Comparative Study into Microstructural and Mechanical Characterization of HVOF-WC-Based Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spray Powders
2.2. Substrate and Preparation
2.3. Coating Procedure and Equipment
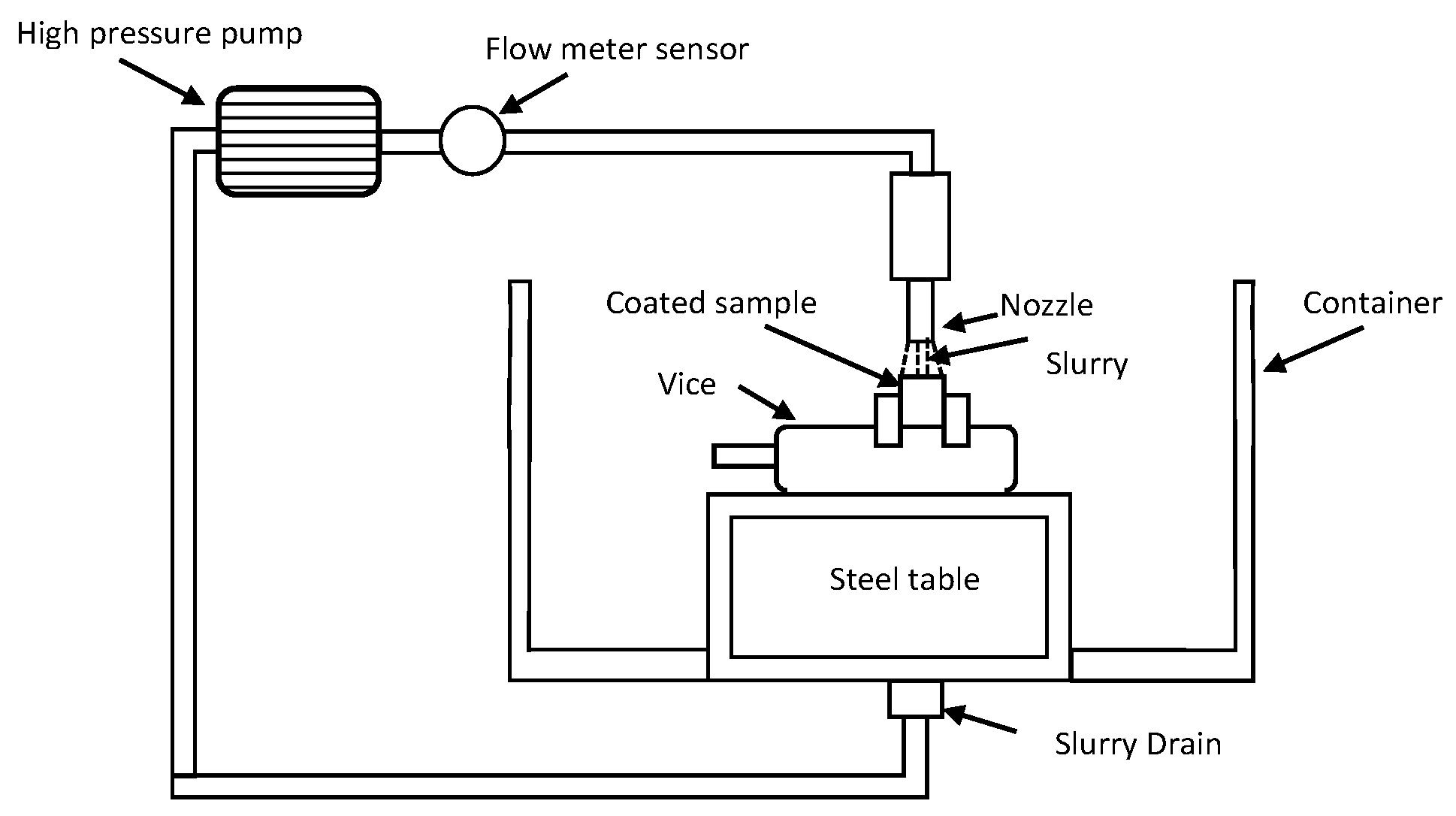
2.4. Erosion Test
3. Results and Discussion
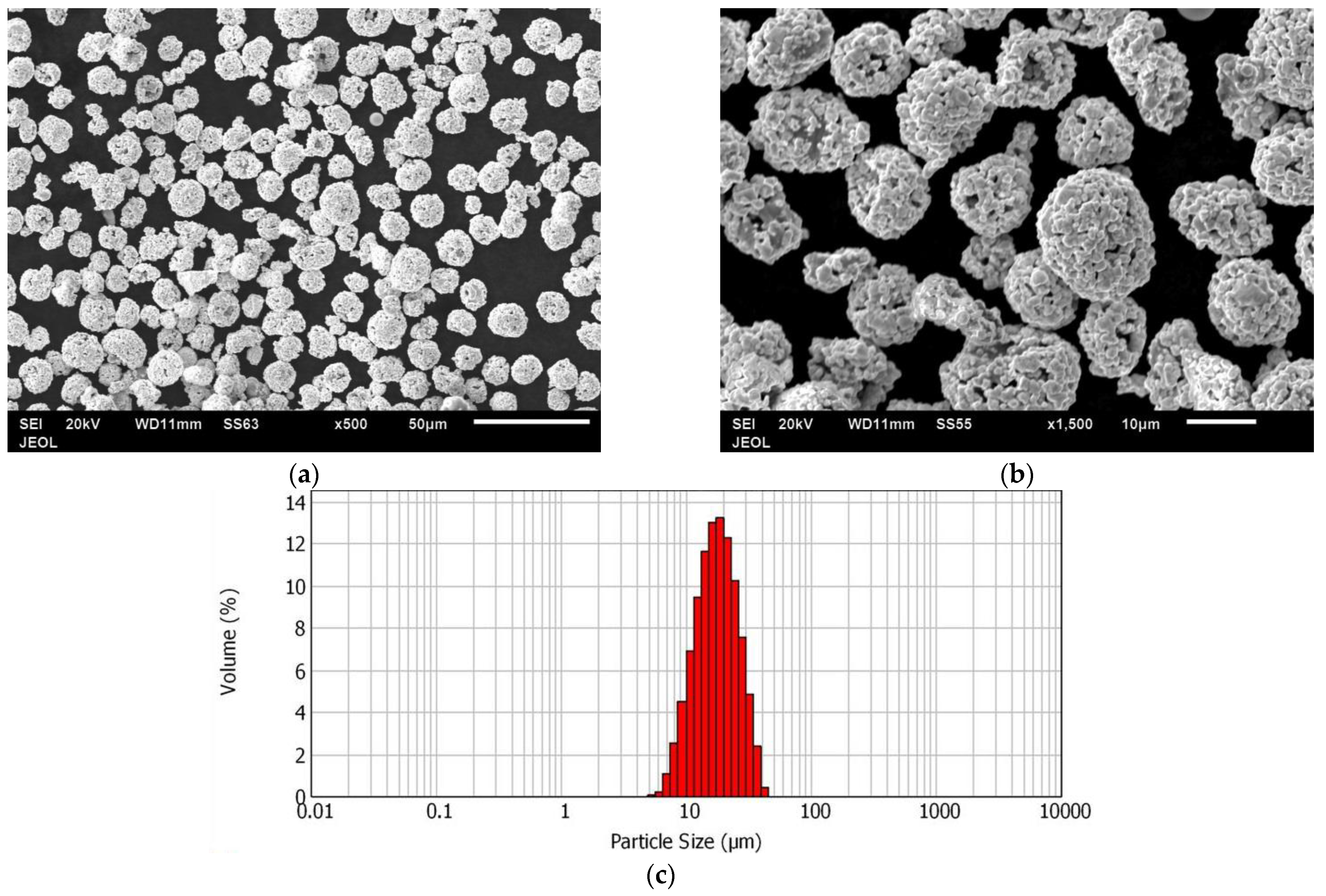
3.1. Powders Characterization
3.1.1. WC-12Co Powder
3.1.2. WC-10Co-4Cr Powder
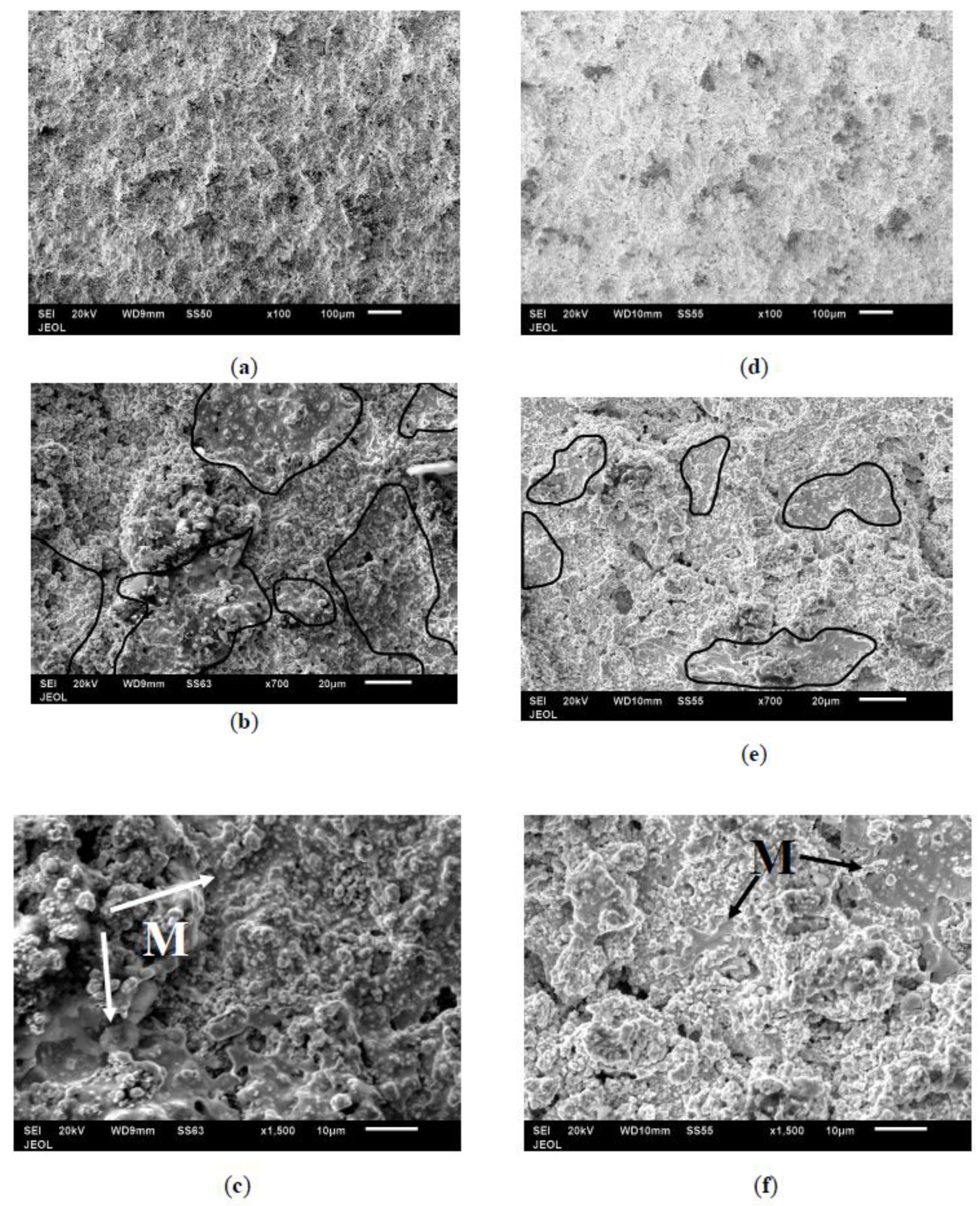
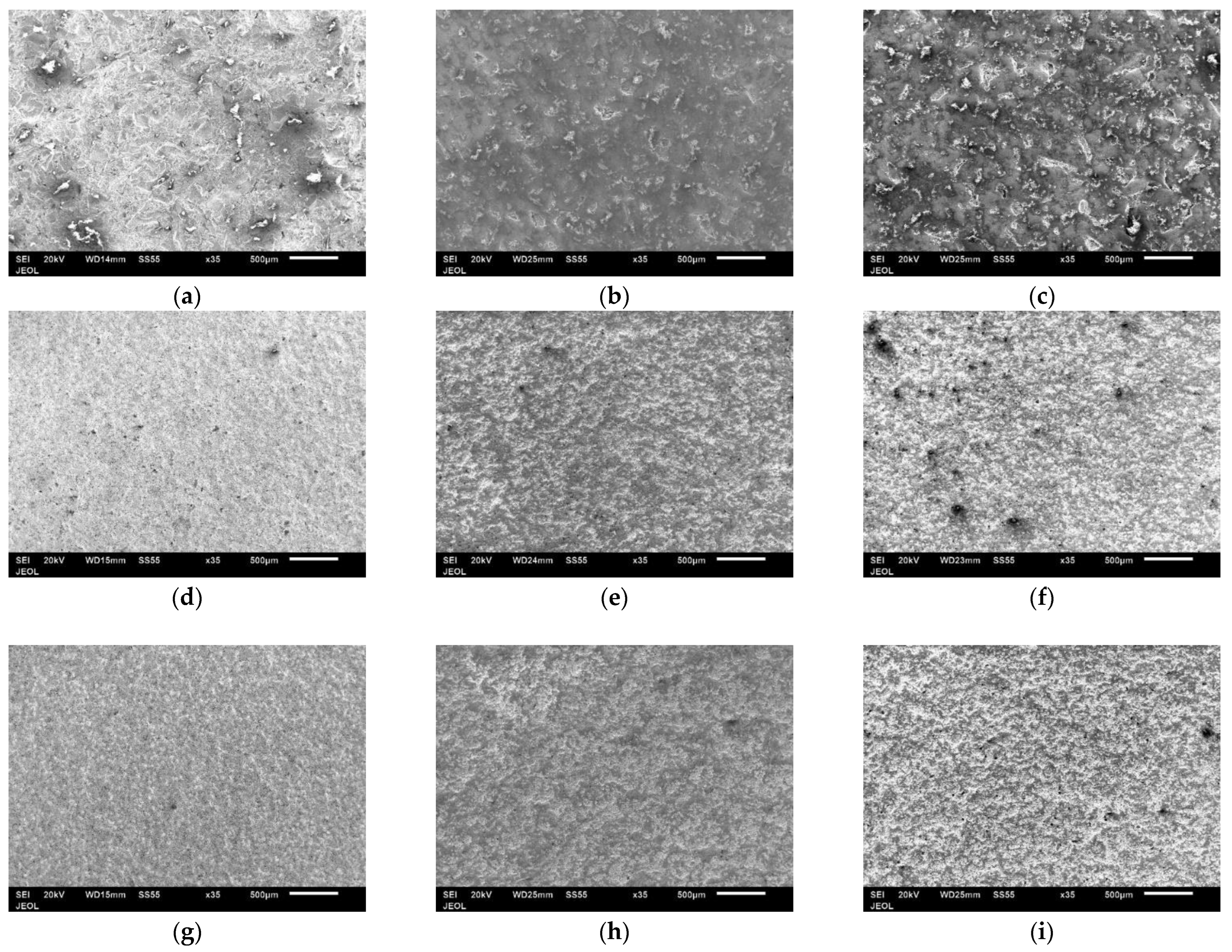
3.2. Surface Characterization of As-Sprayed Coatings
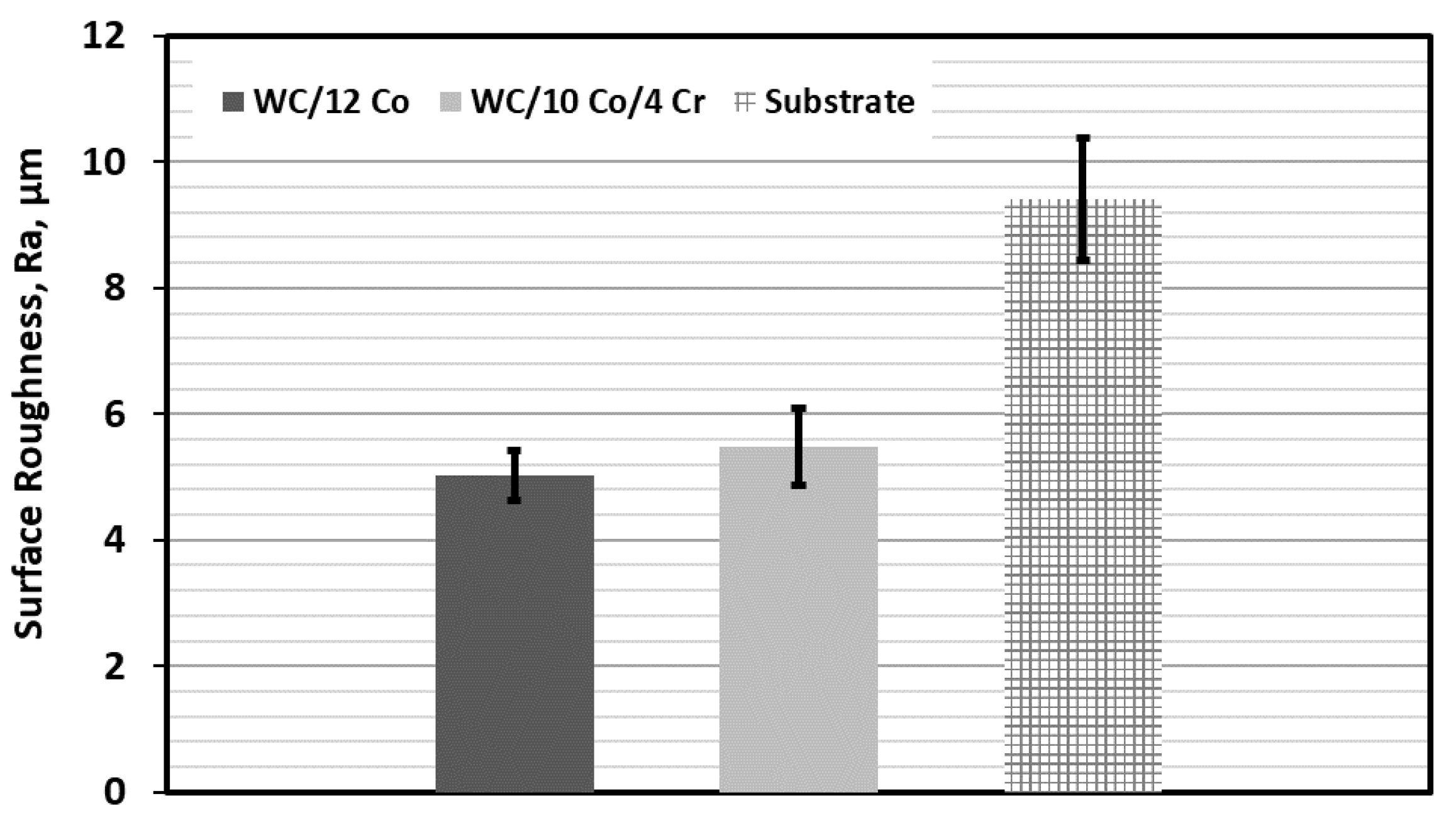
3.3. Surface Roughness
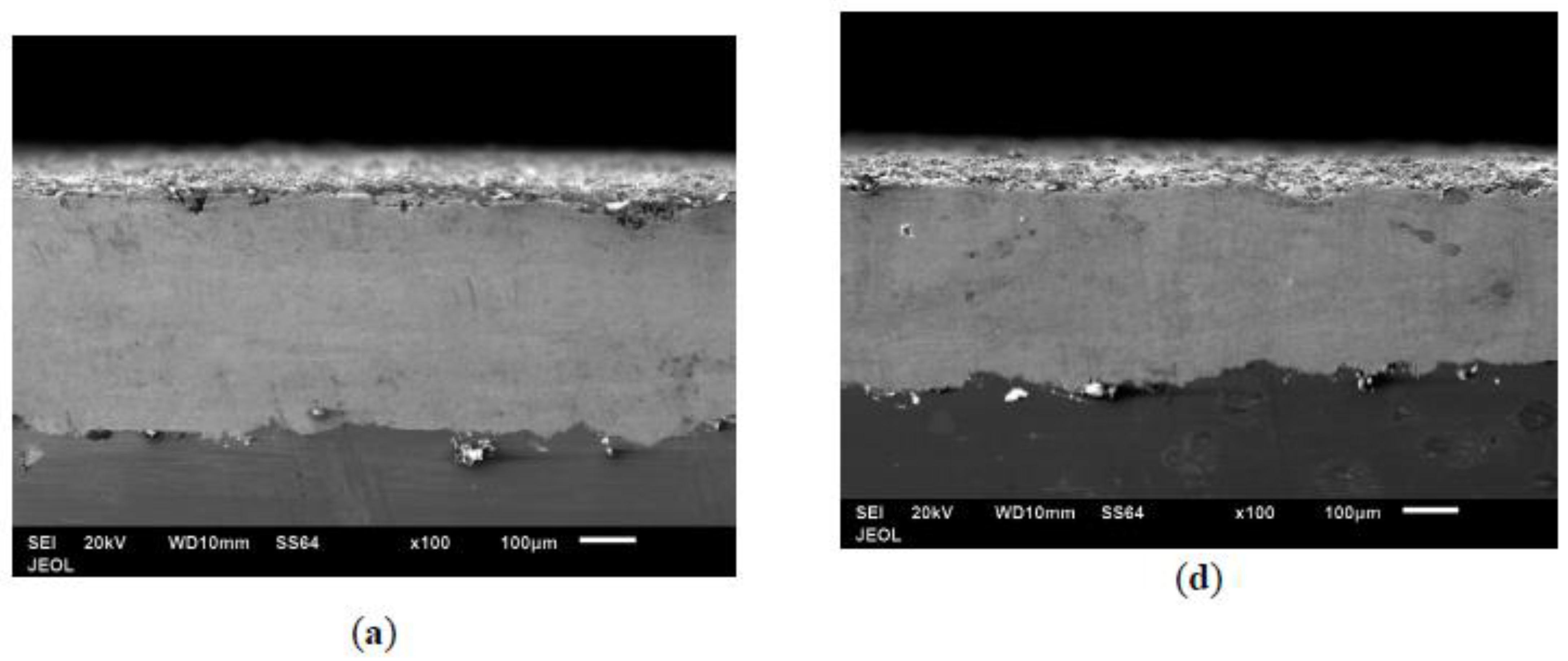
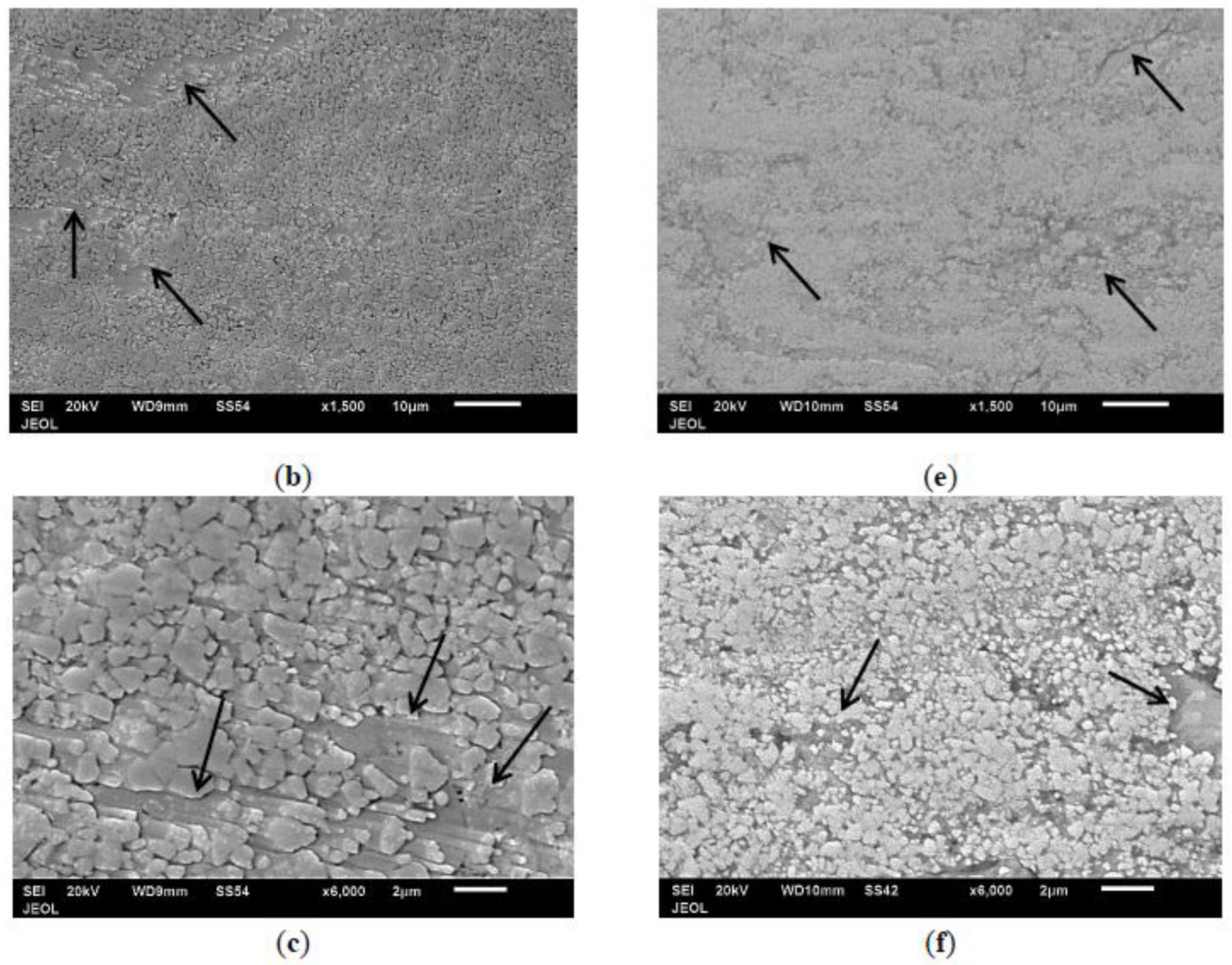
3.4. Morphology of Coating Layer
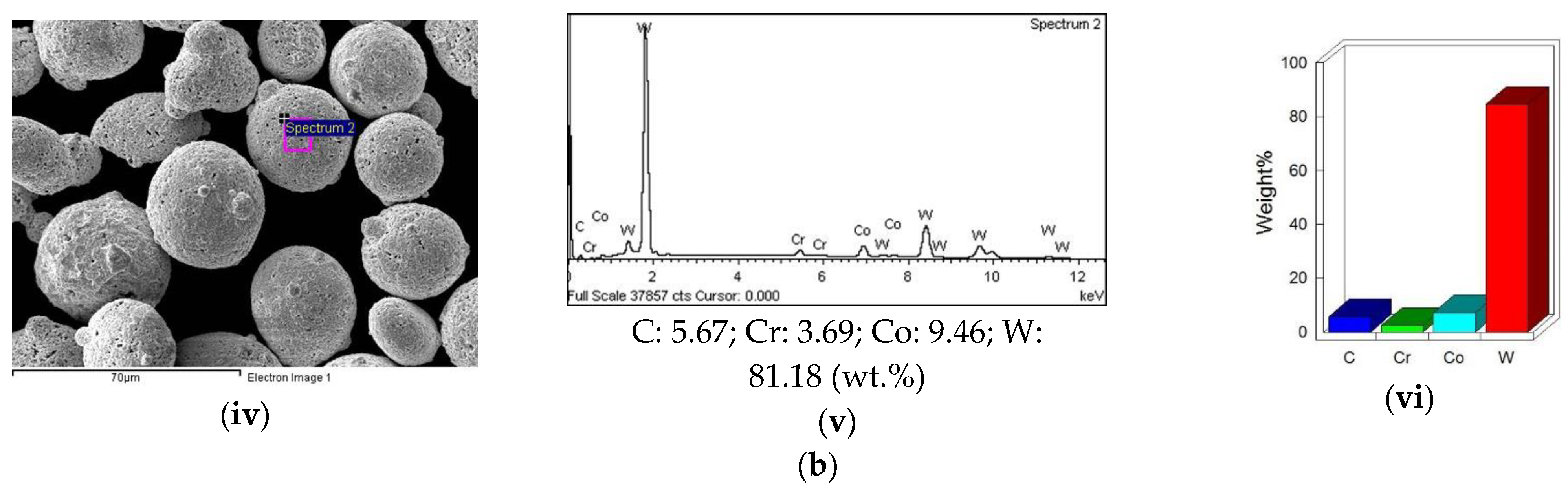
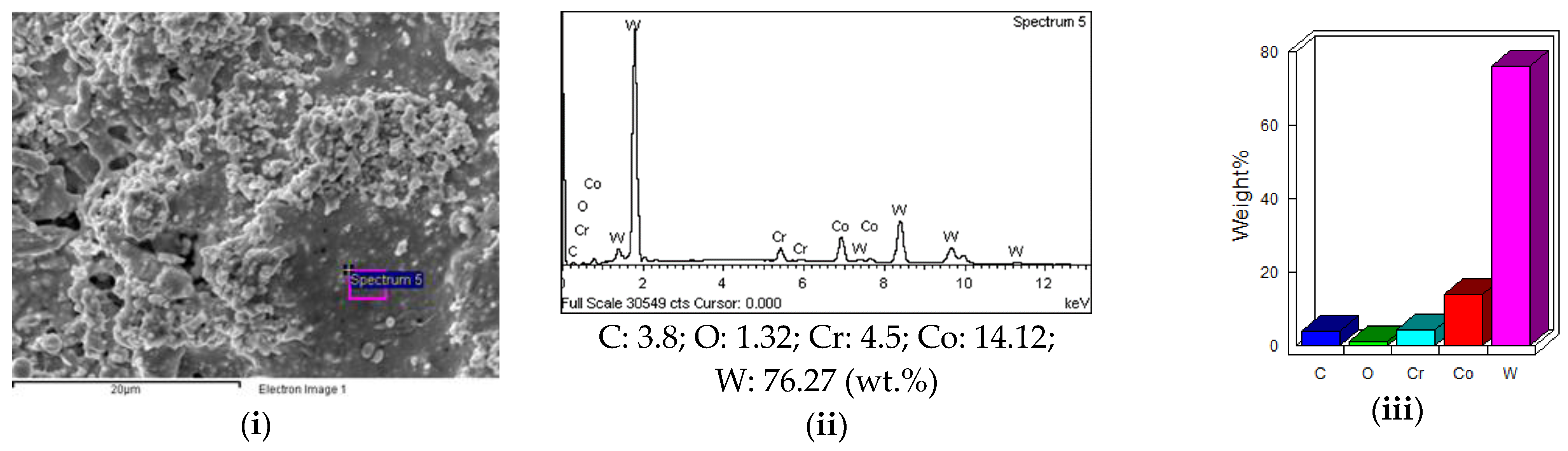
3.5. EDS Analysis of Feed Stock Powders and Coating Layers
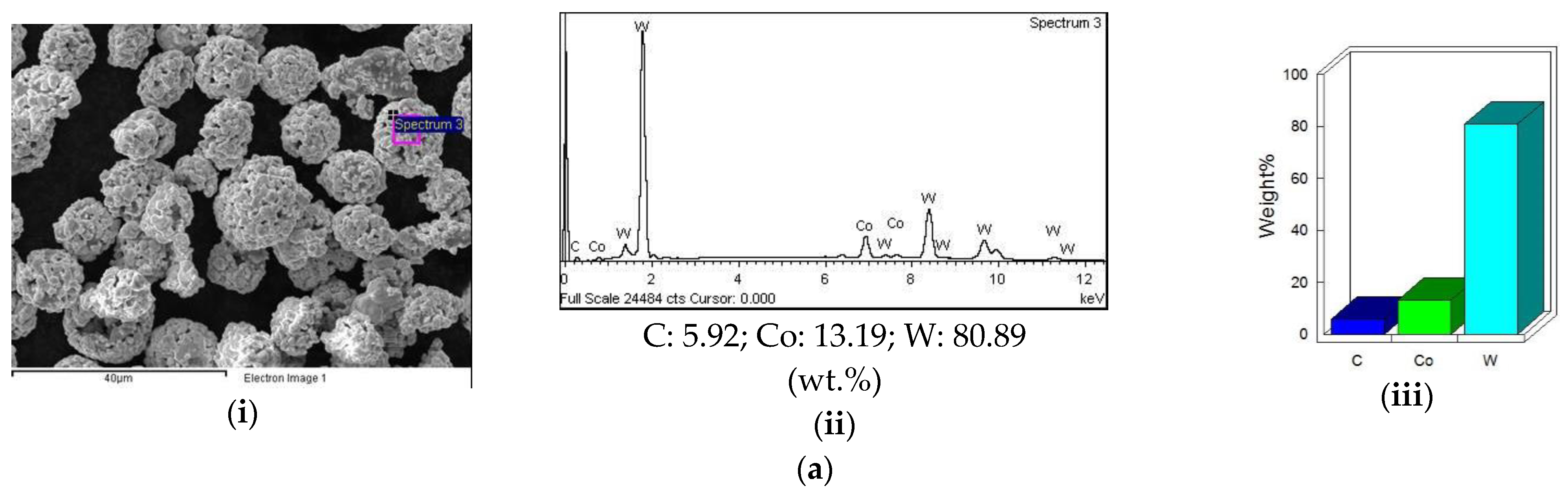
3.5.1. EDS Analysis of Feed Stock Powders
3.5.2. EDS Analysis of the Coating
EDS of WC-12Co Coating
EDS of WC-10Co-4Cr Coating
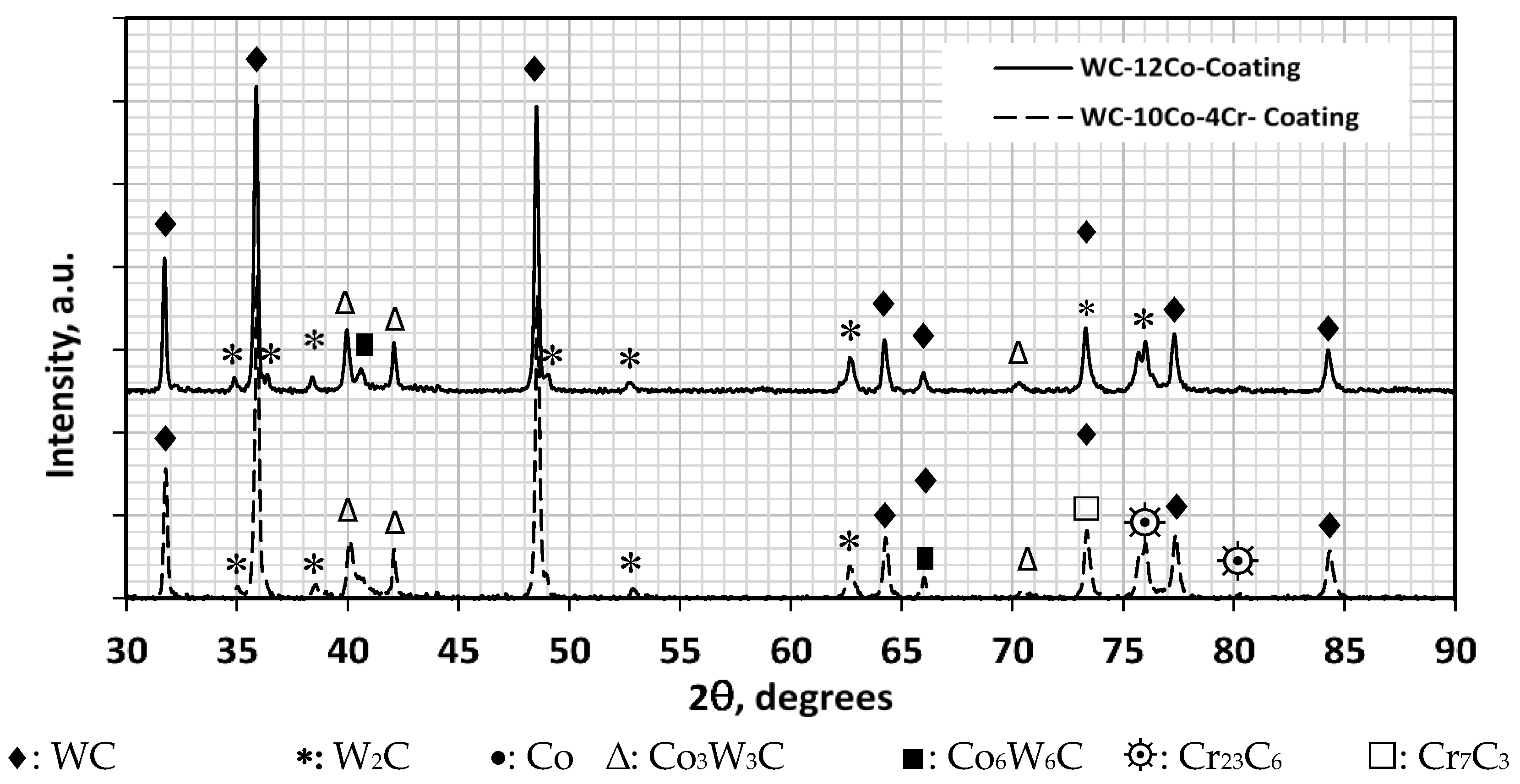
3.6. XRD of WC-12Co in Powder and Coating Conditions
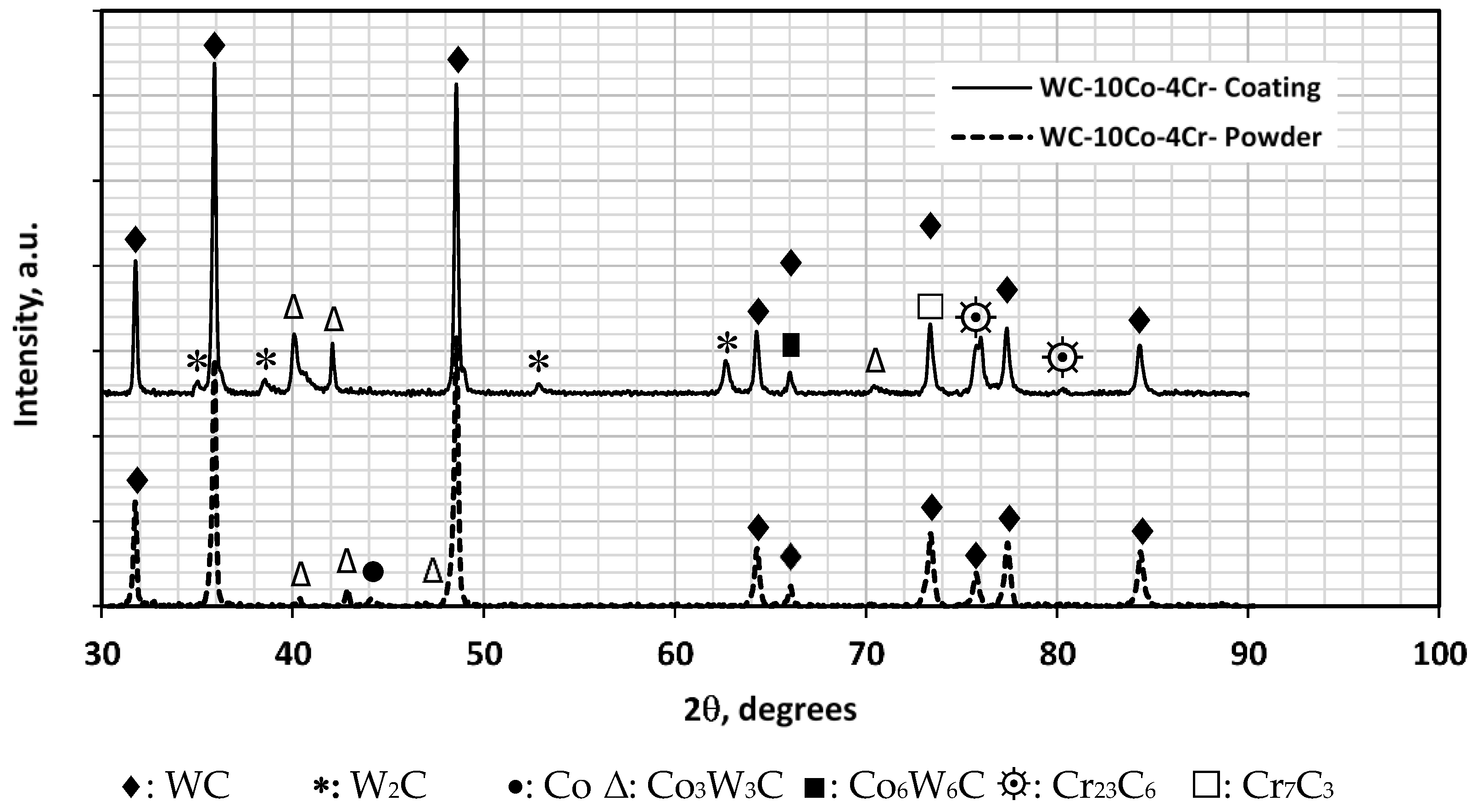
3.7. XRD of WC-10Co-4Cr in Powder and Coating Conditions
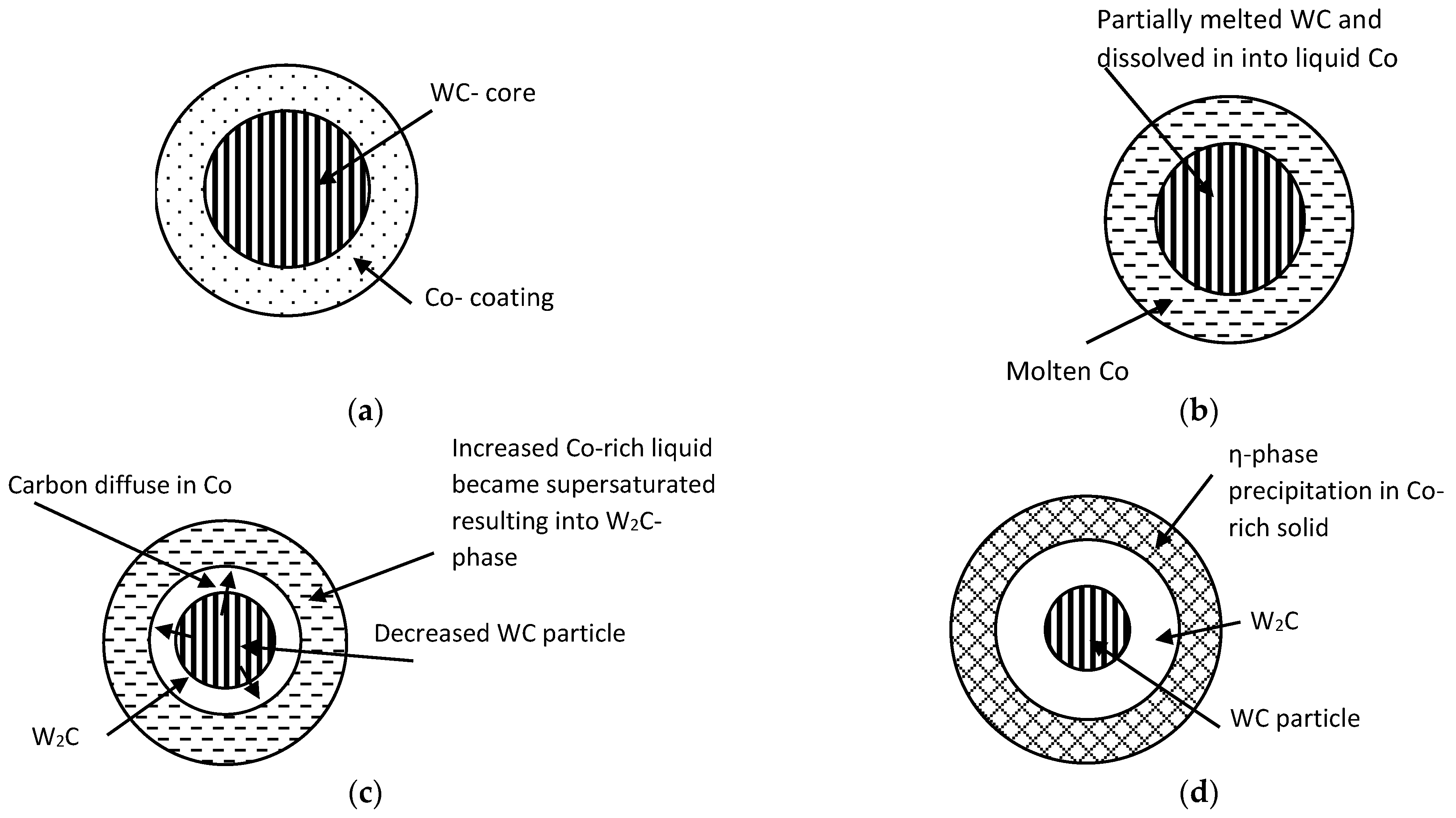
Effect of Particle Size on Decarburization
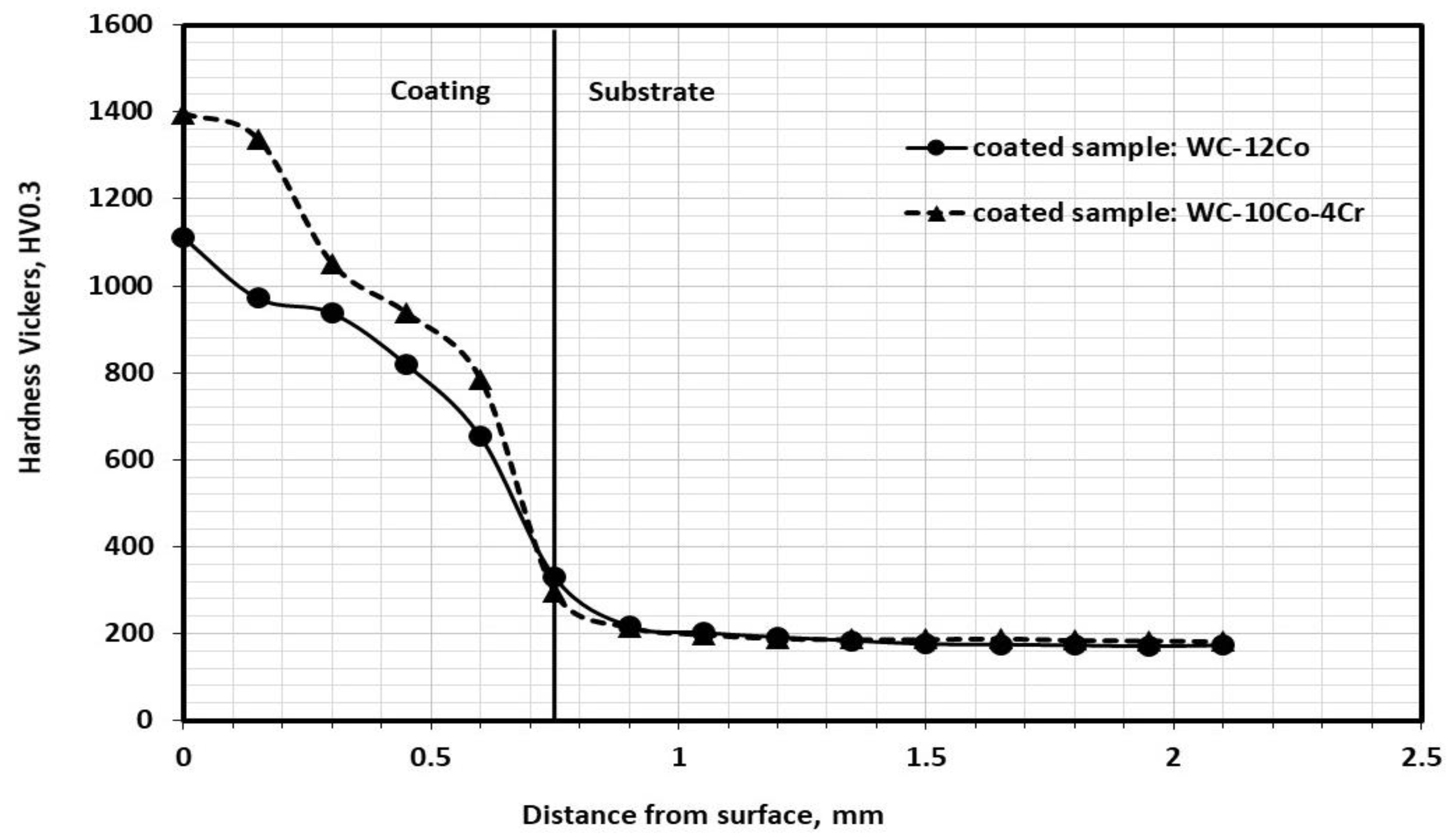
3.8. Microhardness Testing
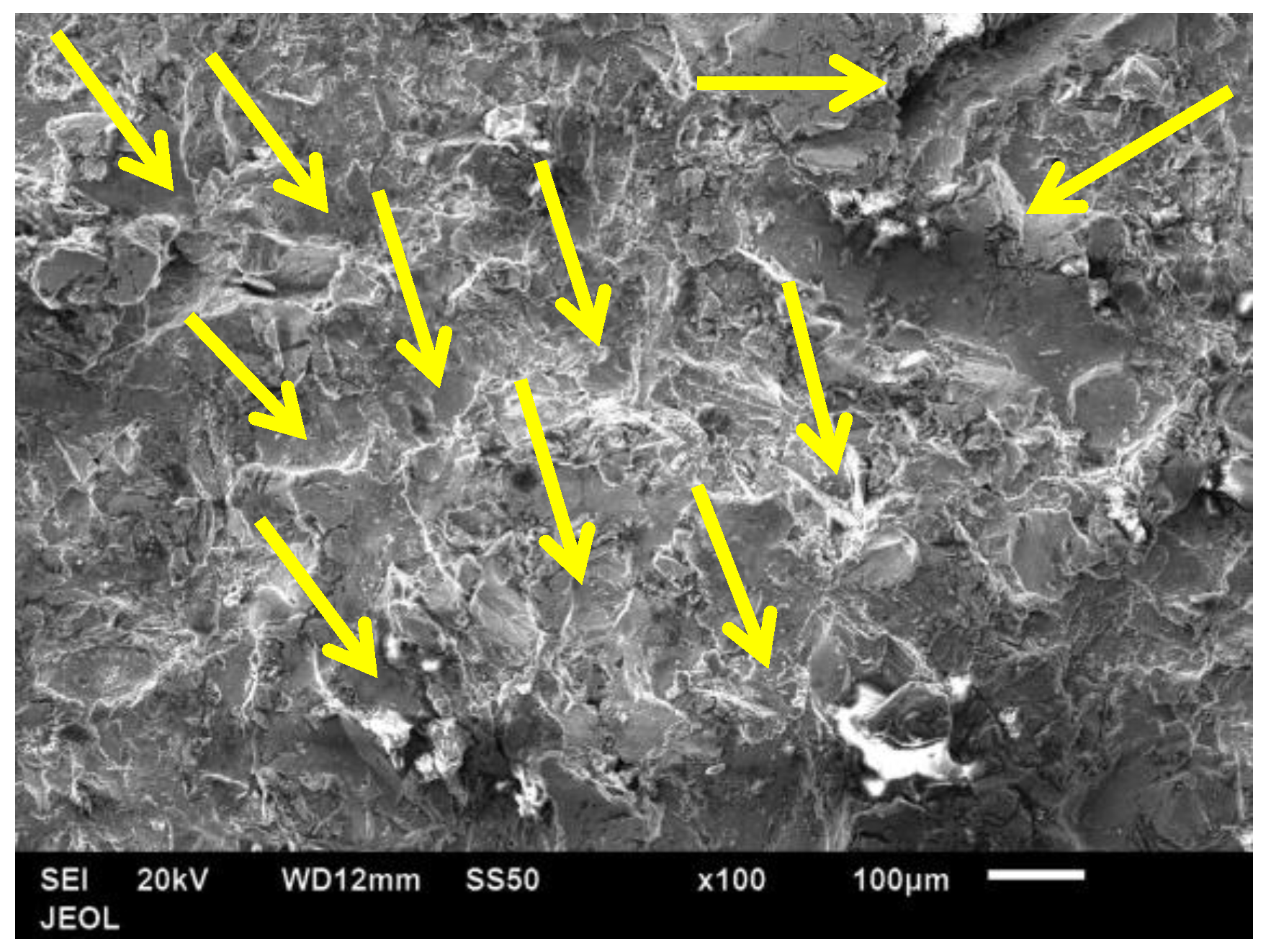
3.9. Erosion Test
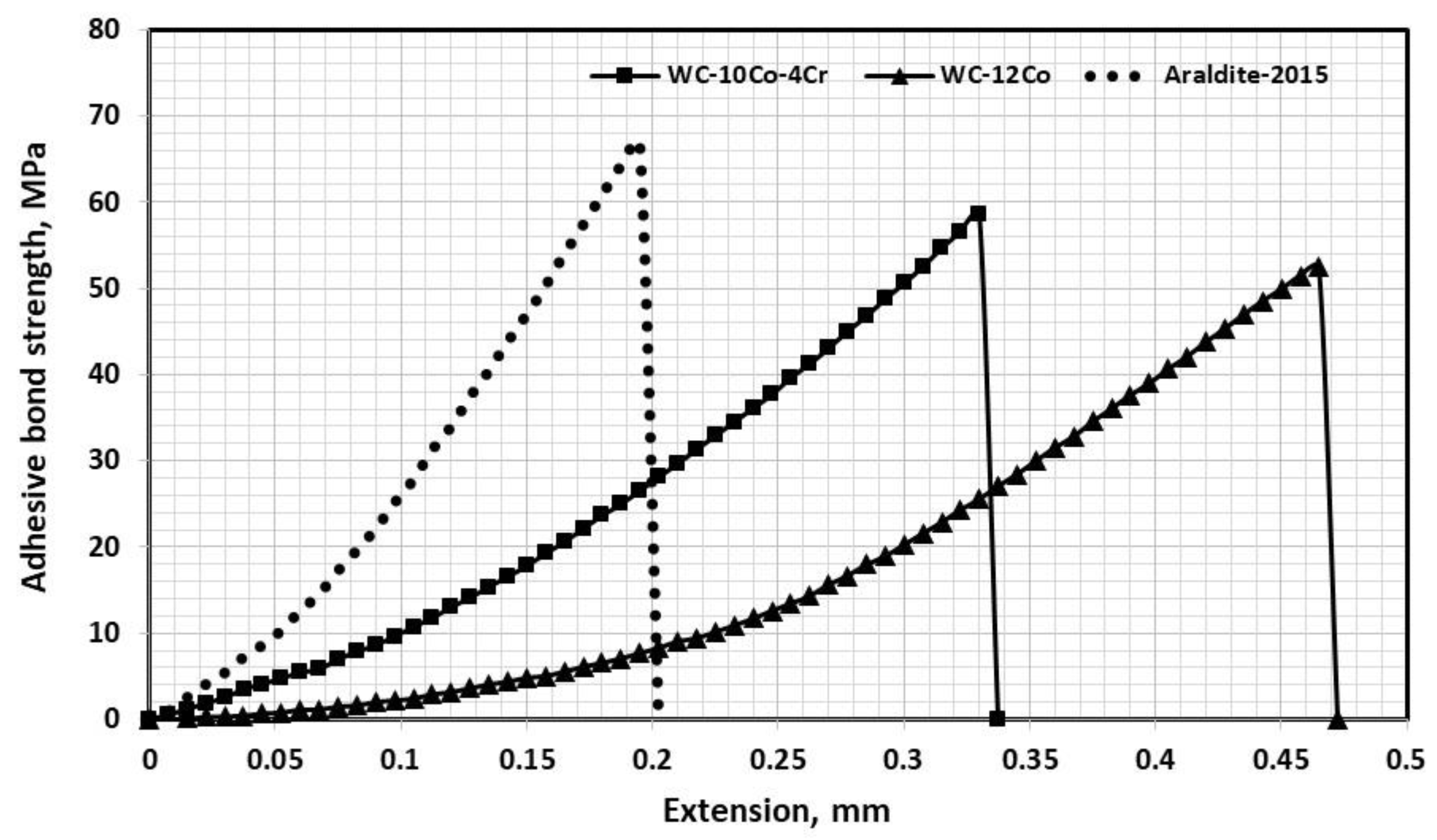
3.10. Tensile Adhesion/Bond Strength
4. Conclusions
- (1)
- Microstructural testing showed that powder with smaller particles size was more prone to melting than the larger one leading to a smoother coating surface.
- (2)
- Some compositional variations were noticed from EDS test results between powder and coating due to decomposition, whereas, XRD indicated the occurrence of decarburization and showed that the extent of decarburization was more pronounced with the smaller particle size powder WC-12Co than with larger one WC-10C0-4Cr.
- (3)
- XRD tests of WC-12Co coating identified the presence of W2C and Co3W3C and Co6W6C (η-phase), whereas the same phases were present with WC-10C0-4Cr coating with the addition of Cr23C6 and Cr7C3 causing the former powder to be softer than the later one.
- (4)
- WC-10C0-4Cr coating is more erosion resistant than WC-12Co one due to its superior hardness as well as possessing less matrix free-path that erodes faster than carbide particles.
- (5)
- Adhesion strength between WC-10C0-4Cr coating layer and substrate was higher than that of WC-12Co due to the smaller carbide size in conjunction with the molten metallic binder being able to lodge itself within the substrate’s rough surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, M.; Zhang, S.; Li, M. Comparative investigation on HVOF sprayed carbide-based coatings. Appl. Surf. Sci. 2013, 273, 799–805. [Google Scholar] [CrossRef]
- Bulnes, A.G.; Fuentes, V.A.; Cano, I.G.; Dosta, S. Understanding the Influence of High Velocity Thermal Spray Techniques on the Properties of Different Anti-Wear WC-Based Coatings. Coatings 2020, 10, 1157. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Marginean, G. Wear and corrosion behavior of thermally sprayed cermet coatings. Surf. Coat. Technol. 2001, 138, 149–158. [Google Scholar] [CrossRef]
- Richert, M.W.; Mikułowski, B.; Pałka, P.; Hotloś, A.; Perek-Nowak, M. The Effect of Chemical Composition and Thermal Sprayed Method on the Chromium and Tungsten Carbides Coatings Microstructure. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Schwetzke, R.; Kreye, H. Microstructure and Properties of Tungsten Carbide Coatings Sprayed with Various High- Velocity Oxygen Fuel Spray Systems. J. Therm. Spray Technol. 1999, 8, 433. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Liu, Y.; Gou, G.; Li, D. Effects of Cr on microstructure and hardness of HVOF-sprayed WC-Co coating. Adv. Mater. Res. 2011, 317–319, 301–306. [Google Scholar] [CrossRef]
- Fang, W.; Cho, T.Y.; Yoon, J.H.; Song, K.O.; Hur, S.K.; Youn, S.J.; Chun, H.G. Processing optimization, surface properties and wear behavior of HVOF spraying WC–CrC–Ni coating. J. Mater. Process. Technol. 2009, 209, 3561–3567. [Google Scholar] [CrossRef]
- Hadad, M.; Hockauf, M.; Meyer, L.W.; Marot, G.; Lesage, J.; Hitzek, R.; Siegmann, S. Adhesion evaluation of multilayered based WC–Co–Cr thermally sprayed coatings. Surf. Coat. Technol. 2008, 202, 4399–4405. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Performance of High-Velocity Oxyfuel-Sprayed Coatings on an Fe-Based Superalloy in Na2SO4-60%V2O5 Environment at 900 °C, Part I: Characterization of the Coatings. J. Mater. Eng. Perform. 2006, 15, 122–129. [Google Scholar] [CrossRef]
- Jafari, M.; Enayati, M.H.; Salehi, M.; Nahvi, S.M.; Park, C.G. Comparison between oxidation kinetics of HVOF sprayed WC-12Co and WC-10Co-4Cr coatings. Int. J. Refract. Met. Hard Mater. 2013, 41, 78–84. [Google Scholar] [CrossRef]
- Yang, Q.; Senda, T.; Ohmori, A. Effect of carbide grain size on microstructure and sliding wear behavior of HVOF-sprayed WC–12% Co coatings. Wear 2003, 254, 23–34. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Cheng, Y.; Xiang, J.; Zhao, X.; Yang, G. Wear and corrosion performance of WC-10Co4Cr coatings deposited by different HVOF and HVAF spraying processes. Surf. Coat. Technol. 2013, 218, 127–136. [Google Scholar] [CrossRef]
- Al-Mutairi, S.; Hashmi, M.S.J.; Yilbas, B.S.; Stokes, J. Microstructural characterization of HVOF/plasma thermal spray of micro/nano WC–12%Co powders. Surf. Coat. Technol. 2015, 264, 175–186. [Google Scholar] [CrossRef]
- Pulsford, J.; Kamnis, S.; Murray, J.; Bai, M.; Hussain, T. Effect of Particle and Carbide Grain Sizes on a HVOAF WC-Co-Cr Coating for the Future Application on Internal Surfaces: Microstructure and Wear. J. Therm. Spray Technol. 2018, 27, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Pauschitz, A.; Bernardi, J.; Koch, T.; Franek, F. Microstructure and Mechanical Properties of HVOF Sprayed Nanocrystalline Cr3C2-25(NiCr) Coating. J. Therm. Spray Technol. 2006, 15, 372–381. [Google Scholar] [CrossRef]
- Mishra, T.K.; Kumar, A.; Sinha, S.K. Experimental investigation and study of HVOF sprayed WC-12Co, WC-10Co-4Cr and Cr3C2-25NiCr coating on its sliding wear behavior. Int. J. Refract. Met. Hard Mater. 2021, 94, 105404. [Google Scholar] [CrossRef]
- Ozbek, Y.Y.; Canikoglu, N.; Ipek, M. The Surface Properties of WC–Co–Cr Based Coatings Deposited by High Velocity Oxygen Fuel Spraying. Acta Phys. Pol. A 2017, 131, 186–189. [Google Scholar] [CrossRef]
- Kear, B.H.; Skandan, G.; Sadangi, R.K. Factors Controlling Decarburization in HVOF Sprayed Nano-WC/Co Hard coatings. Scr. Mater. 2001, 44, 1703–1707. [Google Scholar] [CrossRef]
- SNahvi, M.; Jafari, M. Microstructural and mechanical properties of advanced HVOF-sprayed WC-based cermet coatings. Surf. Coat. Technol. 2016, 286, 95–102. [Google Scholar]
- Stewart, D.A.; Shipway, P.H.; Cartney, D.G.M. Microstructural Evolution in Thermally Sprayed WC-Co Coating: Comparison between Nanocomposite and Conventional Starting Powders. Acta Mater. 2000, 48, 1593–1604. [Google Scholar] [CrossRef]
- Nerz, J.; Kushner, B.; Rotolico, A. Microstructural Evaluation of Tungsten Carbide-Cobalt Coatings. J. Therm. Spray Technol. 1992, 1, 147. [Google Scholar] [CrossRef]
- Guilemany, J.M.; de Paco, J.M.; Nutting, J.; Miguel, J.R. Characterization of the W2C Phase Formed during the High Velocity Oxygen Fuel Spraying of a WC+12 % Co Powder. Metall. Mater. Trans. A 1999, 30, 1913. [Google Scholar] [CrossRef]
- Ludwig, G.A.; Malfatti, C.F.; Schroeder, R.M.; Ferrari, V.Z.; Muller, I.L. WC10Co4Cr coatings deposited by HVOF on martensitic stainless steel for use in hydraulic turbines: Resistance to corrosion and slurry erosion. Surf. Coat. Technol. 2019, 377, 124918. [Google Scholar] [CrossRef]
- Pulsford, J.; Venturi, F.; Pala, Z.; Kamnis, S.; Hussain, T. Application of HVOF WC-Co-Cr coatings on the internal surface of small cylinders: Effect of internal diameter on the wear resistance. Wear 2019, 432, 202965. [Google Scholar] [CrossRef]
- Kumari, K.; Anand, K.; Bellacci, M.; Giannozzi, M. Effect of microstructure on abrasive wear behavior of thermally sprayed WC-10Co-4Cr coatings. Wear 2010, 265, 1309–1319. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Nutting, J.; Llorca-lsern, N. Microstructural Examination of HVOF Chromium Carbide Coatings for High-Temperature Applications. J. Therm. Spray Technol. 1996, 5, 483–489. [Google Scholar] [CrossRef]
- Yuan, J.; Zhan, Q.; Huang, J.; Ding, S.; Li, H. Decarburization mechanisms of WC-Co during thermal spraying: Insights from controlled carbon loss and microstructure characterization. Mater. Chem. Phys. 2013, 142, 165–171. [Google Scholar] [CrossRef]
- ASTM C633-13; Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings. R&B, Inc.: Colmar, PA, USA, 2021.
- Lyphout, C.; Nylen, P.; Östergren, L.G. Adhesion Strength of HVOF Sprayed IN718 Coatings. J. Therm. Spray Technol. 2012, 21, 86–95. [Google Scholar] [CrossRef]






| Chemical composition; wt.% | C: 0.12 | Mn: 1.4 | P: 0.01 | S: 0.01 | Si: 0.3 | Cb: 0.02 | Ti: 0.02 |
| Mechanical properties | Yield strength: 375 MPa | Tensile strength: 525 MPa | Elongation: 18% | ||||
| Coating Parameter | Units |
|---|---|
| Fuel gas type | LPG |
| Oxygen flow rate (pressure) | 250 L/min (10 kg/cm2) |
| Fuel gas flow rate (pressure) | 60 L/min (7 kg/cm2) |
| Airflow rate (pressure) | 600 L/min (6 kg/cm2) |
| Spray distance | ≈250 mm |
| Nitrogen (carrier gas) pressure | 6 kg/cm2 |
| Powder feed rate | 90 g/min |
| WC-12Co | WC-10Co-4Cr | ||
|---|---|---|---|
| 2θ | Intensity | 2θ | Intensity |
| 34.93 | 315 | 35.09 | 216 |
| 38.47 | 323 | 38.58 | 311 |
| 52.8 | 179 | 52.9 | 170 |
| 62.7 | 742 | 62.7 | 735 |
| 70.4 | 171 | 70.7 | 126 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Rayes, M.M.; Sherif, E.-S.M.; Abdo, H.S. Comparative Study into Microstructural and Mechanical Characterization of HVOF-WC-Based Coatings. Crystals 2022, 12, 969. https://doi.org/10.3390/cryst12070969
El Rayes MM, Sherif E-SM, Abdo HS. Comparative Study into Microstructural and Mechanical Characterization of HVOF-WC-Based Coatings. Crystals. 2022; 12(7):969. https://doi.org/10.3390/cryst12070969
Chicago/Turabian StyleEl Rayes, Magdy M., El-Sayed M. Sherif, and Hany S. Abdo. 2022. "Comparative Study into Microstructural and Mechanical Characterization of HVOF-WC-Based Coatings" Crystals 12, no. 7: 969. https://doi.org/10.3390/cryst12070969








