, M.; Schinke, C.D.; Al Hadidi, S. Gender disparities in multiple myeloma publications. EJHaem 2022, 3, 966–969. [Google Scholar]
- Kaushik, R.; Thakur, R.; Gulati, A.; Sharma, S.K. Multiple Myeloma: Clinico-hematological profile in a tertiary care hospital: A three years study. Ann. Pathol. Lab. Med. 2017, 4, A470–A475. [Google Scholar]
- Gahrton, G.; Iacobelli, S.; Apperley, J.F.; Bandini, G.; Björkstrand, B.; Bladé, J.; Boiron, J.M.; Cavo, M.; Cornelissen, J.J.L.M.; Corradini, P.; et al. The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: Reduced relapse risk in female to male transplants. Bone Marrow Transplant. 2005, 35, 609–617. [Google Scholar]
- Posch, D.; Rabitsch, W.; Wohlfarth, P.; Leiner, M.; Porpaczy, E.; Drach, J.; Raderer, M.; Lamm, W. Gender-Specific Aspects in Patients with Multiple Myeloma Undergoing Autologous Stem Cell Transplantation: A Single-Center Experience. Oncology 2017, 93, 295–301. [Google Scholar]
- Heini, A.D.; Kammermann, K.; Bacher, U.; Jeker, B.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Nilius, H.; Pabst, T. Gender-Specific Prognostic Impact of Treosulfan Levels in High-Dose Chemotherapy for Multiple Myeloma. Cancers 2024, 16, 3364. [Google Scholar] [PubMed]
- Arnett, M.; Castro Bórquez, K.M.; Hurtado Martinez, J.A.H.; Robles Espinoza, A.I.; Flores Pérez, P.A.; Cuevas Vicencio, A.J.; Flores Quiroz, F.; Jensen, R.M.; Orlowski, R.Z.; Strain, J.; et al. Exploring gender-based decision-making differences among patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2024, 42, e19524. [Google Scholar] [CrossRef]
- Siddiqui, R.S.; Chaudhary, S.G.; Shahzad, M.; Anwar, I.; Hussain, A.; Ahmed, N.; Abhyankar, S.H.; Shune, L.O.; Hematti, P.; Male, H.J.; et al. Gender disparities in the National Institutes of Health funding for hematologic malignancies and cellular therapies. Leuk. Lymphoma 2022, 63, 1708–1713. [Google Scholar] [PubMed]
- Tinsley-Vance, S.M.; Durosier Mertilus, D.S.; Nodzon, L.A.; Lengacher, C.A. An Integrative Review of Sex Differences in Quality of Life and Symptoms Among Survivors of Hematologic Malignancies. Oncol. Nurs. Forum 2023, 50, 299–312. [Google Scholar]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J. Women’s Health 2014, 23, 112–119. [Google Scholar] [CrossRef]
- Kirtane, K.S.; Lee, S.J. Racial and ethnic disparities in hematologic malignancies. Blood 2017, 130, 1699–1705. [Google Scholar]
- Smith-Graziani, D.; Flowers, C.R. Understanding and Addressing Disparities in Patients with Hematologic Malignancies: Approaches for Clinicians. Am. Soc. Clin. Oncol. Educ. Book. Am. Soc. Clin. Oncol. Annu. Meet. 2021, 41, 1–7. [Google Scholar]
- Loscalzo, M.; Clark, K.L. Abstracts of the 13th Annual Conference of the American Psychosocial Oncology Society, 3–5 March 2016, San Diego, California. Psycho-Oncol. 2016, 25 (Suppl. S2), 1–155. [Google Scholar]
- Liu, L.; Yang, Y.; Wang, Z.-Y.; Wu, H.; Wang, Y.; Wang, L. Prevalence and Positive Correlates of Posttraumatic Stress Disorder Symptoms among Chinese Patients with Hematological Malignancies: A Cross-Sectional Study. PLoS ONE 2015, 10, e0145103. [Google Scholar]
- Tsatsou, I.; Konstantinidis, T.I.; Kalemikerakis, I.; Adamakidou, T.; Vlachou, E.; Govina, O. Unmet Supportive Care Needs of Patients with Hematological Malignancies: A Systematic Review. Asia-Pac. J. Oncol. Nurs. 2020, 8, 5–17. [Google Scholar]
- Hoyt, M.A.; Rubin, L.R. Gender representation of cancer patients in medical treatment and psychosocial survivorship research. Cancer 2012, 118, 4824–4832. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiao, W.; Jiang, Y.; Zhu, M.; Shao, J.; Ren, P.; Liu, D.; Li, W. Effect of sex on the efficacy of patients receiving immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer Med. 2019, 8, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Wesson, W.; Logan, E.; Tabak, C.; Rashid, A.; Mansour, R.; Mushtaq, M.U.; Mcguirk, J.P.; Hashmi, H.; Hamadani, M.; et al. The Glass Wall: Gendered Authorship Disparities in in CD 19 and BCMA CAR-T Clinical Trials for Lymphoma and Myeloma. Blood 2023, 24, e344–e349. [Google Scholar] [CrossRef]
- Bernardi, K.; Lyons, N.B.; Huang, L.; Holihan, J.L.; Olavarria, O.A.; Martin, A.C.; Milton, A.N.; Loor, M.M.; Zheng, F.; Tyson, J.E.; et al. Gender Disparity in Authorship of Peer-Reviewed Medical Publications. Am. J. Med. Sci. 2019, 360, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.D.; Ross, F.M.; Chiecchio, L.; Dagrada, G.P.; Konn, Z.J.; Tapper, W.J.; Walker, B.A.; Wardell, C.P.; Gregory, W.M.; Szubert, A.J.; et al. Gender Disparities in the Tumor Genetics and Clinical Outcome of Multiple Myeloma. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1703–1707. [Google Scholar] [CrossRef]
- Smith, C.J.; Ambs, S.; Landgren, O. Biological determinants of health disparities in multiple myeloma. Blood Cancer J. 2018, 8, 85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansarian, M.A.; Fatahichegeni, M.; Ren, J.; Wang, X. Sex and Gender in Myeloid and Lymphoblastic Leukemias and Multiple Myeloma: From Molecular Mechanisms to Clinical Outcomes. Curr. Oncol. 2025, 32, 204. https://doi.org/10.3390/curroncol32040204
Ansarian MA, Fatahichegeni M, Ren J, Wang X. Sex and Gender in Myeloid and Lymphoblastic Leukemias and Multiple Myeloma: From Molecular Mechanisms to Clinical Outcomes. Current Oncology. 2025; 32(4):204. https://doi.org/10.3390/curroncol32040204
Chicago/Turabian StyleAnsarian, Mohammad Amin, Mahsa Fatahichegeni, Juan Ren, and Xiaoning Wang. 2025. "Sex and Gender in Myeloid and Lymphoblastic Leukemias and Multiple Myeloma: From Molecular Mechanisms to Clinical Outcomes" Current Oncology 32, no. 4: 204. https://doi.org/10.3390/curroncol32040204
APA StyleAnsarian, M. A., Fatahichegeni, M., Ren, J., & Wang, X. (2025). Sex and Gender in Myeloid and Lymphoblastic Leukemias and Multiple Myeloma: From Molecular Mechanisms to Clinical Outcomes. Current Oncology, 32(4), 204. https://doi.org/10.3390/curroncol32040204