Technical Innovations and Complex Cases in Robotic Surgery for Lung Cancer: A Narrative Review
Abstract
1. Introduction
2. State of the Art
- –
- The surgeon console, where the first surgeon stays, with an advanced 3D visor.
- –
- The vision cart, which allows the control of the robotic system.
- –
- The patients’ cart, with four arms that can be adapted to the patient’s position.
- –
- Monopolar cautery instruments, like different-sized hooks and monopolar scissors.
- –
- Bipolar graspers, with different-shaped and sized branches (like “Maryland” branches, fenestrated bipolar, longer or shorter graspers).
- –
- Robotic clip appliers, with the possibility to choose between small, medium, and large clips.
- –
- Needle drivers, for robotic sutures.
- –
- Robotic graspers, with different branches and sizes for different uses.
- –
- Robotic scissors.
- –
- Other special instruments.
- –
- Robotic staplers, with the possibility to choose vascular or parenchymal staplers.
3. Robotic Surgery in Advanced Tumors
4. Robotic Surgery After Neoadjuvant Therapy
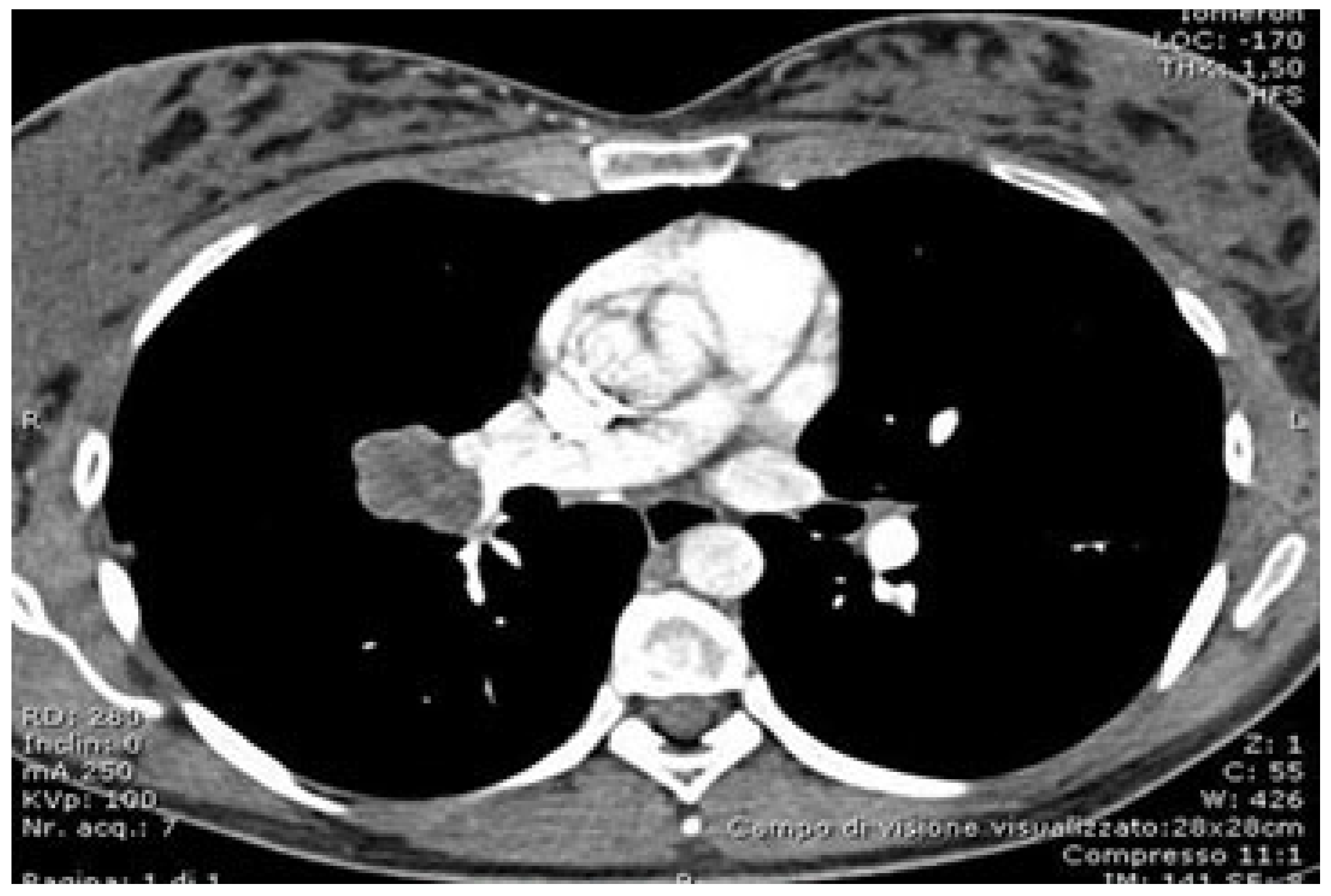
5. Preoperative Evaluation and Software in Thoracic Surgery
5.1. Preoperative Evaluation and Software in Robotic Surgery
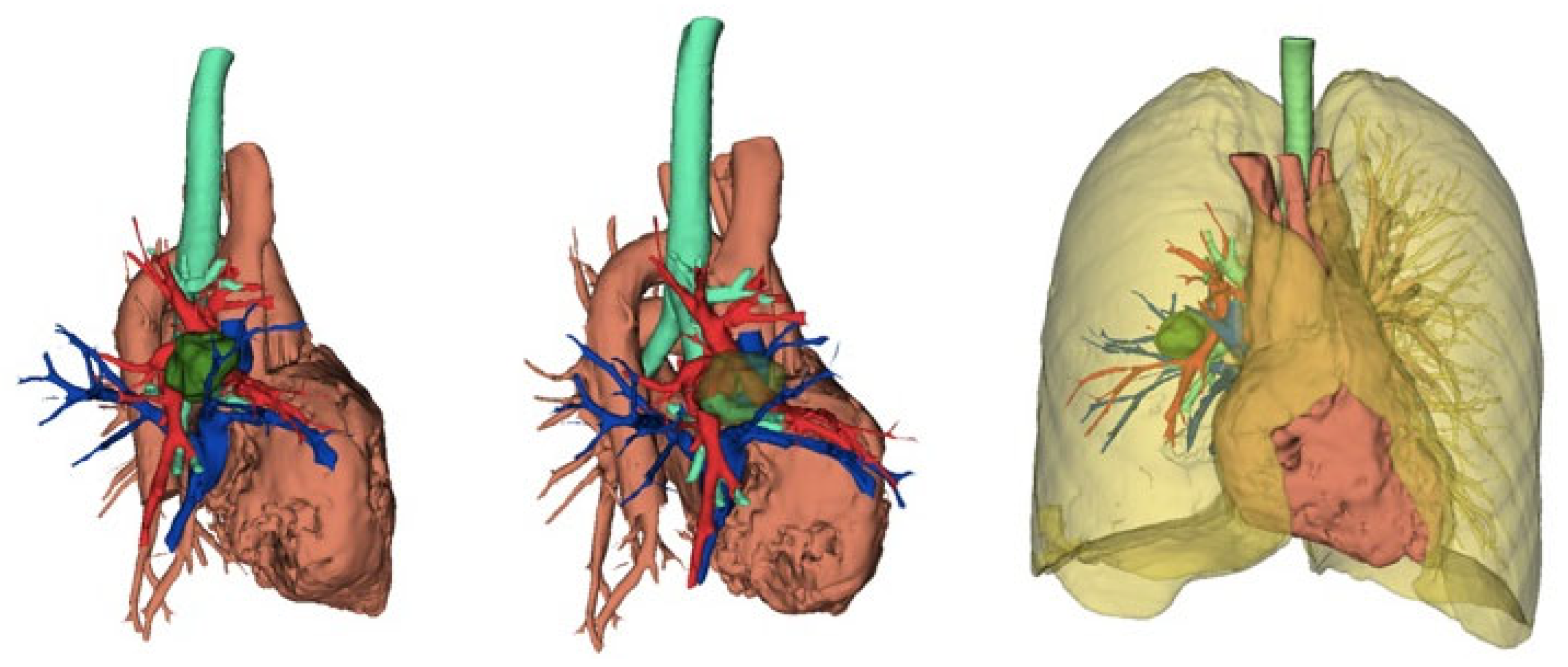
5.2. Role of Advanced Software in Preoperative Planning
5.3. Navigation and Enhanced Visualization
5.4. Predictive Analytics and Artificial Intelligence
5.5. The Impact of Software on Workflow and Training
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ma, J.; Li, X.; Zhao, S.; Wang, J.; Zhang, W.; Sun, G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: A meta-analysis. BMC Cancer 2021, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Mehran, R.J.; Mitchell, K.G.; Rajaram, R.; Correa, A.M.; Bassett, R.L., Jr.; Antonoff, M.B.; Hofstetter, W.L.; Roth, J.A.; Sepesi, B.; et al. Robotic assisted lobectomy for non-small cell lung Cancer: A comprehensive institutional experience. Ann. Thorac. Surg. 2019, 108, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.W.; Jones, D.R.; et al. Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Catelli, C.; Corzani, R.; Zanfrini, E.; Franchi, F.; Ghisalberti, M.; Ligabue, T.; Meniconi, F.; Monaci, N.; Galgano, A.; Mathieu, F.; et al. RoboticAssisted (RATS) versus Video-Assisted (VATS) lobectomy: A monocentric prospective randomized trial. Eur. J. Surg. Oncol. 2023, 49, 107256. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.I.; Lee, J.H.; Kim, H.K. Biportal robotic surgery for anterior mediastinal mass. Ann. Cardiothorac. Surg. 2023, 12, 110–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez-Rivas, D.; Bosinceanu, M.; Manolache, V.; Gallego-Poveda, J.; Garcia, A.; Paradela, M.; Dunning, J.; Bale, M.; Motas, N. Uniportal fully robotic-assisted major pulmonary resections. Ann. Cardiothorac. Surg. 2023, 12, 52–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuzmych, K.; Sassorossi, C.; Nachira, D.; Congedo, M.T.; Margaritora, S.; Meacci, E. Fully Dual-Portal Robotic-Assisted Thoracic Surgery (F-DRATS) and Indocyanine Green-Navigated Segmentectomy. Surg. Tech. Dev. 2024, 13, 294–300. [Google Scholar] [CrossRef]
- Melfi, F.M.; Menconi, G.F.; Mariani, A.M.; Angeletti, C.A. Early experience with robotic technology for thoracoscopic surgery. Eur. J. Cardio-Thoracic Surg. 2002, 21, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Melfi, F.M.; Mussi, A. Robotically assisted lobectomy: Learning curve and complications. Thorac. Surg. Clin. 2008, 18, 289–295, vi–vii. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G.; Galetta, D.; Melfi, F.; Spaggiari, L. First Italian experience of robotic thymectomy for thymoma. Interact. CardioVasc. Thorac. Surg. 2010, 10, 709–712. [Google Scholar]
- Veronesi, G.; Galetta, D.; Maisonneuve, P.; Melfi, F.; Schmid, R.A.; Borri, A.; Vannucci, F.; Spaggiari, L. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Melfi, F.; Mussi, A.; Maisonneuve, P.; Spaggiari, L.; Da Silva, R.K.; Veronesi, G. Robotic lobectomy for non-small cell lung cancer (NSCLC): Long-term oncologic results. J. Thorac. Cardiovasc. Surg. 2012, 143, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, D.; Bosinceanu, M.; Motas, N.; Manolache, V. Uniportal robotic-assisted thoracic surgery for lung resections. Eur. J. Cardiothorac. Surg. 2022, 62, ezac410. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, D.; Manolache, V.; Bosinceanu, M.L.; Gallego-Poveda, J.; Garcia-Perez, A.; de la Torre, M.; Turna, A.; Motas, N. Uniportal pure robotic-assisted thoracic surgery-technical aspects, tips and tricks. Ann. Transl. Med. 2023, 11, 362. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Bale, M.; Bosinceanu, M.L.; Chinthareddy, R. Uniportal robotic-assisted thoracoscopic surgery right upper lobectomy for aspergilloma. Ann. Cardiothorac. Surg. 2023, 12, 142–143. [Google Scholar] [CrossRef]
- Manolache, V.; Motas, N.; Bosinceanu, M.L.; de la Torre, M.; Gallego-Poveda, J.; Dunning, J.; Ismail, M.; Turna, A.; Paradela, M.; Decker, G.; et al. Comparison of uniportal robotic-assisted thoracic surgery pulmonary anatomic resections with multiport robotic-assisted thoracic surgery: A multicenter study of the European experience. Ann. Cardiothorac. Surg. 2023, 12, 102–109. [Google Scholar] [CrossRef]
- NCCN. Clinical Practice Guidelines in Oncology, Version 1.2024, Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 21 February 2025).
- Caso, R.; Watson, T.J.; Khaitan, P.G.; Marshall, M.B. Outcomes of minimally invasive sleeve resection. J. Thorac. Dis. 2018, 10, 6653–6659. [Google Scholar] [CrossRef]
- Zirafa, C.C.; Romano, G.; Sicolo, E.; Bagalà, E.; Manfredini, B.; Alì, G.; Castaldi, A.; Morganti, R.; Davini, F.; Fontanini, G.; et al. Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes. Curr. Oncol. 2023, 30, 9104–9115. [Google Scholar] [CrossRef]
- Lee, M.; Razi, S.S. Pulmonary Sleeve Resection. [Updated 25 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564400/ (accessed on 11 January 2024).
- Verm, R.A.; Vigneswaran, W.T.; Lin, A.; Zywiciel, J.; Freeman, R.; Abdelsattar, Z.M. Robotic chest wall resection for primary benign chest wall tumors and locally advanced lung cancer: An institutional case series and national report. J. Thorac. Dis. 2023, 15, 4849–4858. [Google Scholar] [CrossRef]
- Rojo, M.; Abdelsattar, Z. Robotic resection of a second rib osteochondroma. Multimed. Man. Cardiothorac. Surg. 2023, 2023. [Google Scholar] [CrossRef] [PubMed]
- Egyud, M.R.L.; Holmes, S.; Burt, M.B. Technical Aspects of Robotic First Rib Resection. Thorac. Surg. Clin. 2023, 33, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Egyud, M.R.L.; Burt, M.B. Robotic First Rib Resection and Robotic Chest Wall Resection. Thorac. Surg. Clin. 2023, 33, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Petrella, F.; Sedda, G.; Mazzella, A.; Guarize, J.; Maisonneuve, P.; De Marinis, F.; Spaggiari, L. Preliminary Results of Robotic Lobectomy in Stage IIIA-N2 NSCLC after Induction Treatment: A Case Control Study. J. Clin. Med. 2021, 10, 3465. [Google Scholar] [CrossRef] [PubMed]
- Shahin, G.M.M.; Vos, P.-P.W.K.; Hutteman, M.; Stigt, J.A.; Braun, J. Robot-assisted thoracic surgery for stages IIB-IVA non-small cell lung cancer: Retrospective study of feasibility and outcome. J. Robot. Surg. 2023, 17, 1587–1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kocher, G.; Deckarm, S.; Flury, D. Completely portal robotic Pancoast tumour resection with en bloc resection of the left upper lobe and chest wall. Multimed. Man. Cardiothorac. Surg. 2023, 2023. [Google Scholar] [CrossRef]
- Smith, A.; Wali, A.; Montes, A.; Hadaki, M.; Harrison-Phipps, K.; Karapanagiotou, E.M.; Bille, A. Salvage pulmonary resection in stages IIIb-IV lung cancer after treatment with immune checkpoint inhibitors case series and literature review. J. Surg. Oncol. 2022, 125, 290–298. [Google Scholar] [CrossRef]
- Baig, M.Z.; Razi, S.S.; Agyabeng-Dadzie, K.; Stroever, S.; Muslim, Z.; Weber, J.; Herrera, L.J.; Bhora, F.Y. Robotic-assisted thoracoscopic surgery demonstrates a lower rate of conversion to thoracotomy than video-assisted thoracoscopic surgery for complex lobectomies. Eur. J. Cardiothorac. Surg. 2022, 62, ezac281. [Google Scholar] [CrossRef]
- Asif, A.; Lilley, D.; Howard-Walker, S.; Ajab, S.; Qadri, S.S. The diagnosis and surgical management of pulmonary sequestration in adults: A case series from a single centre in the UK. Indian J. Thorac. Cardiovasc. Surg. 2024, 40, 91–95. [Google Scholar] [CrossRef]
- Tong, X.; Xu, S.; Wang, S.; Meng, H.; Gao, X.; Teng, H.; Ding, R.; Liu, X.; Li, B.; Wang, T.; et al. Clinical experience of the treatment of solitary pulmonary nodules with Da Vinci surgical system. Zhongguo Fei Ai Za Zhi 2014, 17, 541–544. [Google Scholar]
- Emerson, D.; Catarino, P.; Rampolla, R.; Chikwe, J.; Megna, D. Robotic-assisted lung transplantation: First in man. J. Heart Lung Transplant. 2024, 43, 158–161. [Google Scholar] [CrossRef]
- Ascanio, F.; Royo-Crespo, I.; Rosado, J.; Sánchez, L.; Romero, L.; Durán-Rey, D.; Sánchez-Margallo, F.; Jauregui, A. Advances in robotic lung transplantation: Development and validation of a new surgical technique in animal models. Interdiscip. CardioVasc. Thorac. Surg. 2023, 37, ivad179. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Onaitis, M.W.; D’amico, T.A.; Chen, H. Minimally Invasive Thoracic Surgery 3.0: Lessons Learned from the History of Lung Cancer Surgery. Ann. Surg. 2018, 267, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Alban, J.; Kennedy, K.; Hulbert, A.; Lighter, M.; Pasquinelli, M.; Rubinstein, I.; Ghelani, S.; Clayburn, A.; Feldman, L.E. Surgery for early-stage lung cancer with video-assisted thoracoscopic surgery versus open thoracotomy: A narrative review. Semin. Oncol. 2022, 49, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Herb, J.N.; Kindell, D.G.; Strassle, P.D.; Stitzenberg, K.B.; Haithcock, B.E.; Mody, G.N.; Long, J.M. Trends and Outcomes in Minimally Invasive Surgery for Locally Advanced Non-Small-Cell Lung Cancer with N2 Disease. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 547–555. [Google Scholar] [CrossRef]
- Yan, T.D.; Black, D.; Bannon, P.G.; McCaughan, B.C. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 2553–2562. [Google Scholar] [CrossRef]
- Kent, M.; Wang, T.; Whyte, R.; Curran, T.; Flores, R.; Gangadharan, S. Open, video-assisted thoracic surgery, and robotic lobectomy: Review of a national database. Ann. Thorac. Surg. 2014, 97, 236–244. [Google Scholar] [CrossRef]
- Veronesi, G.; Park, B.; Cerfolio, R.; Dylewski, M.; Toker, A.; Fontaine, J.P.; Hanna, W.C.; Morenghi, E.; Novellis, P.; Velez-Cubian, F.O.; et al. Robotic resection of Stage III lung cancer: An international retrospective study. Eur. J. Cardiothorac. Surg. 2018, 54, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J. Robotic sleeve lobectomy: Technical details and early results. J. Thorac. Dis. 2016, 8 (Suppl. S2), S223–S226. [Google Scholar]
- Qiu, T.; Zhao, Y.; Xuan, Y.; Qin, Y.; Niu, Z.; Shen, Y.; Jiao, W. Robotic sleeve lobectomy for centrally located non-small cell lung cancer: A propensity score-weighted comparison with thoracoscopic and open surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 838–846.e2. [Google Scholar] [CrossRef]
- Patton, B.D.; Zarif, D.; Bahroloomi, D.M.; Sarmiento, I.C.; Lee, P.C.; Lazzaro, R.S. Robotic Pneumonectomy for Lung Cancer: Perioperative Outcomes and Factors Leading to Conversion to Thoracotomy. Innovations 2021, 16, 136–141. [Google Scholar] [CrossRef]
- Scheinerman, J.A.; Jiang, J.; Chang, S.H.; Geraci, T.C.; Cerfolio, R.J. Extended Robotic Pulmonary Resections. Front. Surg. 2021, 8, 597416. [Google Scholar] [CrossRef] [PubMed]
- Louie, B.E. Robotic pneumonectomy. Thorac. Surg. Clin. 2014, 24, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Demmy, T.L.; Yendamuri, S.; Hennon, M.W.; Dexter, E.U.; Picone, A.L.; Nwogu, C. Thoracoscopic maneuvers for chest wall resection and reconstruction. J. Thorac. Cardiovasc. Surg. 2012, 144, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Mariolo, A.V.; Casiraghi, M.; Galetta, D.; Spaggiari, L. Robotic Hybrid Approach for an Anterior Pancoast Tumor in a Severely Obese Patient. Ann. Thorac. Surg. 2018, 106, e115–e116. [Google Scholar] [CrossRef]
- Park, B.J.; Yang, H.X.; Woo, K.M.; Sima, C.S. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J. Thorac. Dis. 2016, 8 (Suppl. S4), S406–S413. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Yoshimura, A.; Hagiwara, M.; Akata, S.; Saji, H. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann. Thorac. Cardiovasc. Surg. 2013, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen-Yoshikawa, T.F.; Date, H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J. Thorac. Dis. 2016, 8 (Suppl. S3), S295–S301. [Google Scholar] [CrossRef]
- Mattioni, G.; Palleschi, A.; Mendogni, P.; Tosi, D. Approaches and outcomes of Robotic-Assisted Thoracic Surgery (RATS) for lung cancer: A narrative review. J. Robot Surg. 2023, 17, 797–809. [Google Scholar] [CrossRef]
- Cao, C.; Louie, B.E.; Melfi, F.; Veronesi, G.; Razzak, R.; Romano, G.; Novellis, P.; Ranganath, N.K.; Park, B.J. Outcomes of major complications after robotic anatomic pulmonary resection. J. Thorac. Cardiovasc. Surg. 2020, 159, 681–686. [Google Scholar] [CrossRef]
- Samsonik, S.A.; Esakov, Y.S.; Regushevskaya, D.V.; Banova, Z.I. Planirovanie anatomicheskikh rezektsii legkogo: Preabilitatsiya i funktsional’noe testirovanie [Management before anatomical lung resections: Prehabilitation and functional testing]. Khirurgiia 2024, 91–98. [Google Scholar] [CrossRef]
- Rojas-Solano, J.R.; Ugalde-Gamboa, L.; Machuzak, M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J. Bronchol. Interv. Pulmonol. 2018, 25, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Pastis, N.J., Jr.; Mahajan, A.K.; Khandhar, S.J.; Simoff, M.J.; Machuzak, M.S.; Cicenia, J.; Gildea, T.R.; Silvestri, G.A. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021, 159, 845–852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batirel, H.F. Robotic Surgery in Thoracic Oncology: A Worldwide Perspective. J. Thorac. Dis. 2020, 12, 7026–7042. [Google Scholar]
- Cusumano, G.; D’Arrigo, S.; Terminella, A.; Lococo, F. Artificial Intelligence Applications for Thoracic Surgeons: “The Phenomenal Cosmic Powers of the Magic Lamp”. J. Clin. Med. 2024, 13, 3750. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Gagliano, C.; Lentini, M.; Masiello, E.; Parisi, F.; Iannella, G.; Cilia, N.D.; Salerno, V.; Cusumano, G.; et al. The Integration of Radiomics and Artificial Intelligence in Modern Medicine. Life 2024, 14, 1248. [Google Scholar] [CrossRef]
- Doornbos, M.J.; Peek, J.J.; Maat, A.P.W.M.; Ruurda, J.P.; De Backer, P.; Cornelissen, B.M.W.; Mahtab, E.A.F.; Sadeghi, A.H.; Kluin, J. Augmented Reality Implementation in Minimally Invasive Surgery for Future Application in Pulmonary Surgery: A Systematic Review. Surg. Innov. 2024, 31, 646–658. [Google Scholar] [CrossRef]
- Durand, M.; Nguyen, L.S.; Mbadinga, F.; Pryshchepau, M.; Portefaix, H.; Chaabane, N.; Ropert, S.; Khen-Dunlop, N. Robotic thoracic surgery: Lessons learned from the first 1000 procedures. Front. Surg. 2024, 12, 1417787. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusumano, G.; Calabrese, G.; Gallina, F.T.; Facciolo, F.; Novellis, P.; Veronesi, G.; Viscardi, S.; Lococo, F.; Meacci, E.; Terminella, A.; et al. Technical Innovations and Complex Cases in Robotic Surgery for Lung Cancer: A Narrative Review. Curr. Oncol. 2025, 32, 244. https://doi.org/10.3390/curroncol32050244
Cusumano G, Calabrese G, Gallina FT, Facciolo F, Novellis P, Veronesi G, Viscardi S, Lococo F, Meacci E, Terminella A, et al. Technical Innovations and Complex Cases in Robotic Surgery for Lung Cancer: A Narrative Review. Current Oncology. 2025; 32(5):244. https://doi.org/10.3390/curroncol32050244
Chicago/Turabian StyleCusumano, Giacomo, Giuseppe Calabrese, Filippo Tommaso Gallina, Francesco Facciolo, Pierluigi Novellis, Giulia Veronesi, Stefano Viscardi, Filippo Lococo, Elisa Meacci, Alberto Terminella, and et al. 2025. "Technical Innovations and Complex Cases in Robotic Surgery for Lung Cancer: A Narrative Review" Current Oncology 32, no. 5: 244. https://doi.org/10.3390/curroncol32050244
APA StyleCusumano, G., Calabrese, G., Gallina, F. T., Facciolo, F., Novellis, P., Veronesi, G., Viscardi, S., Lococo, F., Meacci, E., Terminella, A., Romano, G., Zirafa, C., Melfi, F., Margaritora, S., & Chiappetta, M. (2025). Technical Innovations and Complex Cases in Robotic Surgery for Lung Cancer: A Narrative Review. Current Oncology, 32(5), 244. https://doi.org/10.3390/curroncol32050244





