The Influence of Poverty and Rurality on Colorectal Cancer Survival by Race/Ethnicity: An Analysis of SEER Data with a Census Tract-Level Measure of Persistent Poverty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Study Participants
2.3. Measures: Outcome, Exposure, and Covariates
2.4. Statistical Analysis
2.5. Data Availability
3. Results
3.1. Patient Demographics, Tumor Characteristics, and Initial Treatment Modality
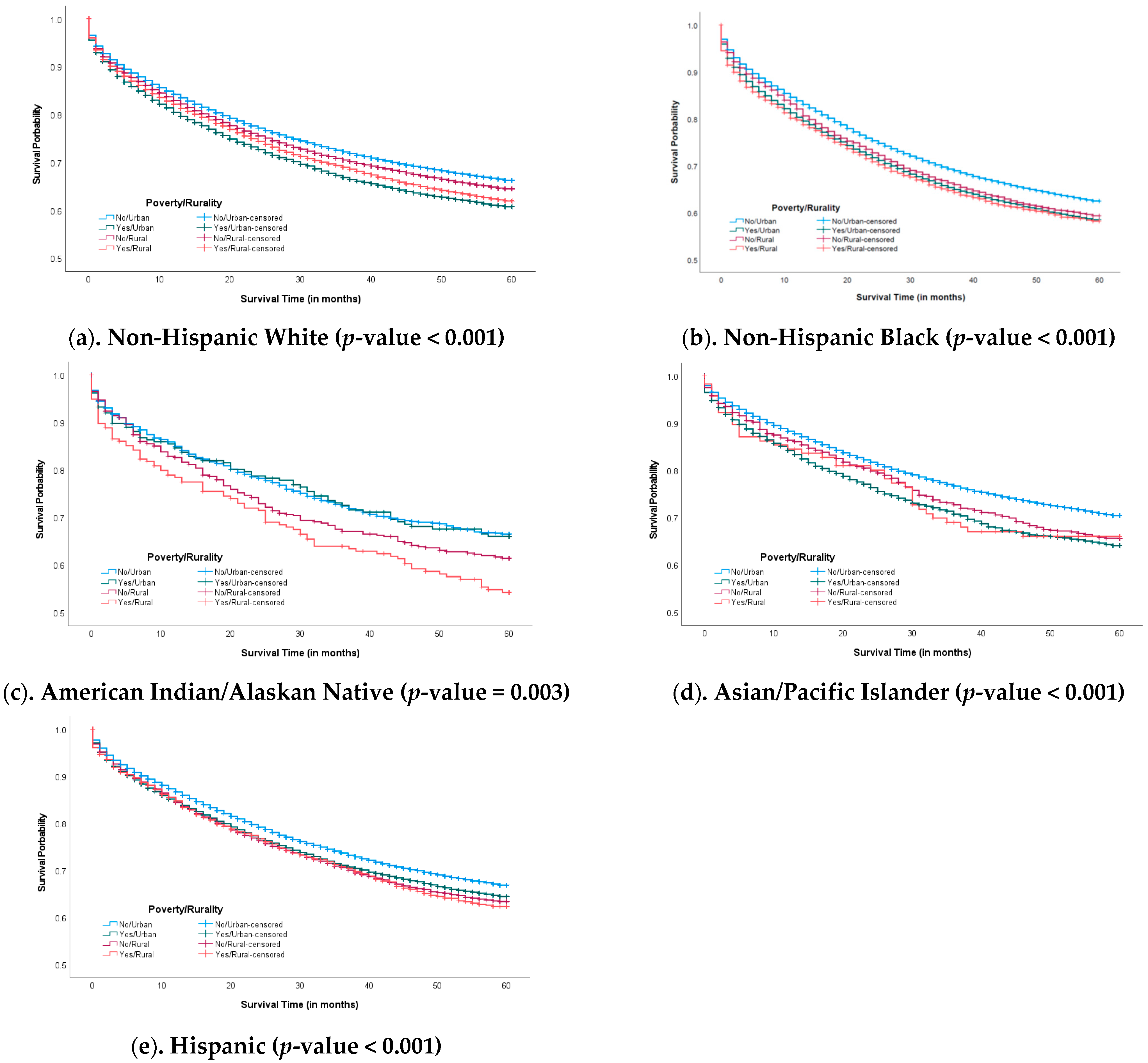
3.2. Five-Year Survival Probability
3.3. Association Between Race/Ethnicity, Persistent Poverty, Rurality, and Cause-Specific CRC Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. STROBE Statement—Checklist of Items That Should Be Included in Reports of Cohort Studies
| Item No | Recommendation | Page No | |
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 1, 2 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 2 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 1, 2 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 2 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | 2, 3 | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 3 |
| Data sources/measurement | 8 * | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 2, 3 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 3, 4 |
| Study size | 10 | Explain how the study size was arrived at | 2, 3 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 3 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | |
| (b) Describe any methods used to examine subgroups and interactions | |||
| (c) Explain how missing data were addressed | 3, 4 | ||
| (d) If applicable, explain how loss to follow-up was addressed | |||
| (e) Describe any sensitivity analyses | |||
| Results | |||
| Participants | 13 * | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 2, 3 |
| (b) Give reasons for non-participation at each stage | |||
| (c) Consider use of a flow diagram | |||
| Descriptive data | 14 * | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | |
| (b) Indicate number of participants with missing data for each variable of interest | 4, 5 | ||
| (c) Summarise follow-up time (eg, average and total amount) | |||
| Outcome data | 15 * | Report numbers of outcome events or summary measures over time | 5, 6 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | |
| (b) Report category boundaries when continuous variables were categorized | 4, 9 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 9 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 9 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 10,11 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 10 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 10 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 11 |
| * Give information separately for exposed and unexposed groups. Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org (accessed on 20 April 2025). | |||
References
- American Cancer Society. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures for African American/Black People 2022–2024; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Coughlin, S.S.; Blumenthal, D.S.; Jordan Seay, S.; Smith, S.A. Toward the elimination of colorectal cancer disparities among African Americans. J. Racial Ethn. Health Disparities 2016, 3, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.; Markossian, T.; Johnson, A.; Dong, F.; Bayakly, R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am. J. Public Health 2014, 104, e63–e71. [Google Scholar] [CrossRef]
- Steinbrecher, A.; Fish, K.; Clarke, C.A.; West, D.W.; Gomez, S.L.; Cheng, I. Examining the association between socioeconomic status and invasive colorectal cancer incidence and morality in California. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1814–1822. [Google Scholar] [CrossRef]
- Enewold, L.; Horner, M.-J.; Shriver, C.D.; Zhu, K. Socioeconomic disparities in colorectal cancer mortality in the United States, 1990–2007. J. Community Health 2014, 39, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Vernon, M.; Su, S.; Coughlin, S.S.; Dong, Y. Racial disparities in the relationship of regional socioeconomic status and colorectal cancer survival in the five regions of Georgia. Cancer Med. 2024, 13, e6954. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Fang, S.; Vernon, S.W.; El-Serag, H.; Shih, Y.T.; Davila, J.; Rasmus, M.L. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer 2007, 110, 660–669. [Google Scholar] [CrossRef]
- Balan, N.; Petrie, B.A.; Chen, K.T. Racial disparities in colorectal cancer care for Black patients: Barriers and solutions. Am. Surg. 2022, 88, 2823–2830. [Google Scholar] [CrossRef]
- Moralez, E.A.; Rao, S.P.; Livaudais, J.C.; Thompson, B. Improving knowledge and screening for colorectal cancer among Hispanics: Overcoming barriers through a PROMOTORA-led home-based educational intervention. Cancer Educ. 2012, 27, 533–539. [Google Scholar] [CrossRef]
- Adams, S.A.; Zahnd, W.E.; Ranganathan, R.; Hung, P.; Brown, M.J.; Truman, S.; Biesecker, C.; Kirksey, V.C.; Eberth, J.M. Rural and racial disparities in colorectal cancer incidence and mortality in South Carolina, 1996–2016. J. Rural Health 2022, 38, 34–39. [Google Scholar] [CrossRef]
- Tobin, E.C.; Nolan, N.; Thompson, S.; Elmore, M.; Richmond, B.K. The intersection of race and rurality and its effect on colorectal cancer survival. Am. Surg. 2023, 89, 3163–3170. [Google Scholar] [CrossRef]
- Andrilla, C.H.; Moore, T.E.; Wong, K.M.; Evans, D.V. Investigating the impact of geographic location on colorectal cancer stage at diagnosis: A national study of the SEER cancer registry. J. Rural Health 2020, 36, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S.; Clary, C.; Johnson, J.A.; Berman, A.; Heboyan, V.; Benevides, T.; Moore, J.; George, V. Continuing challenges in rural health in the United States. J. Environ. Health Sci. 2019, 5, 90–92. [Google Scholar]
- Ricketts, T.C. The changing nature of rural health care. Annu. Rev. Public Health 2000, 21, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Tammana, V.S.; Laiyemo, A.O. Colorectal cancer disparities: Issues, controversies and solutions. World J. Gastroenterol. 2014, 20, 869–876. [Google Scholar] [CrossRef]
- Adams, L.B.; Richmond, J.; Corbie-Smith, G.; Powell, W. Medical mistrust and colorectal cancer screening among African Americans. Community Health 2017, 42, 1044–1061. [Google Scholar] [CrossRef]
- Aspiras, O.; Lucas, T.; Thompson, H.S.; Manning, M.A. Medical mistrust, culturally targeted message framing, and colorectal cancer screening among African Americans. J. Behav. Med. 2023, 46, 871–881. [Google Scholar] [CrossRef]
- Lee, J.; Ewing, B.; Holmes, D. Barriers and Facilitators to Colorectal Cancer Screening in South Asian Immigrants: A Systematic Review. Asian Pac. J. Cancer Prev. 2023, 24, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- James, A.S.; Filippi, M.K.; Pacheco, C.M.; Cully, L.; Perdue, D.; Choi, W.S.; Greiner, K.A.; Daley, C.M. Barriers to colorectal cancer screening among American Indian men aged 50 or older, Kansas and Missouri, 2006–2008. Prev. Chronic Dis. 2013, 10, E170. [Google Scholar] [CrossRef]
- Moss, J.L.; Pinto, C.N.; Srinivasan, S.; Cronin, K.A.; Croyle, R.T. Enduring cancer disparities by persistent poverty, rurality, and race: 1990–1992 to 2014–2018. J. Natl. Cancer Inst. 2022, 114, djac038. [Google Scholar] [CrossRef]
- Ellis, L.; Canchol, J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef]
- Lo, S.H.; Waller, J.; Vrinten, C.; Kobayashi, L.; von Wagner, C. Social cognitive mediators of sociodemographic differences in colorectal cancer screening uptake. BioMed Res. Int. 2015, 2015, 165074. [Google Scholar] [CrossRef] [PubMed]
- Obrochta, C.A.; Murphy, J.D.; Tsou, M.H.; Thompson, C.A. Disentangling racial, ethnic, and socioeconomic disparities in treatment for colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Rugo, H.; Burke, N.J. Navigating a path to equity in cancer care: The role of patient navigation. Am. Soc. Clin. Oncol. Educ. Book. 2021, 41, 3–10. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 532,868) | NHW (n = 363,505) | NHB (n = 63,537) | AI/AN (n = 2236) | Asian/PI (n = 35,453) | Hispanic (n = 68,137) | p-Value | |
|---|---|---|---|---|---|---|---|
| n (%) | |||||||
| Persistent poverty a | <0.001 | ||||||
| No | 476,874 (89.5%) | 343,676 (94.6%) | 45,843 (72.2%) | 1782 (79.7%) | 33,113 (93.4%) | 52,460 (77.0%) | |
| Yes | 55,994 (10.5%) | 19,829 (5.5%) | 17,694 (27.9%) | 454 (20.3%) | 2340 (6.6%) | 15,677 (23.0%) | |
| Rurality a | <0.001 | ||||||
| No | 459,119 (86.2%) | 302,279 (83.2%) | 58,171 (91.6%) | 1585 (70.9%) | 34,273 (96.7%) | 62,811 (92.2%) | |
| Yes | 73,749 (13.8%) | 61,226 (16.8%) | 5366 (8.5%) | 651 (29.1%) | 1180 (3.3%) | 5326 (7.8%) | |
| Poverty/Rurality | <0.001 | ||||||
| Poverty alone | 43,550 (8.2%) | 11,749 (3.2%) | 15,376 (24.2%) | 238 (10.6%) | 2222 (6.3%) | 13,965 (20.5%) | |
| Rural alone | 61,305 (11.5%) | 53,146 (14.6%) | 3048 (4.8%) | 435 (19.5%) | 1062 (3.0%) | 3614 (5.3%) | |
| Poverty & Rural | 12,444 (2.3%) | 8080 (2.2%) | 2318 (3.7%) | 216 (9.7%) | 118 (0.3%) | 1712 (2.5%) | |
| Non-Poverty & Rural | 415,569 (78.0%) | 290,530 (79.9%) | 42,795 (67.4%) | 1347 (60.2%) | 32,051 (90.4%) | 48,846 (71.7%) | |
| Demographic characteristics | |||||||
| Age | <0.001 | ||||||
| 20–39 years | 15,506 (2.9%) | 8412 (2.3%) | 1990 (3.1%) | 107 (4.5%) | 1317 (3.7%) | 3680 (5.4%) | |
| 40–59 years | 147,500 (27.7%) | 88,534 (24.4%) | 22,665 (35.7%) | 773 (34.6%) | 11,543 (32.6%) | 23,985 (35.2%) | |
| 60–79 years | 253,664 (47.6%) | 174,944 (48.1%) | 30,225 (47.6%) | 1097 (49.1%) | 16,537 (46.6%) | 30,861 (45.3%) | |
| 80+ years | 116,198 (21.8%) | 91,615 (25.2%) | 8657 (13.6%) | 259 (11.6%) | 6056 (17.1%) | 9611 (14.1%) | |
| Gender | <0.001 | ||||||
| Male | 277,537 (52.1%) | 189,128 (52.0%) | 31,499 (49.6%) | 1157 (51.7%) | 18,520 (52.2%) | 37,233 (54.6%) | |
| Female | 255,331 (47.9%) | 174,377 (48.0%) | 32,038 (50.4%) | 1079 (48.3%) | 16,933 (47.8%) | 30,904 (45.4%) | |
| Marital status | <0.001 | ||||||
| Single | 69,090 (13.0%) | 39,019 (10.7%) | 15,307 (24.1%) | 417 (18.7%) | 4317 (12.2%) | 10,030 (14.7%) | |
| Married | 227,687 (42.7%) | 163,430 (45.0%) | 18,614 (29.3%) | 868 (38.8%) | 20,695 (58.4%) | 24,080 (35.3%) | |
| Other b | 122,132 (22.9%) | 90,017 (24.8%) | 14,268 (22.5%) | 546 (24.4%) | 6433 (18.2%) | 10,868 (16.0%) | |
| Unknown | 113,959 (21.4%) | 71,039 (19.5%) | 15,348 (24.2%) | 405 (18.1%) | 4008 (11.3%) | 23,159 (34.0%) | |
| Year of diagnosis | <0.001 | ||||||
| 2006–2010 | 269,396 (50.6%) | 188,606 (51.9%) | 31,641 (49.8%) | 972 (43.5%) | 16,760 (47.3%) | 31,417 (46.1%) | |
| 2011–2015 | 263,472 (49.4%) | 174,899 (48.1%) | 31,896 (50.2%) | 1264 (56.5%) | 18,693 (52.7%) | 36,720 (53.9%) | |
| Tumor features | |||||||
| Grade c | <0.001 | ||||||
| Grade 1 | 47,731 (9.0%) | 31,022 (8.5%) | 6472 (10.2%) | 206 (9.2%) | 3136 (8.9%) | 6895 (10.1%) | |
| Grade 2 | 305,250 (57.3%) | 208,444 (57.3%) | 35,787 (56.3%) | 1283 (57.4%) | 21,136 (59.6%) | 38,600 (56.7%) | |
| Grade 3 | 79,249 (14.9%) | 57,303 (15.8%) | 7613 (12.0%) | 284 (12.7%) | 4740 (12.4%) | 9309 (13.7%) | |
| Grade 4 | 10,285 (1.9%) | 7841 (2.2%) | 882 (1.4%) | 40 (1.8%) | 506 (1.4%) | 1016 (1.5%) | |
| Unknown | 90,353 (17.0%) | 58,895 (16.2%) | 12,783 (20.1%) | 423 (18.9%) | 5935 (16.7%) | 12,317 (18.1%) | |
| SEER Summary Stage | <0.001 | ||||||
| Localized | 208,901 (39.2%) | 144,787 (39.8%) | 24,077 (37.9%) | 821 (36.7%) | 13,969 (39.4%) | 25,247 (37.1%) | |
| Regionalized | 185,436 (34.8%) | 127,683 (35.1%) | 20,304 (32.0%) | 772 (34.5%) | 12,705 (35.8%) | 23,972 (35.2%) | |
| Distant | 108,900 (20.4%) | 71,773 (19.7%) | 15,229 (24.0%) | 501 (22.4%) | 6788 (19.2%) | 14,609 (21.4%) | |
| Unknown | 29,631 (5.6%) | 19,262 (5.3%) | 3927 (6.2%) | 142 (6.4%) | 1991 (5.6%) | 4309 (6.3%) | |
| Primary site d | <0.001 | ||||||
| Right | 217,640 (40.8%) | 155,220 (42.7%) | 26,871 (42.3%) | 801 (35.8%) | 10,514 (29.7%) | 24,234 (35.6%) | |
| Left | 315,228 (59.2%) | 208,285 (57.3%) | 36,666 (57.7%) | 1435 (64.2%) | 24,939 (70.3%) | 43,903 (64.4%) | |
| Treatment modality | |||||||
| Surgery | <0.001 | ||||||
| No | 97,699 (18.3%) | 63,682 (17.5%) | 13,892 (21.9%) | 473 (21.2%) | 5971 (16.8%) | 13,681 (20.1%) | |
| Yes | 435,169 (81.7%) | 299,823 (82.5%) | 49,645 (78.1%) | 1763 (78.9%) | 29,482 (83.2%) | 54,456 (80.0%) | |
| Radiation | <0.001 | ||||||
| No | 474,252 (89.0%) | 323,109 (88.9%) | 57,807 (91.0%) | 1866 (83.5%) | 30,577 (86.3%) | 60,893 (89.4%) | |
| Yes | 58,616 (11.0%) | 40,396 (11.1%) | 5730 (9.0%) | 370 (16.6%) | 4876 (13.8%) | 7244 (10.6%) | |
| Chemotherapy | <0.001 | ||||||
| No | 346,719 (65.1%) | 239,971 (66.0%) | 41,370 (65.1%) | 1332 (59.6%) | 22,145 (62.5%) | 41,901 (61.5%) | |
| Yes | 186,149 (34.9%) | 123,534 (34.0%) | 22,167 (34.9%) | 904 (40.4%) | 13,308 (37.5%) | 26,236 (38.5%) | |
| Crude Model a | Model 1 a | Model 2 a | Model 3 a | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Race/ethnicity | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| NHW | Reference | Reference | Reference | Reference | ||||
| NHB | 1.13 (1.11, 1.15) | 1.20 (1.18, 1.22) | 1.16 (1.14, 1.18) | 1.12 (1.10, 1.13) | ||||
| AI/AN | 1.02 (0.95, 1.09) | 1.11 (1.03, 1.19) | 1.09 (1.02, 1.17) | 1.05 (0.97, 1.12) | ||||
| Asian/PI | 0.84 (0.82, 0.86) | 0.92 (0.90, 0.94) | 0.92 (0.90, 0.94) | 0.91 (0.89, 0.93) | ||||
| Hispanic | 0.95 (0.93, 0.96) | 1.04 (1.02, 1.05) | 1.00 (0.98, 1.01) | 0.97 (0.96, 0.99) | ||||
| Persistent poverty b | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.15 (1.13, 1.16) | 1.12 (1.10, 1.14) | 1.11 (1.09, 1.13) | 1.10 (1.08, 1.12) | ||||
| Rurality b | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.07 (1.05, 1.08) | 1.09 (1.07, 1.10) | 1.10 (1.09, 1.12) | 1.11 (1.10, 1.13) | ||||
| Rurality b No | Rurality b Yes | ||||
|---|---|---|---|---|---|
| 5-Year CRC Survival Statusc No/Yes n (%) | HR (95% CI) a | 5-Year CRC Survival Status c No/Yes n (%) | HR (95% CI) a | HR (95% CI) for Rurality Within Strata of Poverty | |
| Persistent poverty b | |||||
| Non-Hispanic White | |||||
| No (n = 343,676) | 90,702 (31.2%)/199,828 (66.8%) | Reference | 17,491 (32.9%)/35,655 (67.1%) | 1.10 (1.08,1.12) | 1.10 (1.08, 1.12) |
| Yes (n = 19,829) | 4160 (35.4%)/7589 (64.6%) | 1.13 (1.10, 1.17) | 2819 (34.9%)/5261 (65.1%) | 1.26 (1.21,1.31) | 1.12 (1.06, 1.17) |
| HR (95% CI) for poverty within strata of rurality | 1.13 (1.10, 1.17) | 1.15 (1.10, 1.19) | |||
| Non-Hispanic Black | |||||
| No (n = 45,843) | 14,859 (34.7%)/27,936 (65.3%) | Reference | 1145 (37.6%)/1903 (62.4%) | 1.11 (1.05, 1.18) | 1.11 (1.05, 1.18) |
| Yes (n = 17,694) | 5859 (38.1%)/9517 (61.9%) | 1.10 (1.07, 1.14) | 901 (38.9%)/1417 (61.1%) | 1.20 (1.12, 1.28) | 1.09 (1.01, 1.16) |
| HR (95% CI) for poverty within strata of rurality | 1.10 (1.07, 1.14) | 1.07 (0.98, 1.17) | |||
| AI/AN | |||||
| No (n = 1782) | 425 (31.6%)/922 (68.5%) | Reference | 157 (36.1%)/278 (63.9%) | 1.19 (0.98, 1.43) | 1.19 (0.98, 1.43) |
| Yes (n = 454) | 75 (31.5%)/163 (68.5%) | 0.99 (0.77, 1.27) | 93 (43.1%)/123 (56.9%) | 1.66 (1.32, 2.08) | 1.67 (1.23, 2.28) |
| HR (95% CI) for poverty within strata of rurality | 0.99 (0.77, 1.27) | 1.40 (1.08, 1.82) | |||
| Asian/PI | |||||
| No (n = 33,113) | 8787 (27.4%)/23,264 (72.6%) | Reference | 338 (31.8%)/724 (68.2%) | 1.17 (1.05, 1.31) | 1.17 (1.05, 1.31) |
| Yes (n = 2340) | 721 (32.5%)/1501 (67.6%) | 1.11 (1.03, 1.19) | 38 (32.2%)/80 (67.8%) | 1.19 (0.87, 1.64) | 1.08 (0.78, 1.50) |
| HR (95% CI) for poverty within strata of rurality | 1.11 (1.03, 1.19) | 1.02 (0.73, 1.42) | |||
| Hispanic | |||||
| No (n = 52,460) | 14,959 (30.6%)/33,887 (69.4%) | Reference | 1219 (33.7%)/2395 (66.3%) | 1.15 (1.09, 1.22) | 1.15 (1.09, 1.22) |
| Yes (n = 15,677) | 4546 (32.6%)/9419 (67.5%) | 1.05 (1.01, 1.09) | 586 (34.2%)/1126 (65.8%) | 1.18 (1.09, 1.29) | 1.12 (1.03, 1.23) |
| HR (95% CI) for poverty within strata of rurality | 1.05 (1.02, 1.09) | 1.03 (0.93, 1.14) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coughlin, S.S.; Tsai, M.-H.; Cortes, J.; Bevel, M.; Vernon, M. The Influence of Poverty and Rurality on Colorectal Cancer Survival by Race/Ethnicity: An Analysis of SEER Data with a Census Tract-Level Measure of Persistent Poverty. Curr. Oncol. 2025, 32, 248. https://doi.org/10.3390/curroncol32050248
Coughlin SS, Tsai M-H, Cortes J, Bevel M, Vernon M. The Influence of Poverty and Rurality on Colorectal Cancer Survival by Race/Ethnicity: An Analysis of SEER Data with a Census Tract-Level Measure of Persistent Poverty. Current Oncology. 2025; 32(5):248. https://doi.org/10.3390/curroncol32050248
Chicago/Turabian StyleCoughlin, Steven S., Meng-Han Tsai, Jorge Cortes, Malcolm Bevel, and Marlo Vernon. 2025. "The Influence of Poverty and Rurality on Colorectal Cancer Survival by Race/Ethnicity: An Analysis of SEER Data with a Census Tract-Level Measure of Persistent Poverty" Current Oncology 32, no. 5: 248. https://doi.org/10.3390/curroncol32050248
APA StyleCoughlin, S. S., Tsai, M.-H., Cortes, J., Bevel, M., & Vernon, M. (2025). The Influence of Poverty and Rurality on Colorectal Cancer Survival by Race/Ethnicity: An Analysis of SEER Data with a Census Tract-Level Measure of Persistent Poverty. Current Oncology, 32(5), 248. https://doi.org/10.3390/curroncol32050248






